3.1. Characteristics of NP Powders, AM Powders and FE Powders
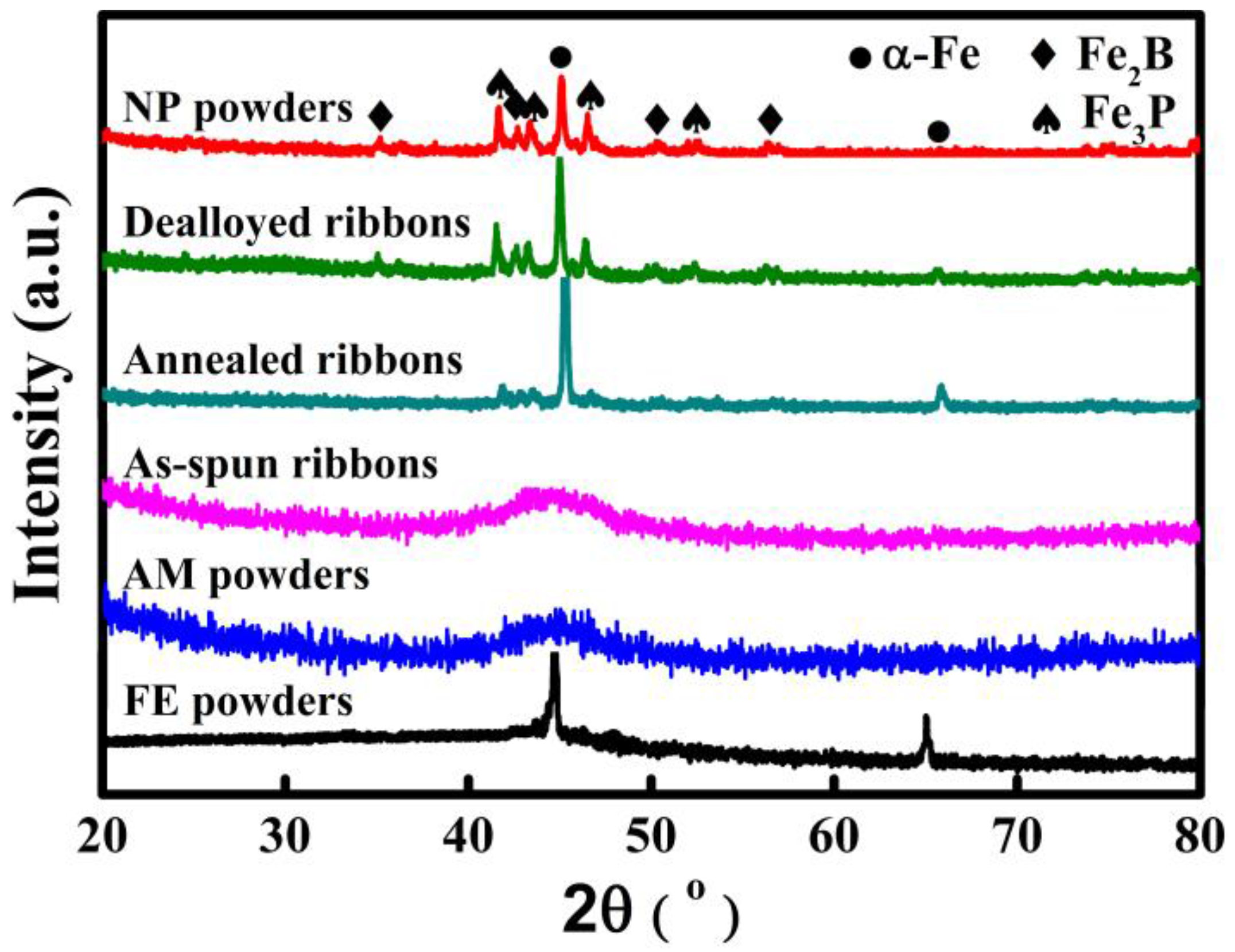
The XRD patterns of NP powders, AM powders and FE powders as well as as-spun ribbons, annealed ribbons and dealloyed ribbons are shown in
Figure 1. The appearance of a single halo diffraction peak typical for amorphous alloys indicates that both AM powders and as-spun ribbons are in an amorphous state. The diffraction peaks at about 45.3° and 65.9° of FE powders are assigned to α-Fe phase. Some diffraction peaks originating from α-Fe, Fe
2B and Fe
3P are confirmed in the pattern of annealed ribbons, indicating that the α-Fe, Fe
2B and Fe
3P phases precipitate after annealing of the as-spun ribbons. The presence of two diffraction peaks at 45.3° and 65.9° from α-Fe crystals in the pattern of NP powders and dealloyed ribbons suggests that some α-Fe phases are residual after dealloying. Meanwhile, the relative diffraction peaks of Fe
2B and Fe
3P phases are stronger in the patterns of NP powders and dealloyed ribbons than in as-annealed ribbons. It is thus predicted that α-Fe grains are partially preferentially dissolved in 0.05 M H
2SO
4 solution, which may be attributed to the potential difference between α-Fe, Fe
2B, as well as Fe
3P phases.
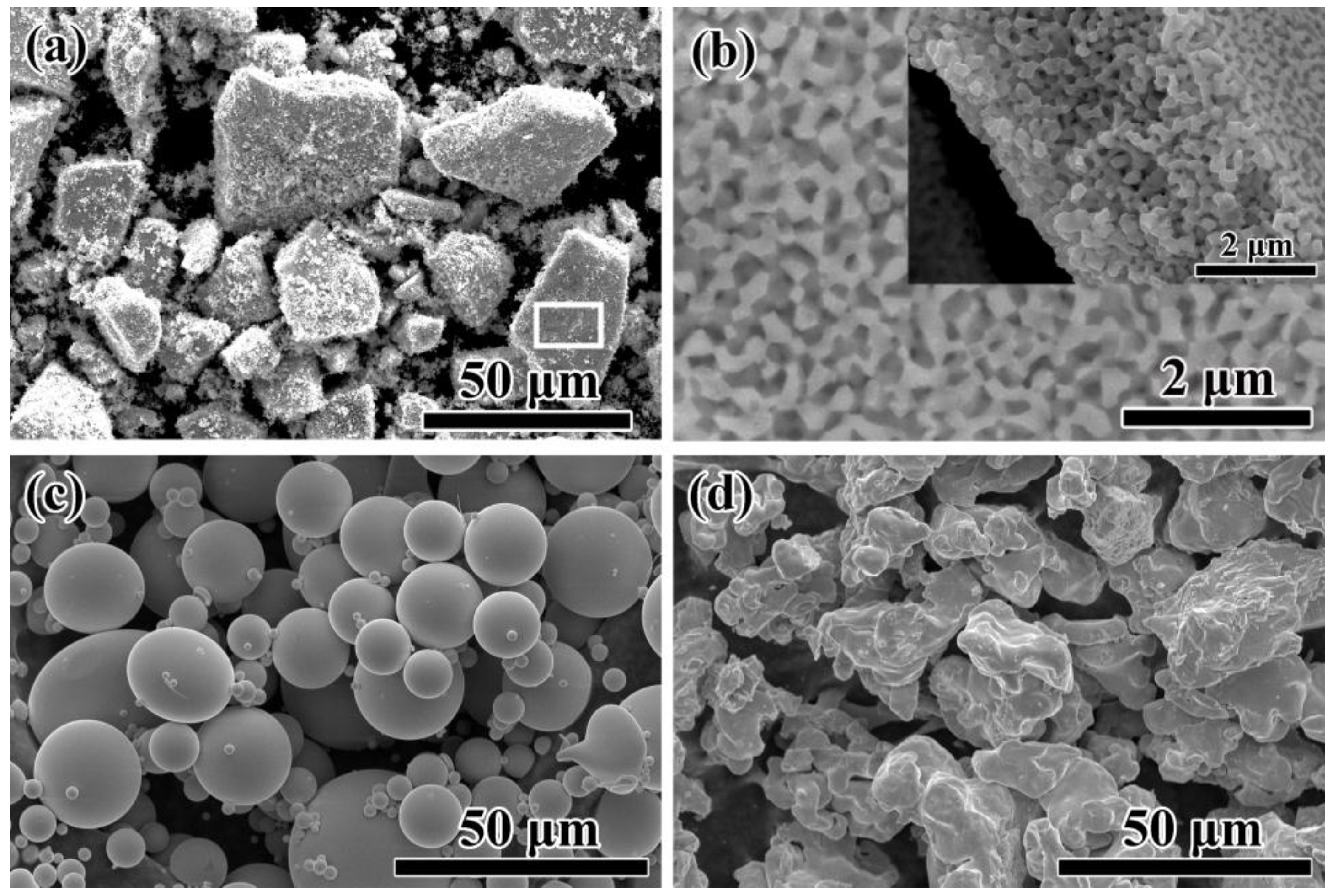
The morphologies of NP powders, AM powders and FE powders are shown in
Figure 2. The dealloyed ribbons were broken into small powders with the average size of about 20 μm as irregular shapes by milling (
Figure 2a). As shown in high magnification image of
Figure 2b, a homogeneously nanoporous structure with nanopores and continuous ligaments with a porosity of about 53% formed after dealloying in 0.05 M H
2SO
4 solution for 3.6 ks (
Figure 2b). The cross-sectional SEM image of NP powders shows that inner part of the specimen is also in porous structure, meaning that the specimen is fully dealloyed (inset of
Figure 2b). The AM powders prepared by gas atomization process are in a spherical shape with an average diameter of 18 μm (
Figure 2c). Commercial Fe powders with a rough surface are in irregular shapes with a large particle size (about 42 μm) than AM powder (
Figure 2d). The TEM images of dealloyed specimen show the microstructure with bi-continuous pores and ligament networks in
Figure 3a. The selective area diffraction pattern (SADP) of NP powders in
Figure 3b suggests the existence of α-Fe crystals based on the diffraction pattern from α-Fe (1–11), (220) and (311) crystal planes. Nanopores with a pore size of about 100 nm can be observed in the magnified TEM image in
Figure 3c. The difference in the contrast might be attributed to the difference in the chemical composition. The SADPs in
Figure 3d,e are conducted at Sites 1# and 2# in
Figure 3c. Residual phases in gray contrast at Site 1# were identified as Fe
2B with crystal planes of (110), (211) and (222) and α-Fe phases with diffraction indexed as (100), (101) and (104) at Site 2#. The high resolution TEM image in
Figure 3f at Site 3# denotes that there exists small amount of residual amorphous phase after dealloying and Fe
3P phases with crystal plane spacing of 3.05 Å, 2.18 Å and 2.02 Å. The results of TEM are consistent with the results of XRD patterns in
Figure 1 which also demonstrates the complex existence of different phases in the NP powders. Although most of the residual phases are confirmed as crystalline α-Fe, Fe
2B and Fe
3P phases, small amount of amorphous phase coexists in the ligaments.
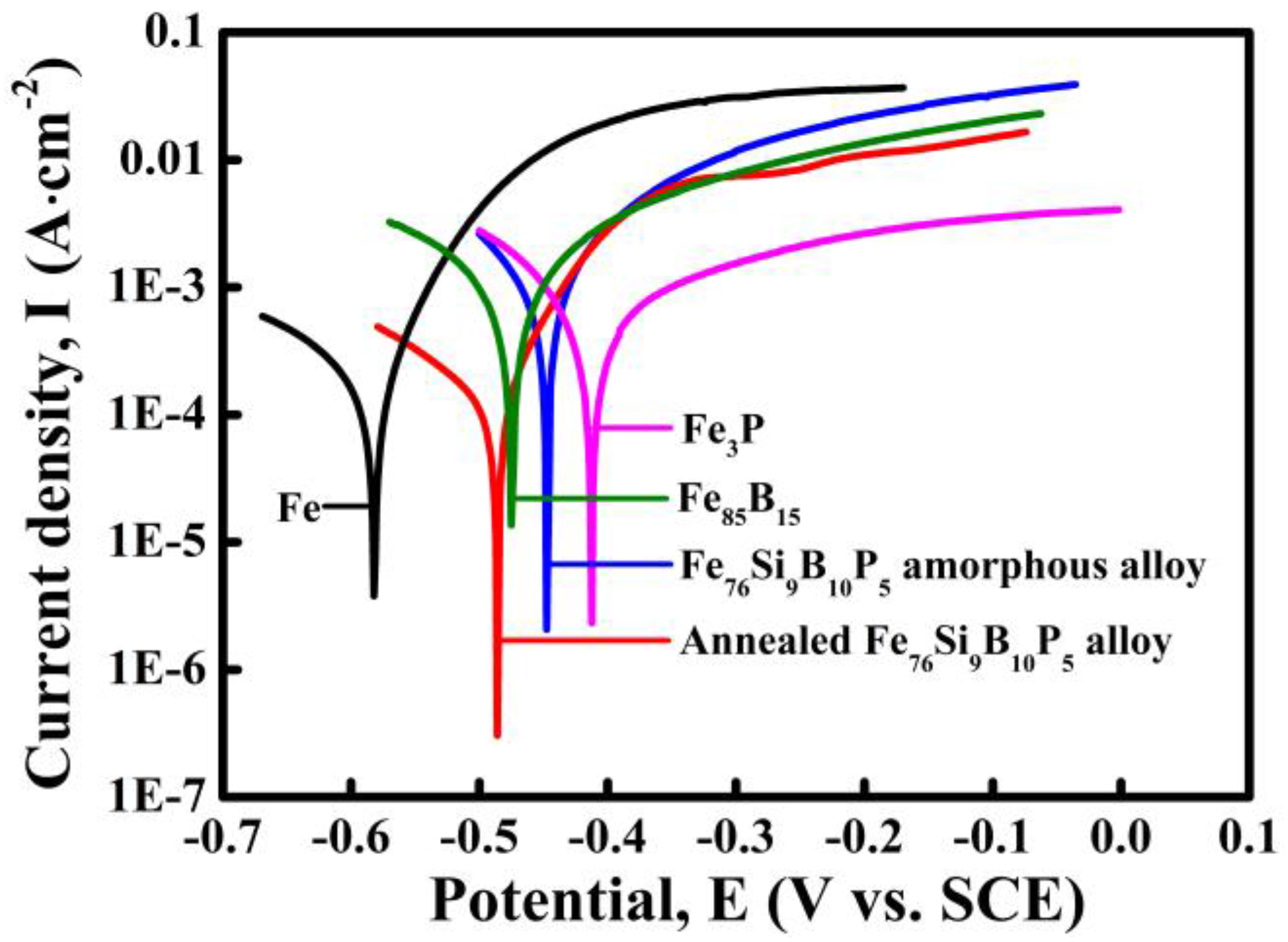
The potentiodynamic polarization behavior of the high-purity Fe plate (α-Fe), Fe
85B
15 alloy, Fe
3P alloy, as-spun Fe
76Si
9B
10P
5 amorphous alloy and annealed Fe
76Si
9B
10P
5 alloy was investigated in 0.05 M H
2SO
4 solution. As shown in
Figure 4, all the tested alloys are actively dissolved in 0.05 M H
2SO
4 solution with different corrosion potentials. The corrosion potential of Fe plate is −0.58 V, which is much lower than those of Fe
85B
15 alloy (−0.47 V), Fe
3P alloy (−0.41 V), as-spun Fe
76Si
9B
10P
5 amorphous alloy (−0.45 V) and annealed Fe
76Si
9B
10P
5 alloy (−0.48 V). It is concluded that high-purity Fe plate has the highest dissolution activity in 0.05 M H
2SO
4 solution. From the electrochemical aspects, high-purity Fe plate tends to start the preferential dissolution once Fe plate couples with Fe
85B
15 alloy, Fe
3P alloy, as-spun Fe
76Si
9B
10P
5 amorphous alloy or annealed Fe
76Si
9B
10P
5 alloy. On the one hand, the negative shift of corrosion potential of annealed Fe
76Si
9B
10P
5 alloy is considered to result from the precipitation of higher electrochemical-active α-Fe nanocrystals during annealing. On the other hand, the decrease in the intensity of the main diffraction peak of α-Fe phase after dealloying in
Figure 1 to some extent proves the preferential dissolution of the α-Fe phase in annealed alloy. The electrochemical stability of these tested alloys reflects the order of the preferential reactions in the dealloying process which might influence the degradation behavior of azo dye-containing industrial wastewater.
3.2. Enhanced Azo Dye Degradation Performance of NP Powders
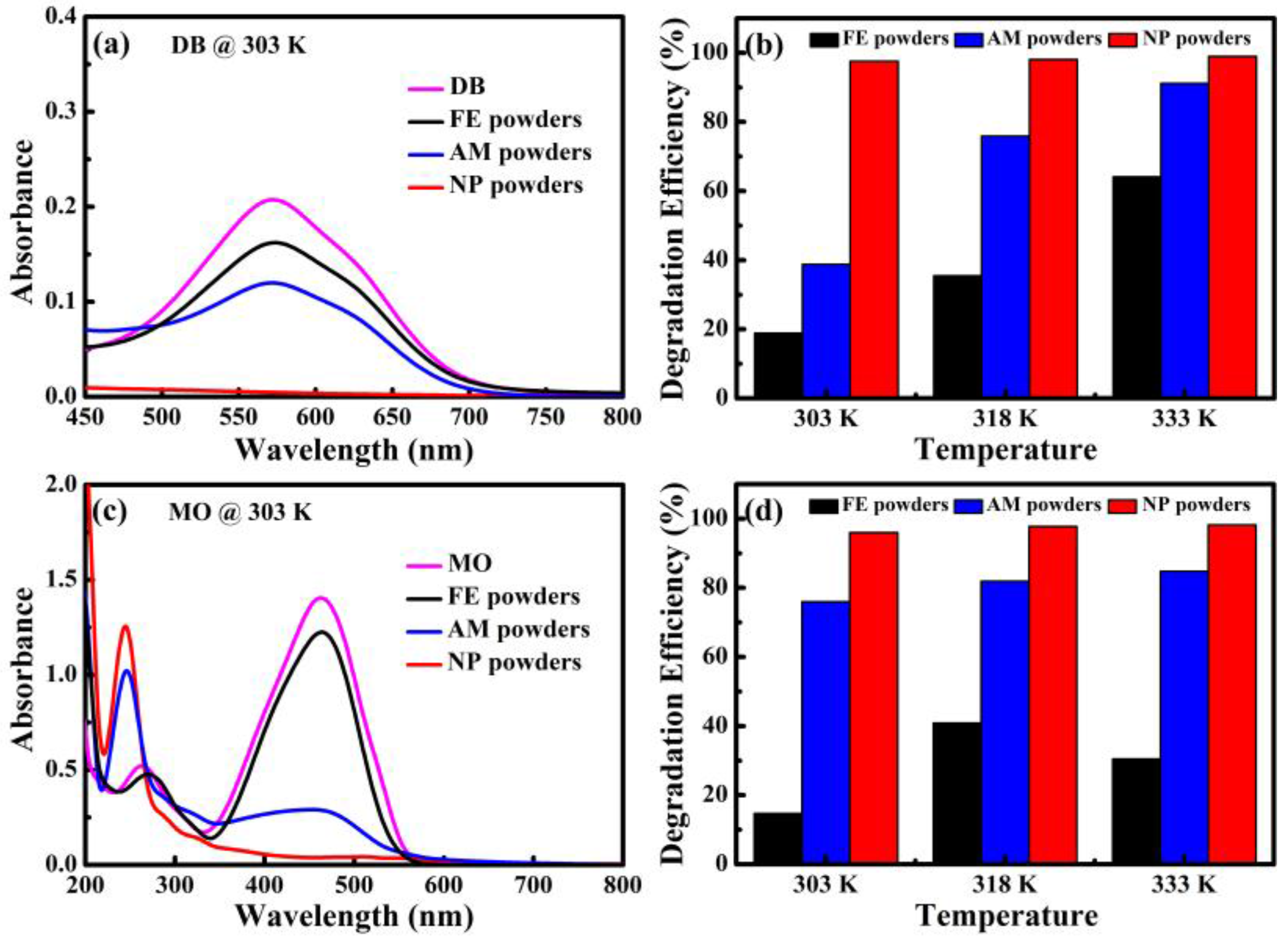
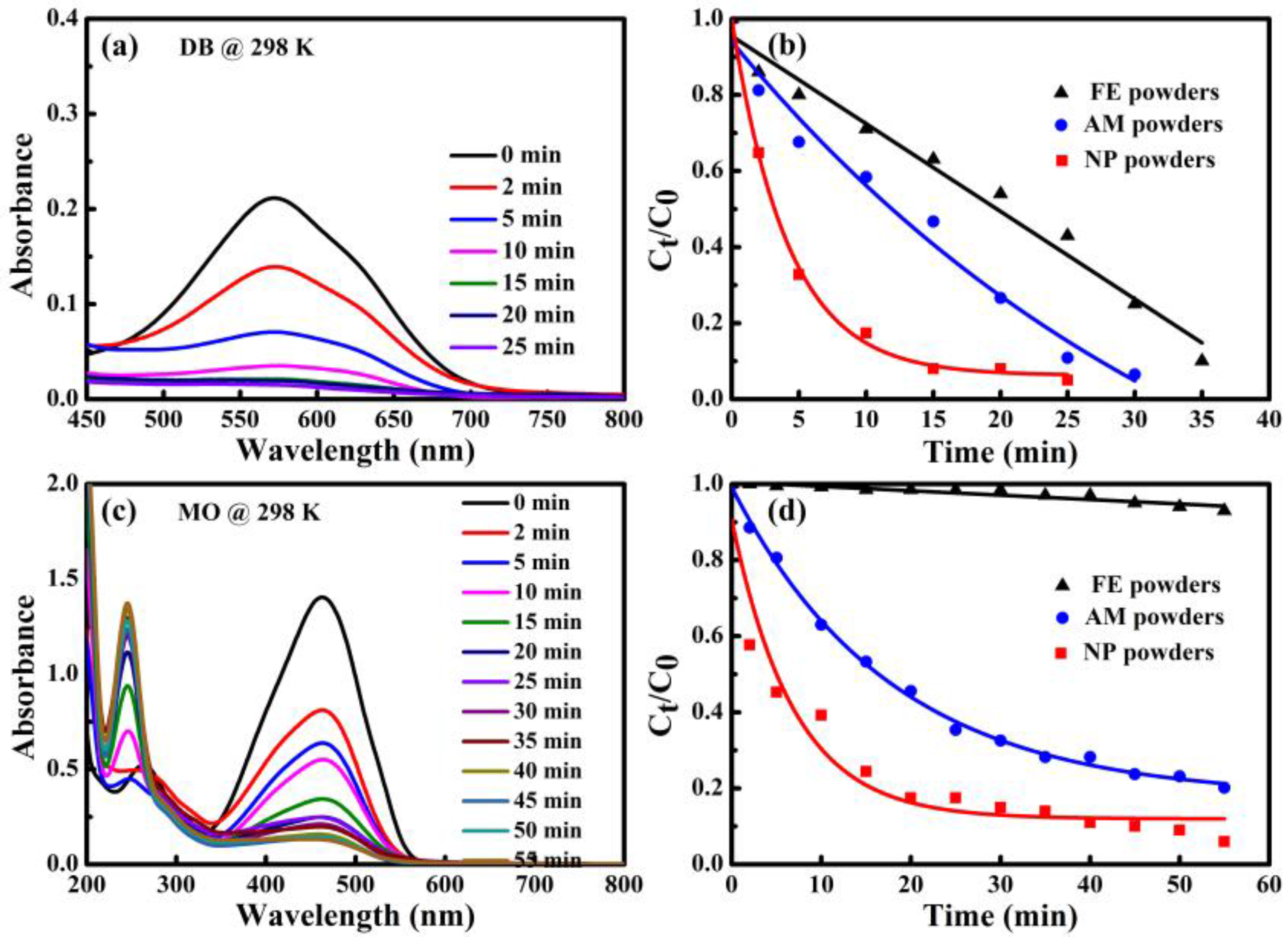
The UV-Vis spectra and degradation efficiency of MO and DB degraded by NP powders, AM powders and FE powders are shown in
Figure 5. Degradation efficiencies of azo dye by NP powders, AM powders and FE powders were calculated using Equation (1) [
18]:
where η is degradation efficiency, A
0 is the original absorbance and A is the absorbance of degraded solutions. The corresponding degradation efficiencies of DB and MO solutions at different cases are listed in
Table 1. Only one peak at 576 nm corresponding to the wavelength of green and yellow color could be observed in the UV-Vis spectra of DB solution in
Figure 5a. The absorbance peak of the DB solution after reacting with NP powders is much lower than those of the two other kinds of powders within the same degradation time of 25 min. The degradation efficiencies of DB for NP powders are 97.6%, 98.1% and 99.0%, at 303 K, 318 K and 333 K, respectively. The degradation of DB solution until colorless for NP powders takes 8 min at 303 K. When the degradation is performed at higher temperature, the degradation of DB solution until colorless for NP powders decreases to 4 min and 3 min, at 318 K and 333 K, respectively. Meanwhile, degradation efficiencies of AM powders and FE powders are 38.8% and 18.9% at 303 K in
Figure 5b. The degradation efficiencies of DB by AM powders and FE powders also increase with the increasing of the temperature. It is concluded that the NP powders degrade the DB solution much faster than AM powders and FE powders. In addition, two absorbance peaks in the UV-Vis spectra of MO solution degraded by three kinds of powders at 303 K are shown in
Figure 5c. The broad peak at 463 nm is originated from –N=N– bonding of MO and a new absorbance peak at about 245 nm appearing after degradation reaction is considered to be reaction products after degradation because some intermediates forms during the process of degradation reaction [
7]. The peak intensity at 463 nm is much weaker while the peaks at 245 nm become stronger after reacting with MO by NP powders compared with those by AM and FE powders, suggesting that NP powders exhibit much better degradation performance in the same condition. The degradation times till colorless of MO solution for NP powders are 60, 34 and 25 min at 303 K, 318 K and 333 K, respectively. The degradation efficiencies of NP powders are higher than 96% at all temperatures (
Table 1). It is obvious that NP powders demonstrate higher degradation efficiency for MO compared with AM and FE powders. Similarly, the degradation efficiency of MO solution by NP powders is further improved with the increasing of the temperatures, accompanying with the degradation time decreasing significantly.
The degradation behavior of DB and MO by three kinds of powders is investigated based on UV-Vis spectra taken at different degradation times at 298 K. For DB solution, as shown in
Figure 6a, the absorbance peak at about 576 nm corresponding to color radical decayed with the evolution of time in the UV-vis spectra. The peaks almost totally disappear after degradation of 25 min. The normalized concentration
Ct/
C0 variation of DB against time derived from UV-Vis spectra is shown in
Figure 6b. Here,
Ct and
C0 are real-time and initial concentration of DB solution. It is found that
Ct/
C0 decreases drastically in the case of degradation by NP powders, especially at the beginning of degradation. The value of
Ct/
C0 is less than 0.2 after degradation for 10 min, implying that more than 80% of DB azo dye has been decomposed. Accordingly, about 40% and 25% of DB are degraded completely by AM powders and FE powders, respectively. In contrast, the UV-Vis spectra for MO have two peaks centered at 463 nm and 269 nm before degradation treatment in
Figure 6c. As the degradation reactions undergo, the absorption peaks at 463 nm of the chromophore of the –N=N– bonding become lower, while the peaks at 269 nm shift to 245 nm and the peaks become stronger and stronger, suggesting that the azo –N=N– bonding has been broken, accompanying with the formation of some intermediates containing the benzene ring [
7,
19]. By using the absorption intensity at 463 nm, the normalized concentration change against time of MO was acquired (
Figure 6d). The NP powders exhibits the much better degradation performance than AM powders and FE powders. Compared with
Figure 6b, the plots of
Ct/
C0 vs. time of FE powders in
Figure 6d is different. The degradation of DB and MO solutions by both NP powders and AM powders follows the pseudo-first-order kinetic model (
C = C0 exp (−
kobst),
R2 > 0.95), and FE powders obeys the pseudo-zero-order kinetic model (
C =
C0(1
− kobst)). All the calculated reaction rate constants (
kobs) are presented in
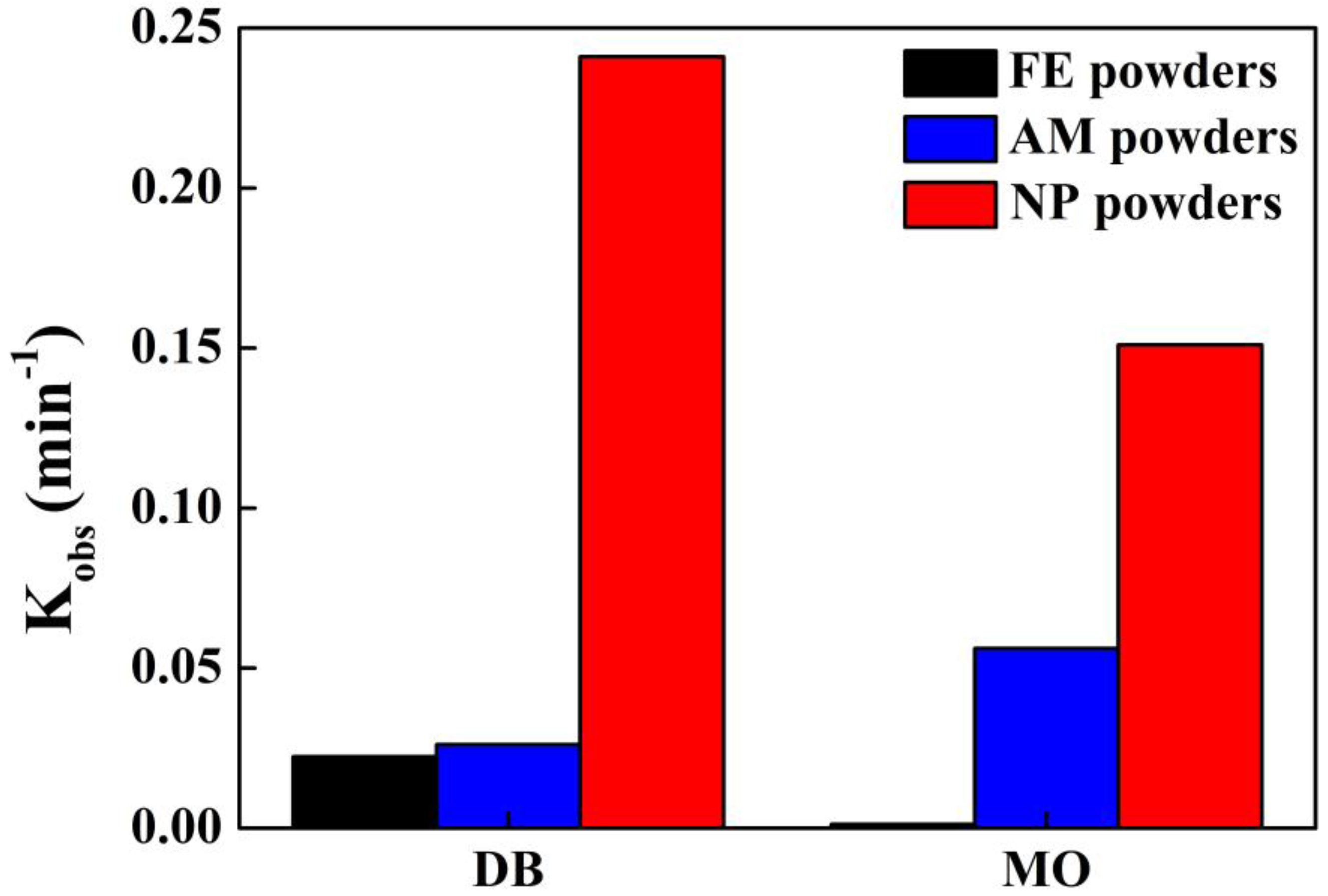
Figure 7 and
Table 1. The
kobs value of the degradation of DB molecule by NP powders could be about 9 and 10 times higher than those of AM powders and FE powders, respectively. The
kobs value of the degradation of MO by NP powders is three times higher than AM powders and 131 times higher than FE powder. The MO azo dye (C
14H
14N
3SO
3Na) and DB (C
32H
20N
6S
4O
10Na
4) have molecular weights of 360 g mol
−1 and 1029 g mol
−1, respectively. DB azo dye is regarded as a representative large molecule and MO azo dye does as a medium molecule. FE powders show a better degradation ability for large molecules than medium molecules, while AM powders perform better on medium molecules. Among the three powders, the NP powders have the best degradation performance on both large molecules of DB and medium molecules of MO, which might be due to the diversity in the chemical composition of the nanoporous structure because of the coexistence of amorphous phase and crystalline α-Fe, Fe
2B and Fe
3P phases.
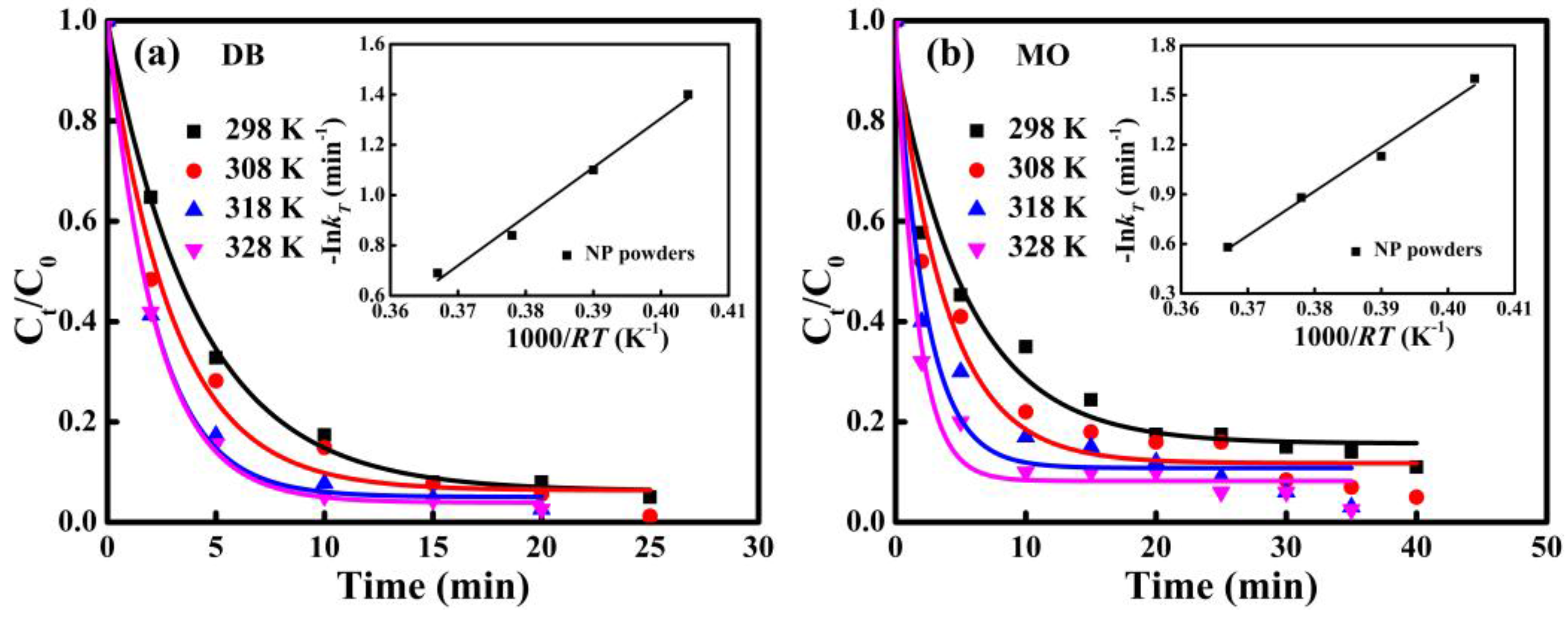
The normalized concentration
Ct/
C0 and the activation energy of the degradation reactions of DB and MO azo dyes by NP powders from 298 K to 328 K are shown in
Figure 8. Reaction rate constants at different temperatures can be deduced by fitted data from the pseudo-first-order model. According to the Arrhenius equation, the activation energy can be derived from the change of temperature using Equation (2) [
20,
21]:
where
kT is the kinetic rate constant at different temperatures (
T), Δ
E is the activation energy (kJ mol
−1),
R is the gas constant and A is a constant. The plot of -ln
kT versus 1000/
RT for DB and MO solutions are inserted in
Figure 8a,b. Good linear relationships have been obtained (
R2 > 0.95), and the calculated activation energies for the degradation of DB and MO by NP powders are 19.5 kJ mol
−1 and 26.8 kJ mol
−1, which are much lower than those of commercial [
7], demonstrating excellent degradation performance of NP powders.
3.3. Enhanced Degradation Mechanism of NP Powders
To explore the degradation mechanism of NP powders, the surface morphology and elemental distribution of the powders after reaction with the azo dye solutions were analyzed.
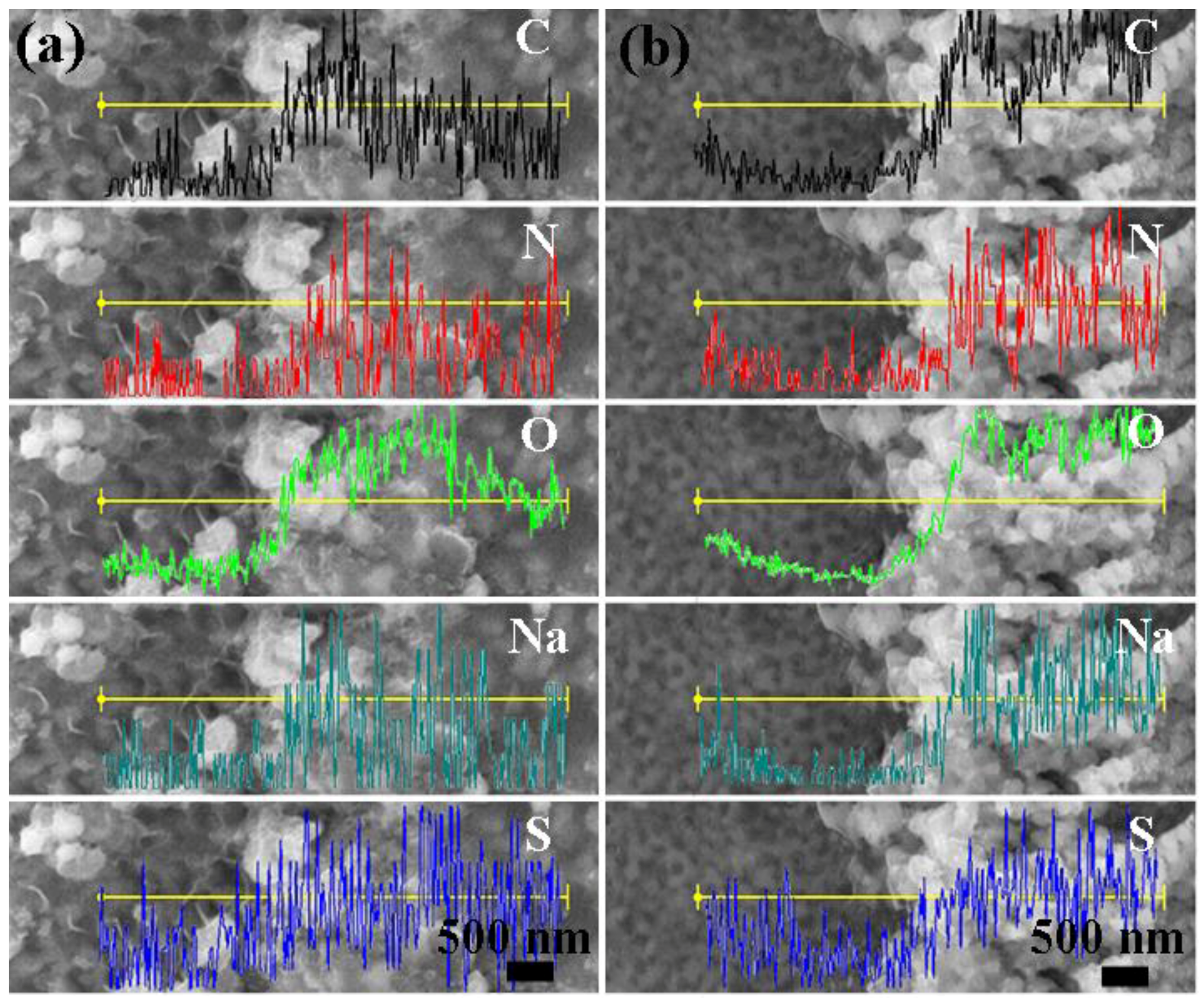
Figure 9 displays the surface morphology of NP powders after reacting with DB (
Figure 9a) and MO (
Figure 9b) solutions. Instead of fine and clear pores in the full scale, as shown in
Figure 2b, some white particles and floccule-like products, which might be the decomposition products of azo dyes, cover the porous surface. The EDS line scanning results of the specimens reacted with azo dye solutions illustrate the variation of the composition along the yellow line. After reaction with azo dyes solutions, the amount of Fe is decreased from 86.4% to 15.4%, while small amounts of C, N, S and Na elements can be detected in the white products, which are constituent elements of azo dyes.
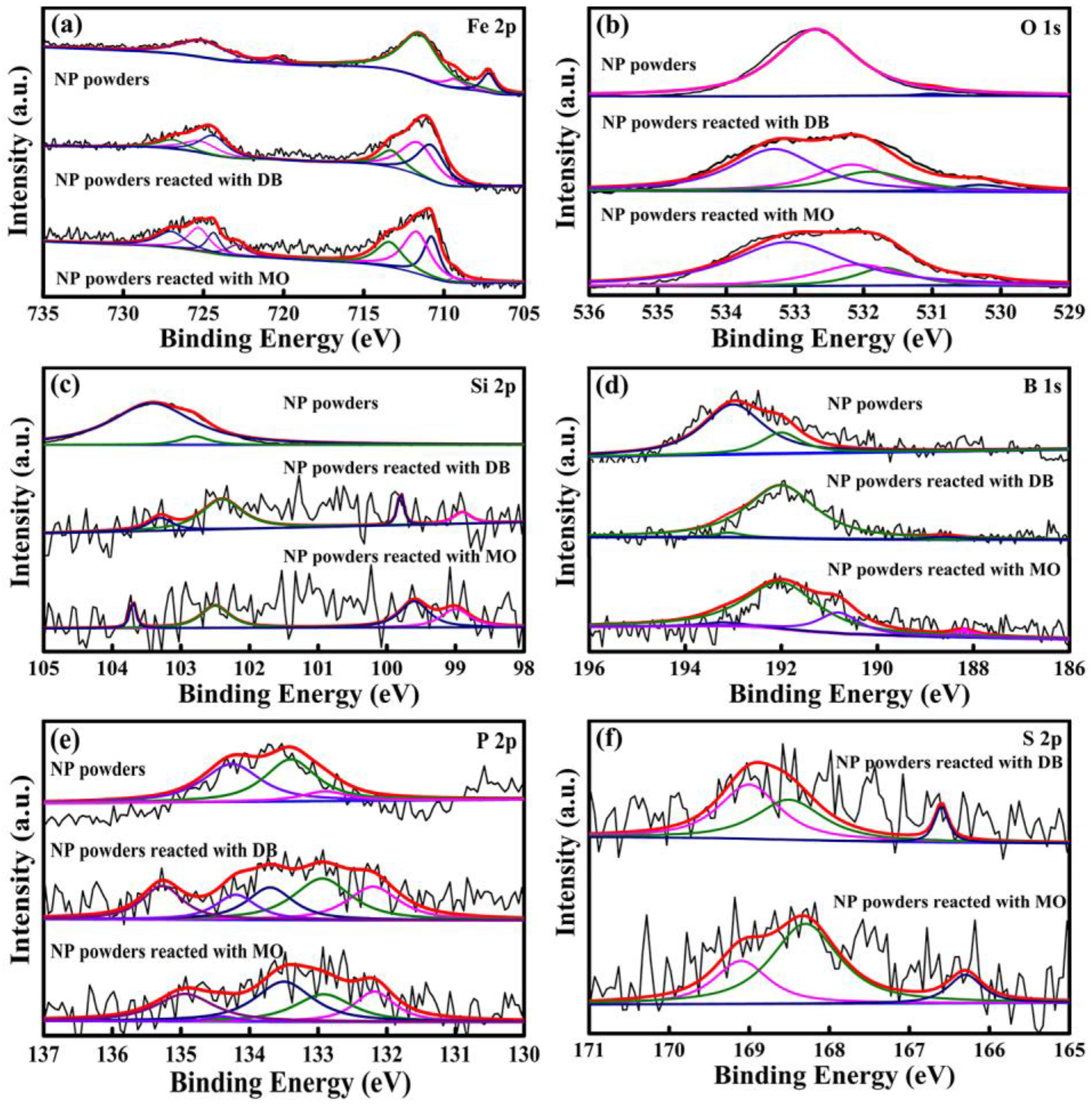
Moreover, to obtain the detailed elemental information from the outermost atomic layers, XPS measurement was implemented to identify the surface chemical states of NP powders before and after reaction. The XPS spectra are summarized in
Figure 10. Before reaction, all of the alloy elements (Fe, Si, B, P) as well as absorbed C and O elements are detected. Fe 2p spectrum in original NP powders is composed of a mixture of Fe
0 (707.2 eV), Fe
2+(709.3 eV) and Fe
3+(711.5 eV), as displayed in
Figure 10a. B
xO
y (B
3+, 192.2 eV), B
2O
3 (B
3+, 193.1 eV), Si
0 (98.9 eV), SiO (Si
2+, 102.3 eV) and SiO
2 (Si
4+, 103.8 eV) are also observed. In contrast, the concentrations of Si, B, and P elements are far lower than those in the original surface of NP powders after reaction, hinting that these metalloids are involved in the degradation reaction. The existence of Si
4+/Si
2+, B
3+ and P
5+ indicates that the metalloids are oxidized during degradation reaction. Fe 2
p spectra in
Figure 10 are mainly composed of Fe
3+ from Fe
2O
3 (Fe
3+, 710.8 eV), γ-FeOOH (Fe
3+, 711.7 eV), and Fe
2(SO
4)
3 (Fe
3+, 713.4 eV) after reacting with azo dyes. It should be noted that small amount of S and Na elements (0.18–0.22 at %) rising from SO
42+/SO
32+ and Na
+1 species are confirmed on the surface of NP powders after degradation with azo dyes, demonstrating the formation of Na
2SO
4 and Na
2SO
3 [
7,
22].
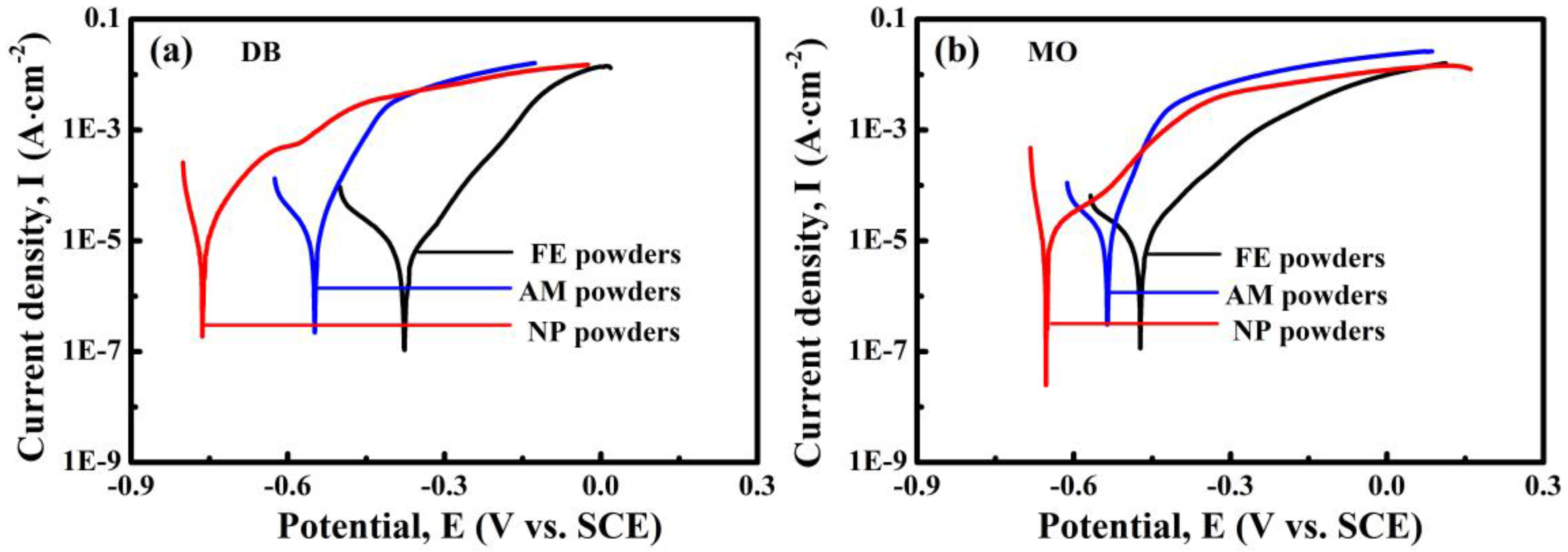
The potentiodynamic polarization curves of NP powders, AM powders and FE powders in 50 mg/L DB and 20 mg/L MO solutions were investigated to discuss the chemical stability of the different powders, as shown in
Figure 11. All the tested specimens are actively dissolved during the anodic polarization process. In both solutions, NP powders show much lower corrosion potential than those of AM powders and FE powders, hinting that NP powders exhibit higher electrochemical activity in both the azo dye solutions, accompanied with more active catalytic activity during the process of degradation.
3.4. Discussion
As mentioned above, Fe
76Si
9B
10P
5 amorphous alloy was crystallized to form α-Fe, Fe
2B and Fe
3P phases after annealing. During the process of dealloying in 0.05 M H
2SO
4 solution, a nanoporous structure formed because of the partially preferential dissolution of α-Fe phase. In our previous research [
23,
24], α-Fe grains in nanocrystalline Fe
83.3Si
3B
10P
3Cu
0.7 and Fe
85.2B
10–14P
0–4Cu
0.8 alloys are preferentially dissolved in the form of micro-coupling cells between anodic α-Fe grains and cathodic residual amorphous phases. In this research, active dissolution of high-purity Fe plate, Fe
85B
15 alloy, Fe
3P alloy, and as-spun Fe
76Si
9B
10P
5 amorphous alloy in
Figure 4 to some extent reflects the active dissolution of α-Fe, Fe
2B and Fe
3P phases in the matrix of the annealed Fe
76Si
9B
10P
5 precursor alloys during dealloying. It is predictable that Fe
2B phase in the annealed alloys exhibits a higher corrosion potential than that of Fe
85B
15 alloy because the ratio of B/Fe in Fe
2B is higher than that in Fe
85B
15 alloy. Thus, α-Fe grains own the highest electrochemical activity among α-Fe, Fe
2B, Fe
3P and residual amorphous phases (confirmed by XRD and SADPs in
Figure 1 and
Figure 3) and undergo the preferential dissolution of α-Fe grains in 0.05 M H
2SO
4 solution. The selective dissolution behavior of α-Fe grains in annealed Fe
76Si
9B
10P
5 alloy is similar to the reported mechanism although the present reactions are more complicated due to the involvement of other Fe
2B and Fe
3P phases [
23,
24]. In other words, α-Fe grains dissolved quickly once exposed to H
2SO
4 solution, while the residual amorphous phases, Fe
2B and Fe
3P, with higher corrosion potentials, dissolve slowly, as shown in the results presented in
Figure 3 and
Figure 4.
On the other hand, the NP powders with a nanoporous structure exhibit higher degradation efficiency to azo dye solutions than the AM powders and FE powders. The enhanced degradation performance of NP powder may be elaborated by the following reasons. One reason is that the surface area of the NP powders with the formation nanoporous architecture increases significantly which are beneficial to the adsorption of dye molecules, supporting more reduction and oxidation sites for degradation reaction. The Fe element, as well as the metalloids (Si, B and P), in the ligaments of the nanoporous structure, acting as electron donors, will be oxidized when degradation reaction occurs. At the same time, the azo-dye molecular chains as acceptors are broken after the capture of electrons. Furthermore, based on the TEM image (
Figure 3a) and cross-sectional SEM image (
Figure 2b), the nanopores inside NP powders interconnected together which may form the fluid channels for azo dye solution, which further improve the degradation efficiency. From the view of electrochemical activity, it is also possible to explain why NP powders show higher degradation performance to azo dyes than AM powders and FE powders. The differences in the corrosion potential between FE powders and AM powders in DB and MO solutions in
Figure 11 are 170 mV and 110 mV, respectively, which mainly result from the difference in the chemical composition and microstructure. The differences in the corrosion potential between AM powders and NP powders in DB and MO solution in
Figure 11 are more than 290 mV and 110 mV, respectively, which are believed to originate from the difference in microstructure. The lower corrosion potentials of NP powders in azo dye solutions imply that NP powders exhibit higher electrochemical activity, accompanied with more active catalytic activity during the process of degradation with a lower activation energy. Another reason is that the coexistences of the residue amorphous phase in metastable state and metalloid elements such as Si, B and P after dealloying may enhance the catalytic activity of degradation reaction more by forming loose metalloid-containing intermediate products [
8]. Here, the excellent catalytic activity of amorphous electrodes results from the abundance of the active sites induced by the special electronic structure [
25]. Thus, the degradation process by NP powders is accelerated compared with AM powders and FE powders.
According to the above mentioned results, the degradation process of NP powders can be summarized as followings: MO or DB molecules are firstly absorbed to the nanoporous architecture, which are bicontinuous pore-and-ligament networks with large reactive surface area, then Fe elements were oxidized to Fe
3+. Meanwhile, the cleavage of nitrogen to nitrogen double bonds (–N=N–), which are the most active bonds in azo dye molecules, leads to the decoloration of azo dyes. Whenazo dyes are degraded to intermediates, which are partially attached on the porous structure, the intermediates are subsequently further oxidized to small molecules, such as CO
2, H
2O and Na
2SO
4/Na
2SO
3 [
22]. In addition, the coexistence of residue amorphous phase in metastable state and metalloids in porous ligament, which makes the surface more active by forming loose oxide layer in the process of degradation, may also play an important role to enhance the degradation activity [
7,
8].