Rapid Removal of Zinc(II) from Aqueous Solutions Using a Mesoporous Activated Carbon Prepared from Agricultural Waste
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. AC Preparation
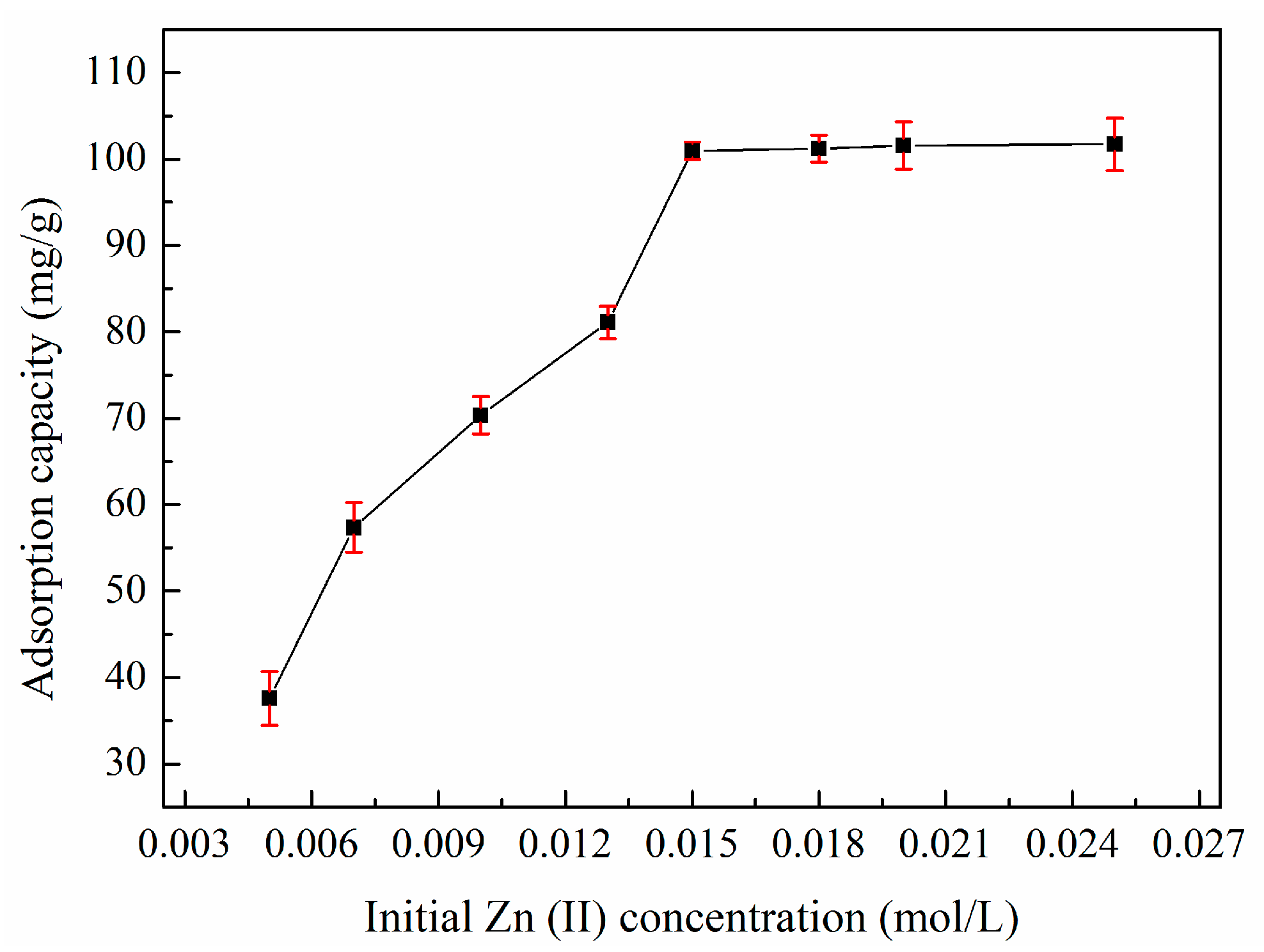
2.3. Adsorption Studies
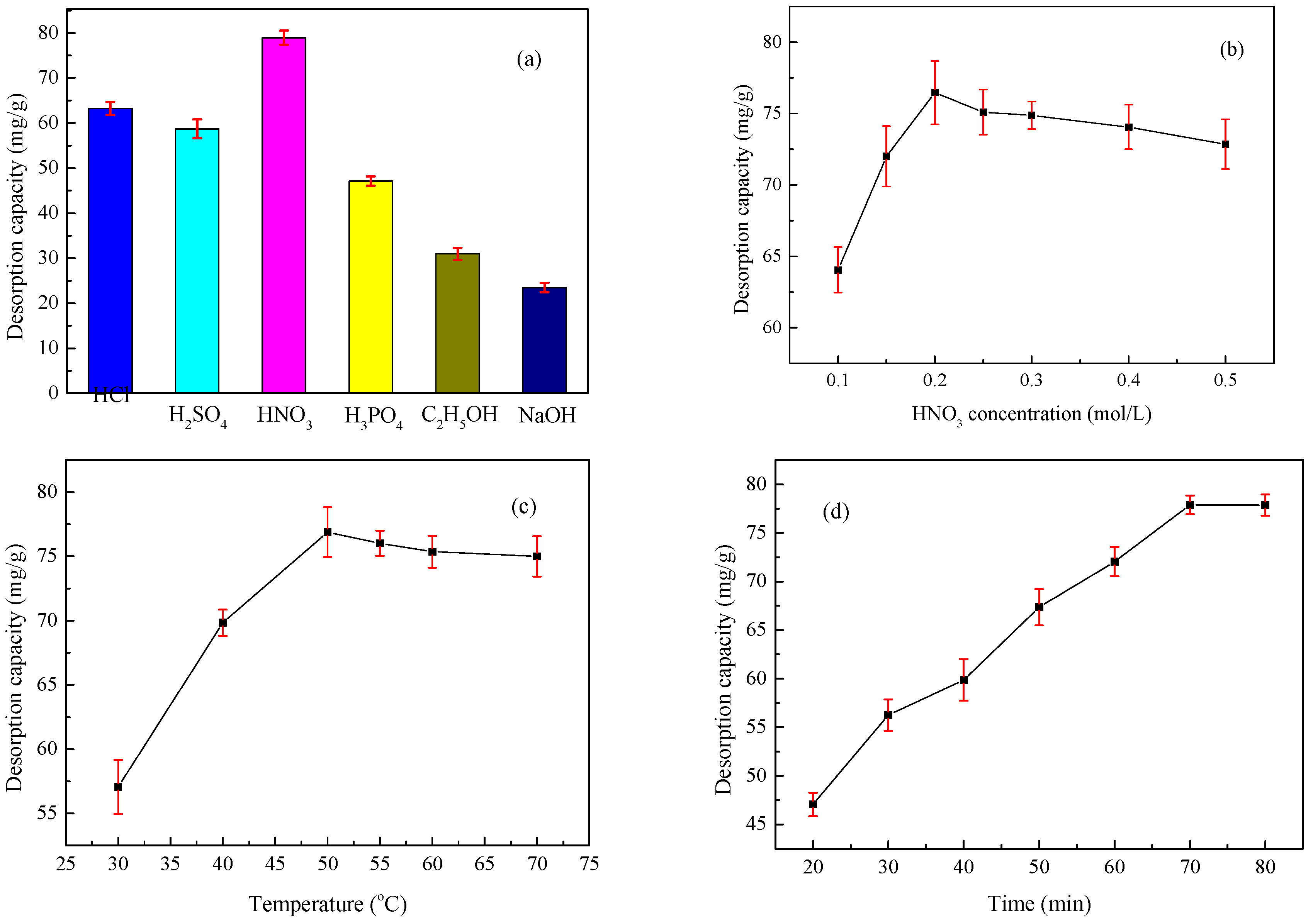
2.4. Desorption and Regeneration Studies
3. Results and Discussion
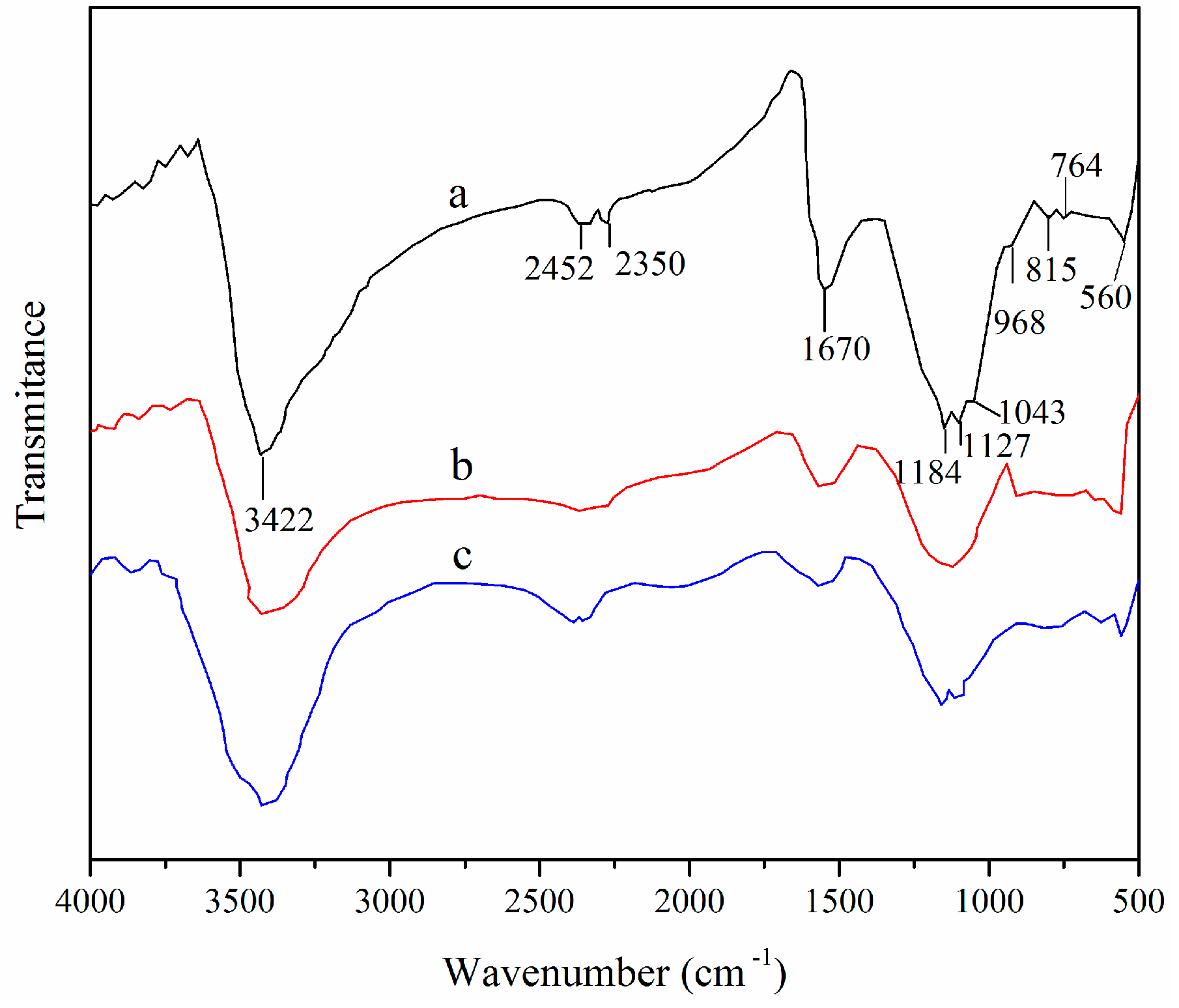
3.1. Adsorbent Characterization
3.2. Properties of XSBLAC
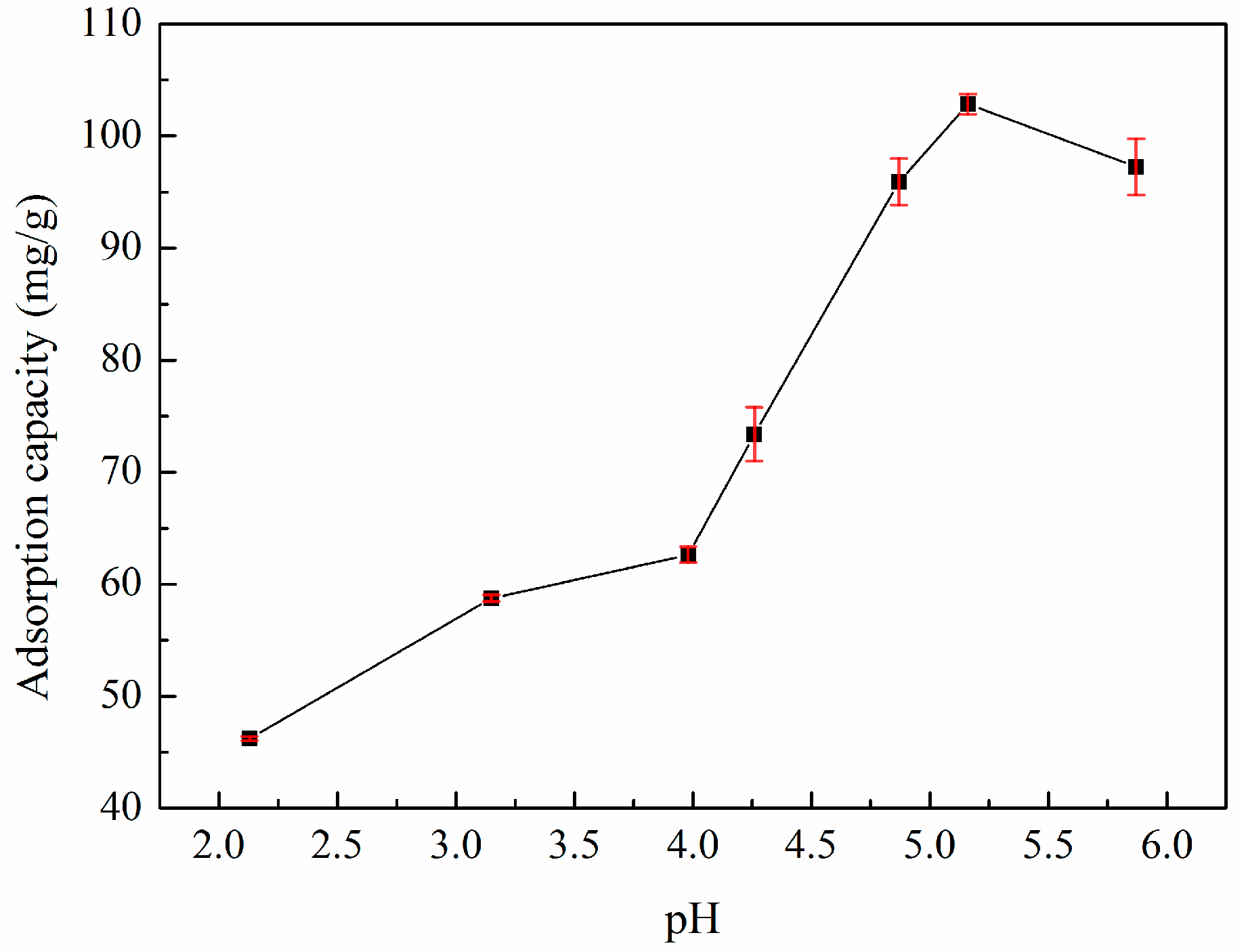
3.3. Effect of pH Value on Adsorption
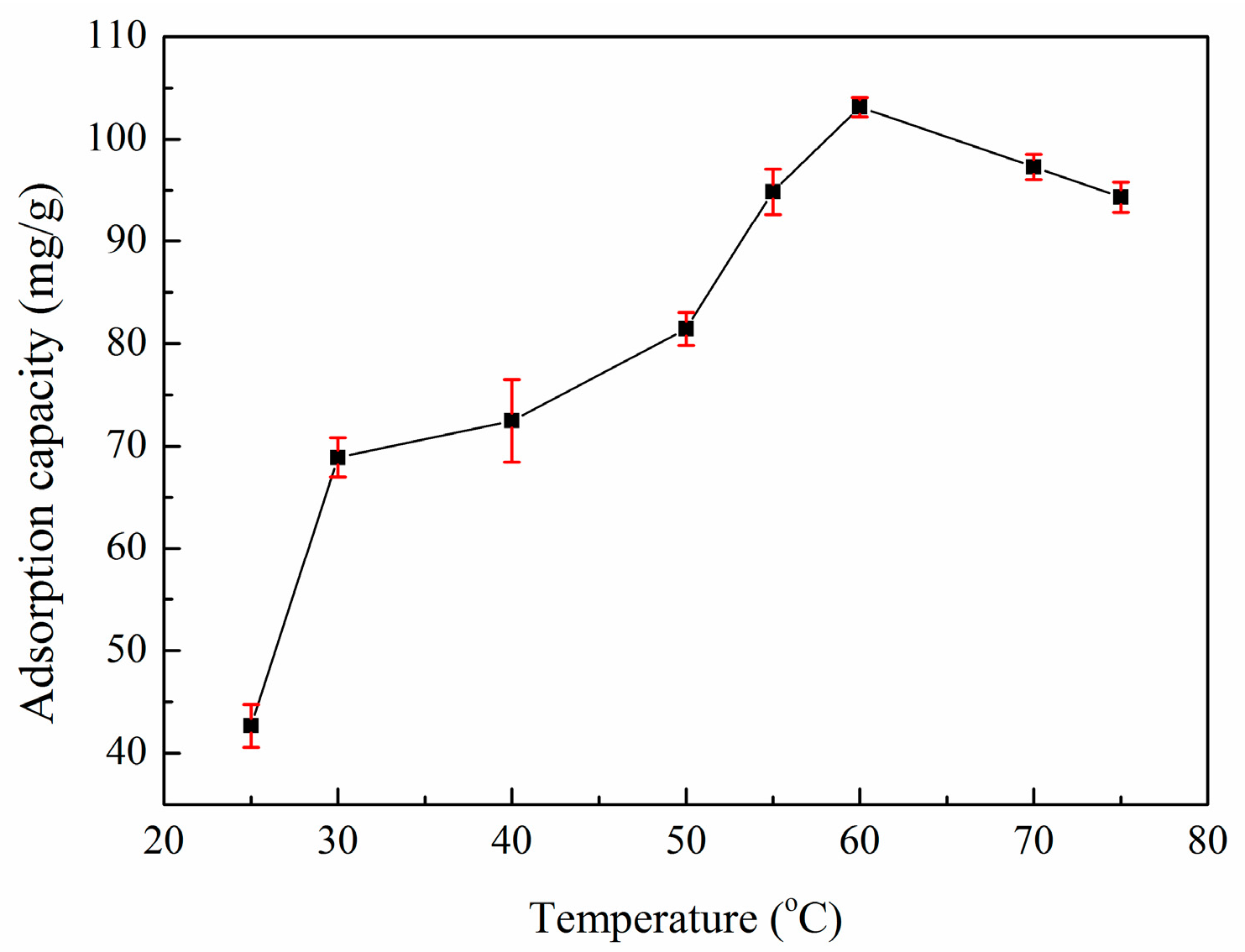
3.4. Effect of Temperature on Adsorption
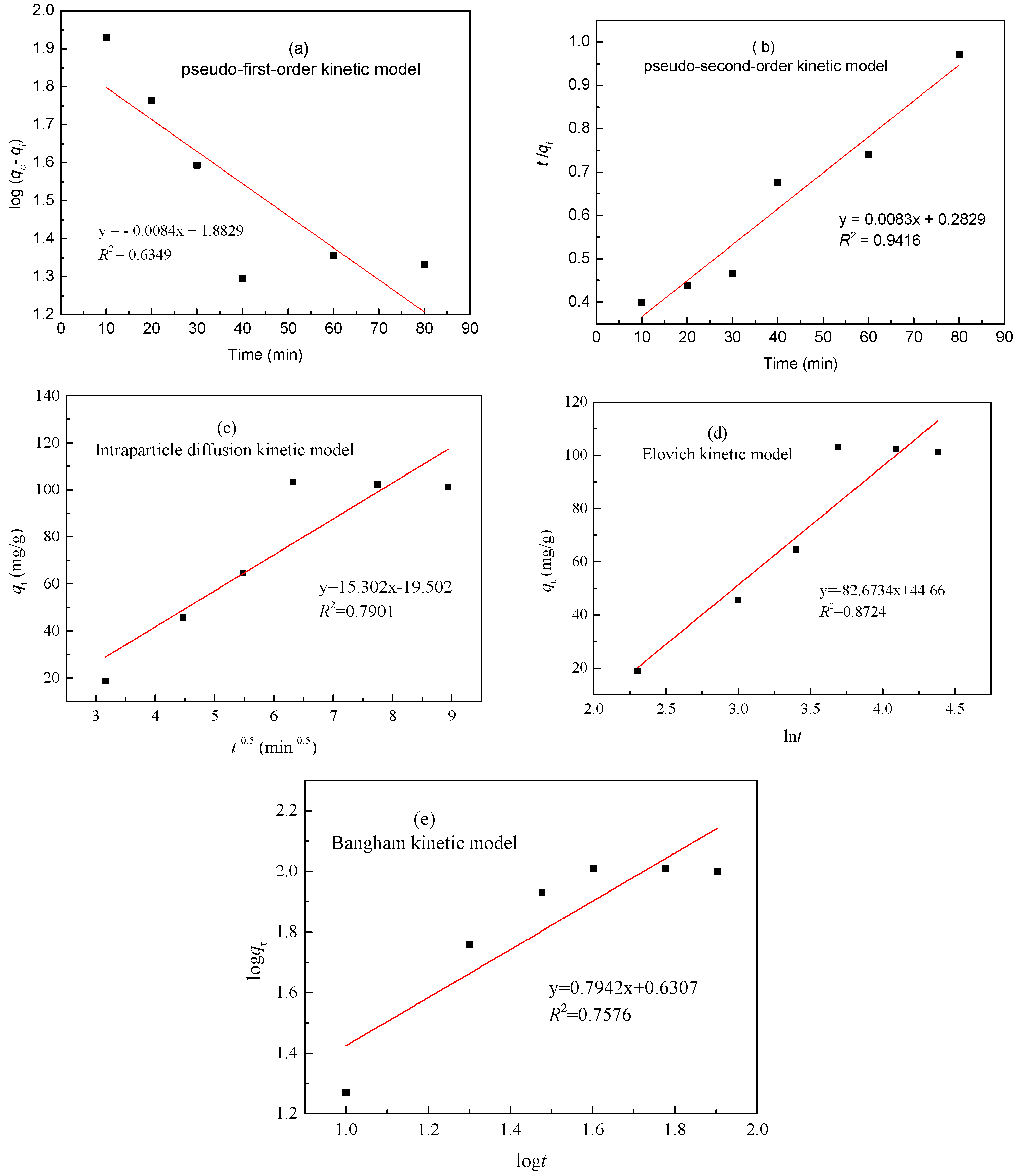
3.5. Adsorption Kinetics
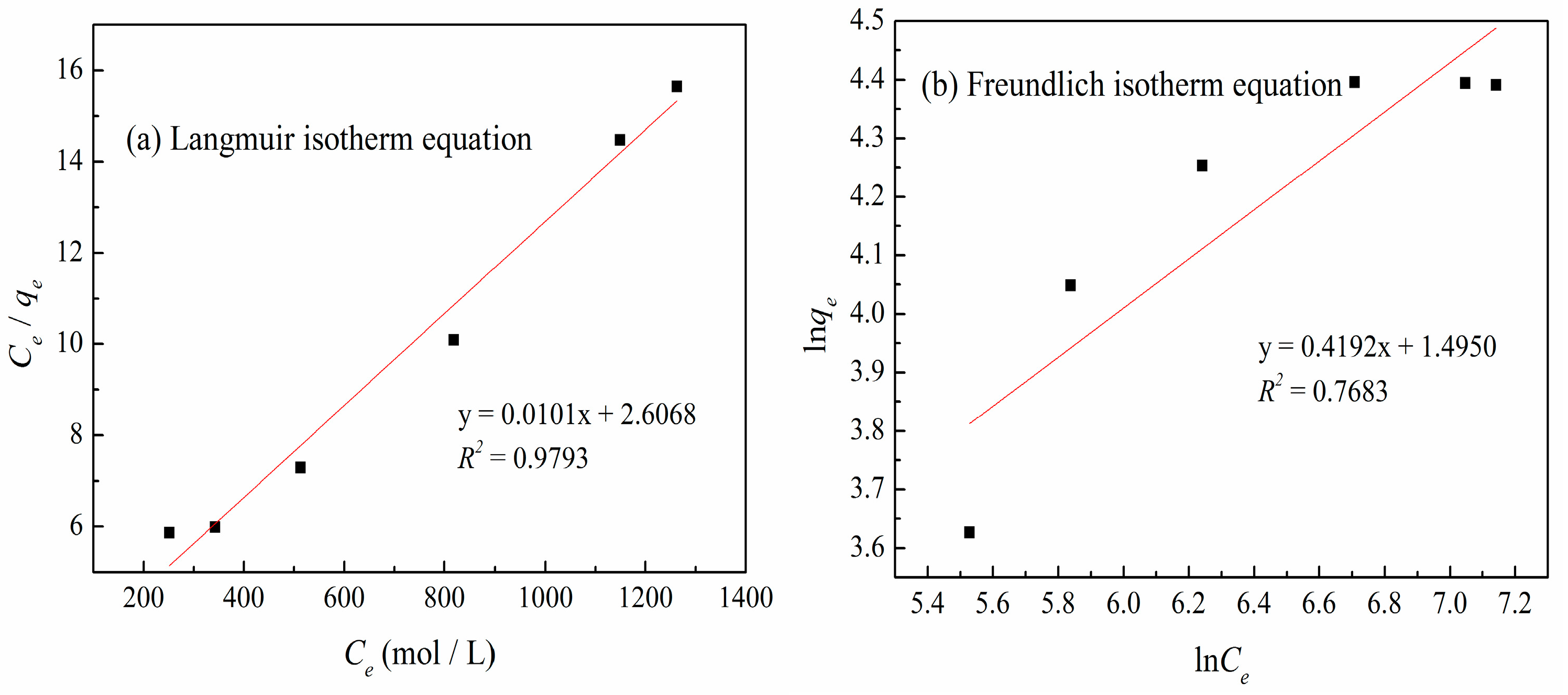
3.6. Adsorption Isotherms
3.7. Desorption and Regeneration
3.8. Adsorption Mechanism
3.9. Comparison with Previously Reported Data for Zinc(II) Adsorption
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hawari, A.; Rawajfih, Z.; Nsour, N. Equilibrium and thermodynamic analysis of zinc ions adsorption by olive oil mill solid residues. J. Hazard. Mater. 2009, 168, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Lalhruaitluanga, H.; Jayaram, K.; Prasad, M.N.; Kumar, K.K. Lead(II) adsorption from aqueous solutions by raw and activated charcoals of Melocanna baccifera Roxburgh (bamboo)—A comparative study. J. Hazard. Mater. 2010, 175, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Wahby, A.; Abdelouahab-Reddam, Z.; El Mail, R.; Stitou, M.; Silvestre-Albero, J.; Sepúlveda-Escribano, A. Mercury removal from aqueous solution by adsorption on activated carbons prepared from olive stones. Adsorption 2011, 17, 603–609. [Google Scholar] [CrossRef]
- Shinde, N.R.; Bankar, A.V.; Kumar, A.R.; Zinjarde, S.S. Removal of Ni(II) ions from aqueous solutions by biosorption onto two strains of Yarrowia lipolytica. J. Environ. Manag. 2012, 102, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Minceva, M.; Fajgar, R.; Markovska, L.; Meshko, V. Comparative study of Zn2+, Cd2+, Pb2+ removal from water solution using natural clinoptilolitic zeolite and commercial granulated activated carbon equilibrium of adsorption. Sep. Sci. Technol. 2008, 43, 2117–2143. [Google Scholar] [CrossRef]
- Puzii, A.M.; Stavitskaya, S.S.; Poddubnaya, O.I.; Vikarchuk, V.M.; Tsyba, N.N. Structural and adsorption properties of active carbon from coconut shells modified with phosphorus heteroatoms. J. Therm. Anal. Calorim. 2016, 124, 1–16. [Google Scholar] [CrossRef]
- Lin, J.; Wu, Z.; Tseng, W. Extraction of environmental pollutants using magnetic nanomaterials. Anal. Methods 2010, 2, 1874–1879. [Google Scholar] [CrossRef]
- Depci, T.; Kul, A.R.; Önal, Y. Competitive adsorption of lead and zinc from aqueous solution on activated carbon prepared from Van apple pulp: Study in single- and multi-solute systems. Chem. Eng. J. 2012, 200, 224–236. [Google Scholar] [CrossRef]
- Linares-Solano, A.; López-González, J.D.D.; Molina-Sabio, M.; Rodriguez-Reinoso, F. Active carbons from almond shells as adsorbents in gas and liquid phases. J. Chem. Technol. Biotechnol. 2010, 30, 65–72. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Qian, Q.; Machida, M.; Tatsumoto, H. Effect of ZnO loading to activated carbon on Pb(II) adsorption from aqueous solution. Carbon 2006, 44, 195–202. [Google Scholar] [CrossRef]
- Bansode, R.R.; Losso, J.N.; Marshall, W.E.; Rao, R.M.; Portier, R.J. Adsorption of metal ions by pecan shell-based granular activated carbons. Bioresour. Technol. 2003, 89, 115–119. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Molina-Sabio, M.; Rodríguez-Reinoso, F. Modification of the porous structure along the preparation of activated carbon monoliths with H3PO4 and ZnCl2. Microporous Mesoporous Mater. 2007, 103, 29–34. [Google Scholar] [CrossRef]
- Manjula, D.M. Heavy Metals Removal from Industrial Wastewater by Nano Adsorbent Prepared from Cucumis Melopeel Activated Carbon. J. Nanomed. Res. 2017, 5, 00102. [Google Scholar] [CrossRef]
- Cronje, K.J.; Chetty, K.; Carsky, M.; Sahu, J.N.; Meikap, B.C. Optimization of chromium (VI) sorption potential using developed activated carbon from sugarcane bagasse with chemical activation by zinc chloride. Desalination 2011, 275, 276–284. [Google Scholar] [CrossRef]
- Issabayeva, G.; Aroua, M.K.; Sulaiman, N.M. Removal of lead from aqueous solutions on palm shell activated carbon. Bioresour. Technol. 2006, 97, 2350–2355. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Awang, M.; Mohosina, B.S.; Kamaruzzaman, B.Y.; Nik, W.B.W.; Adnan, C.M.C. Waste palm shell converted to high efficient activated carbon by chemical activation method and its adsorption capacity tested by water filtration. APCBEE Procedia 2012, 1, 293–298. [Google Scholar] [CrossRef]
- Gonçalves, M.; Sánchez-García, L.; Oliveira Jardim, E.D.; Silvestre-Albero, J.; Rodríguez-Reinoso, F. Ammonia removal using activated carbons: Effect of the surface chemistry in dry and moist conditions. Environ. Sci. Technol. 2014, 45, 10605–10610. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Hao, Y.; Ding, L.; Xue, Z. The Preparation Method and Application of Xanthoceras Sorbifolia Bunge Hull Activated Carbon Loaded with KOH. Chiana Patent ZL2011100844764, 17 January 2013. [Google Scholar]
- Romero-Anaya, A.J.; Lillo-Ródenas, M.A.; Lecea, S.M.D.; Linares-Solano, A. Hydrothermal and conventional H3PO4 activation of two natural bio-fibers. Carbon 2012, 50, 3158–3169. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, A. Adsorption of Pb(II) from aqueous solution by Chitosan-g-poly(acrylic acid)/Attapulgite/Sodium humate composite hydrogels. J. Chem. Eng. Data 2010, 55, 2379–2384. [Google Scholar] [CrossRef]
- Yang, J.G. Heavy metal removal and crude bio-oil upgrading from sedum plumbizincicola harvest using hydrothermal upgrading process. Bioresour. Technol. 2010, 101, 7653–7657. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, J.; Chen, C.; Ren, X.; Hu, J.; Wang, X. Removal of cobalt from aqueous solution by magnetic multiwalled carbon nanotube/iron oxide composites. Chem. Eng. J. 2011, 174, 126–133. [Google Scholar] [CrossRef]
- Myglovetsa, M.; Poddubnayaa, O.I.; Sevastyanovac, O.; Lindströmc, M.E.; Gawdzikd, B.; Sobiesiakd, M.; Tsybaa, M.M.; Sapsaye, V.I.; Klymchuke, D.O.; Puziy, A.M. Preparation of carbon adsorbents from lignosulfonate by phosphoric acid activation for the adsorption of metal ions. Carbon 2014, 80, 771–783. [Google Scholar] [CrossRef]
- Khan, M.A.; Ngabura, M.; Choong, T.S.; Masood, H.; Chuah, L.A. Biosorption and desorption of nickel on oil cake: Batch and column studies. Bioresour. Technol. 2012, 103, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Horsfall, M.; Abia, A.A. Sorption of cadmium(II) and zinc(II) ions from aqueous solutions by cassava waste biomass (Manihot sculenta Cranz). Water Res. 2003, 37, 4913–4923. [Google Scholar] [CrossRef] [PubMed]
- Shahryari, Z.; Goharrizi, A.S.; Azadi, M. Experimental study of methylene blue adsorption from aqueous solutions onto carbon nano tubes. Int. J. Water Resour. Environ. Eng. 2010, 2, 016–028. [Google Scholar]
- Karagoz, S.; Tay, T.; Ucar, S.; Erdem, M. Activated carbon from waste biomass by sulfuric acid activation and their use on methylene blue adsorption. Bioresour. Technol. 2008, 99, 6214–6222. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Garcia, J.; Ovejero, G.; Mestanza, M. Adsorption of anionic and cationic dyes on activated carbon from aqueous solutions: Equilibrium and kinetics. J. Hazard. Mater. 2009, 172, 1311–1320. [Google Scholar] [CrossRef]
- Ramalingam, S.; Parthiban, L.; Rangasamy, P. Biosorption Modeling with Multilayer Perceptron for Removal of Lead and Zinc Ions Using Crab Shell Particles. Arab. J. Sci. Eng. 2014, 39, 8465–8475. [Google Scholar] [CrossRef]
- Mishra, V.; Balomajumder, C.; Agarwal, V.K. Biosorption of Zn(II) onto the surface of non-living biomasses: A comparative study of adsorbent particle size and removal capacity of three different biomasses. Water Air Soil Pollut. 2010, 211, 489–500. [Google Scholar] [CrossRef]
- Fernandez, E.; Hugi-Cleary, D.; López-Ramón, M.V.; Stoeckli, F. Adsorption of phenol from dilute and concentrated aqueous solutions by activated carbons. Langmuir 2003, 19, 9719–9723. [Google Scholar] [CrossRef]
- Rozada, F.; Otero, M.; García, A.I.; Morán, A. Application in fixed-bed systems of adsorbents obtained from sewage sludge and discarded tyres. Dyes Pigment 2007, 72, 47–56. [Google Scholar] [CrossRef]
- Sandesh, K.; Kumar, R.S.; Jagadeeshbabu, P.E. Rapid removal of cobalt (II) from aqueous solution using cuttle fish bones; equilibrium, kinetics, and thermodynamic study. Asia-Pac. J. Chem. Eng. 2013, 8, 144–153. [Google Scholar] [CrossRef]
- Singha, B.; Das, S.K. Biosorption of Cr(VI) ions from aqueous solutions: Kinetics, equilibrium, thermodynamics and desorption studies. Colloids Surf. B Biointerfaces 2011, 84, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Tan, X.L.; Chen, C.L.; Wang, X.K. Adsorption of Pb(II) from aqueous solution to MX-80 bentonite: Effect of pH, ionic strength, foreign ions and temperature. Appl. Clay Sci. 2008, 41, 37–46. [Google Scholar] [CrossRef]
- Chakraborty, S.S.; Maiti, N.; Mandal, B.M.; Bhattacharyya, S.N. Miscibility and phase diagrams for poly(vinyl methyl ether) and polyacrylate blend systems. Polymer 1993, 34, 111–114. [Google Scholar] [CrossRef]
- Rana, D.; Mandal, B.M.; Bhattacharyya, S.N. Analogue calorimetry of polymer blends: Poly(styrene-co-acrylonitrile) and poly(phenyl acrylate) or poly(vinyl benzoate). Polymer 1996, 37, 2439–2443. [Google Scholar] [CrossRef]
- Rana, D.; Mandal, B.M.; Bhattacharyya, S.N. Analogue Calorimetric Studies of Blends of Poly(vinyl ester)s and Polyacrylates. Macromolecules 1996, 29, 1579–1583. [Google Scholar] [CrossRef]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.H.; Indraswati, N.; Ismadji, S. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.; Mahmood, A.; Nazir, R. Biosorption potential and kinetic studies of vegetable waste mixture for the removal of nickel(II). J. Mater. Cycles Waste Manag. 2013, 15, 115–121. [Google Scholar] [CrossRef]
- Morsy, R. Synthesis and physicochemical evaluation of hydroxyapatite gel biosorbent for toxic Pb(II) removal from wastewater. Arab. J. Sci. Eng. 2016, 41, 2185–2191. [Google Scholar] [CrossRef]
- Daifullah, A.A.M.; Girgis, B.S. Impact of surface characteristics of activated carbon on adsorption of BTEX. Colloids Surf. A 2003, 214, 181–193. [Google Scholar] [CrossRef]
- Deng, L.; Su, Y.; Su, H.; Wang, X.; Zhu, X. Sorption and desorption of lead (II) from wastewater by green algae Cladophora fascicularis. J. Hazard. Mater. 2007, 143, 220–225. [Google Scholar] [CrossRef]
- Bulut, Y.; Tez, Z. Adsorption studies on ground shells of hazelnut and almond. J. Hazard. Mater. 2007, 149, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Molina-Sabio, M.; Gonçalves, M.; Rodríguez-Reinoso, F. Oxidation of activated carbon with aqueous solution of sodium dichloroisocyanurate: Effect on ammonia adsorption. Microporous Mesoporous Mater. 2011, 142, 577–584. [Google Scholar] [CrossRef]
- Lu, C.Y.; Chiu, H.S. Adsorption of zinc(II) from water with purified carbon nanotubes. Chem. Eng. Sci. 2006, 61, 1138–1145. [Google Scholar] [CrossRef]
- Kathrine, C.; Hansen, H.C.B. Sorption of zinc and lead on coir. Bioresour. Technol. 2007, 98, 89–97. [Google Scholar] [CrossRef]
- Ghoul, M.; Bacquet, M.; Morcellet, M. Uptake of heavy metals from synthetic aqueous solutions using modified PE1-silica gels. Water Res. 2003, 37, 729–734. [Google Scholar] [CrossRef]
- Kargi, F.; Cikla, S. Biosorption of zinc(II) ions onto powdered waste sludge (PWS): Kientics and isotherm. Enzym. Microb. Technol. 2006, 38, 705–710. [Google Scholar] [CrossRef]
- Coleman, N.J.; Lee, W.E.; Slipper, I.J. Interactions of aqueous Cu2+, Zn2+ and Pb2+ ions with crushed concrete fines. J. Hazard. Mater. 2005, 121, 203–213. [Google Scholar] [CrossRef] [PubMed]
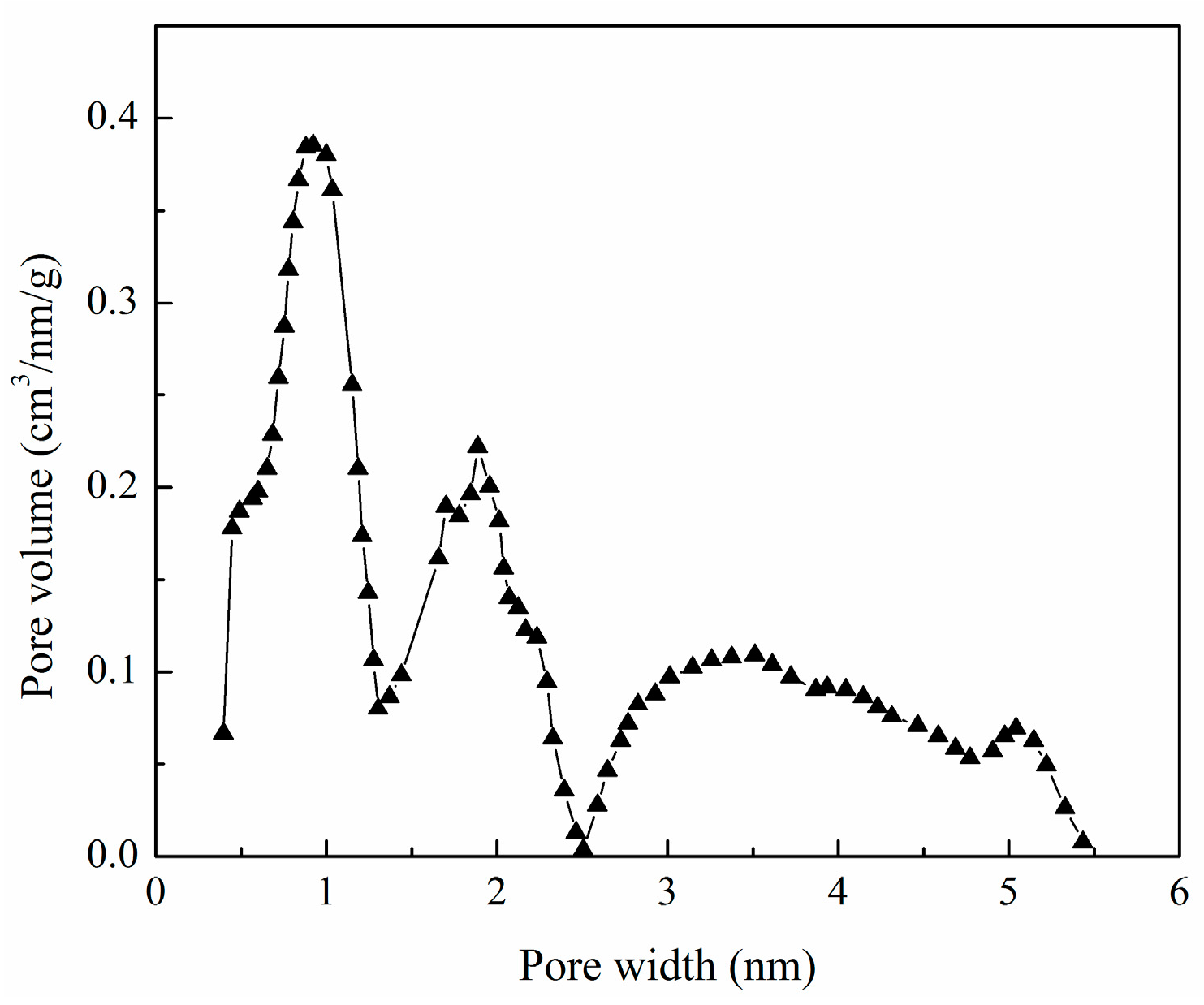
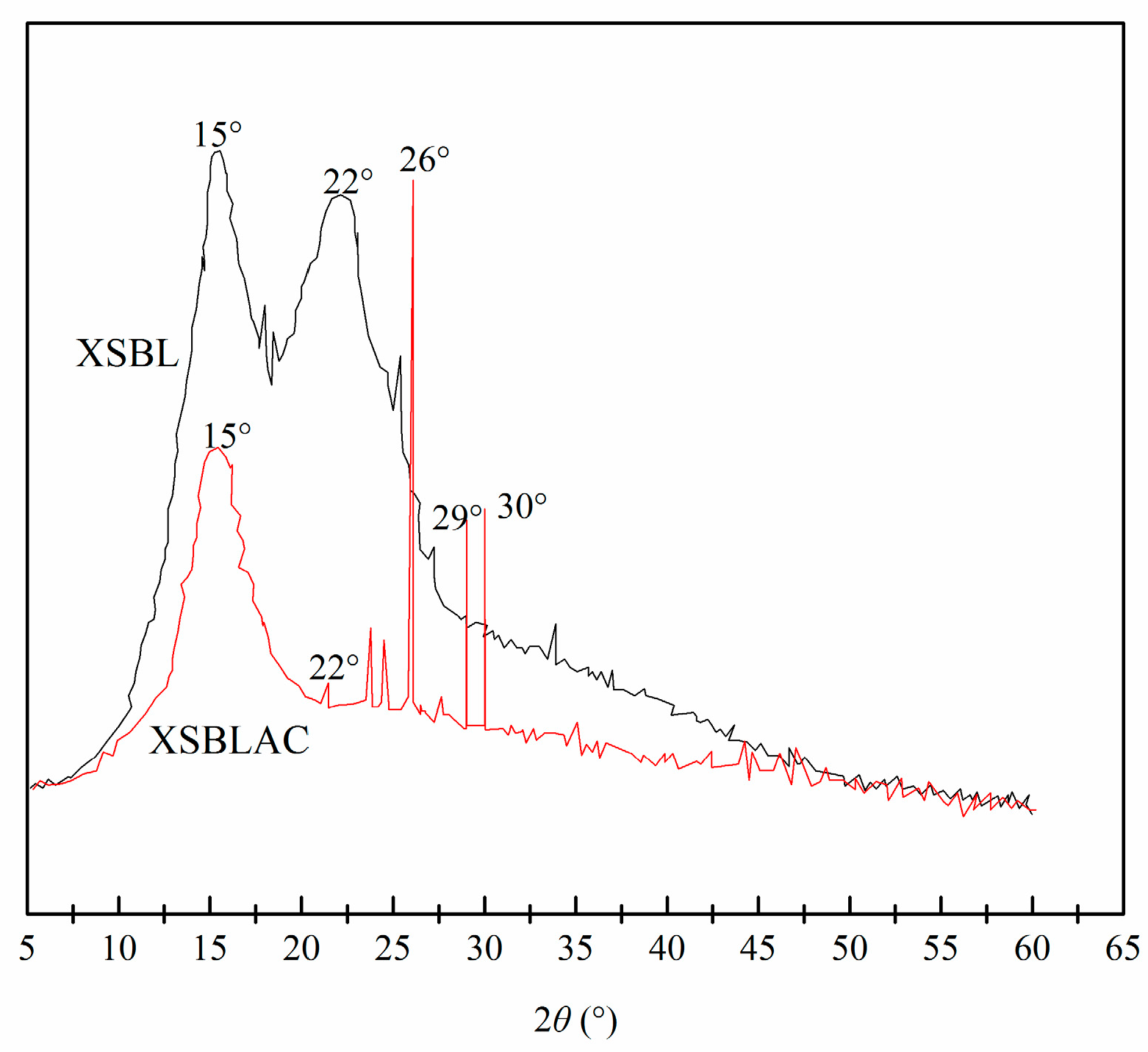
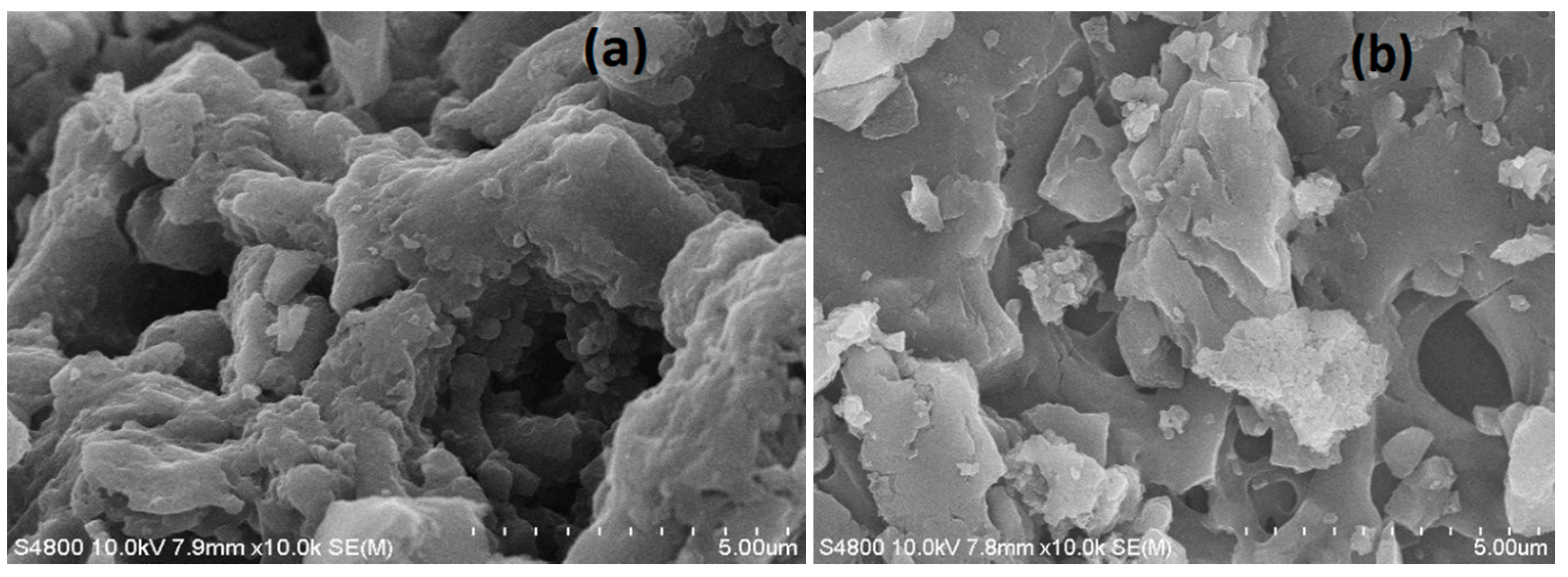



| Sample | SBET (m2/g) | Sext (m2/g) | Sext/SBET (%) | Smic (m2/g) | Vtot (cm3/g) | Vmeso (cm3/g) | Vmeso/Vtot (%) | Dp (nm) |
|---|---|---|---|---|---|---|---|---|
| XSBLAC | 688.62 | 477.87 | 69.4 | 210.43 | 0.377 | 0.252 | 66.8 | 2.2 |
| Sample | C (at %) | O (at %) | N (at %) | Cl (at %) | Na (at %) | O/C (%) | N/C (%) |
|---|---|---|---|---|---|---|---|
| XSBLAC | 80.26 | 12.92 | 5.62 | 0.75 | 0.45 | 16.1 | 0.07 |
| XSBL | 60.33 | 34.96 | 3.41 | 0.93 | 0.37 | 60.0 | 0.06 |
| Metal | qe (exp) (mg/g) | Parameters | Pseudo-First-Order | Pseudo-First-Order | Intraparticle Diffusion | Elovich | Bangham Model | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zinc(II) | 103.82 | R2 | 0.6349 | 0.9416 | 0.7901 | 0.8724 | 0.7576 | |||||
| Constants | k1 | 0.01329 min−1 | K2 | 2.764 × 10−4 min−1 | ki | 15.302 mg/(g min0.5) | α | 11.2501 mg/(g min) | Kr (mg·g−1·min−1) | 0.7942 | ||
| qe (cal) | 76.36 mg/g | qe (cal) | 110.48 mg/g | β | 0.01327 g/mg | m | 0.1004 | |||||
| qm (mg/g) | Langmuir Isotherm Equation | Freundlich Isotherm Equation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| qe (mg/g) | b (mg/L) | RL | R2 | p | 1/n | kf (mg/L) | R2 | p | |
| 103.82 | 100.76 | 0.0039 | 0.1362 | 0.9793 | 1.327 × 10−4 | 0.4133 | 4.614 | 0.7683 | 0.0026 |
| Recycle Times | 1st | 2nd | 3rd | 4th | 5th |
|---|---|---|---|---|---|
| Adsorption capacity (mg/g) | 103.82 | 94.06 | 92.62 | 80.83 | 42.44 |
| Desorption capacity (mg/g) | 78.52 | 73.31 | 71.05 | 60.76 | 19.17 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Hao, Y.; Wang, X.; Chen, Z. Rapid Removal of Zinc(II) from Aqueous Solutions Using a Mesoporous Activated Carbon Prepared from Agricultural Waste. Materials 2017, 10, 1002. https://doi.org/10.3390/ma10091002
Zhang X, Hao Y, Wang X, Chen Z. Rapid Removal of Zinc(II) from Aqueous Solutions Using a Mesoporous Activated Carbon Prepared from Agricultural Waste. Materials. 2017; 10(9):1002. https://doi.org/10.3390/ma10091002
Chicago/Turabian StyleZhang, Xiaotao, Yinan Hao, Ximing Wang, and Zhangjing Chen. 2017. "Rapid Removal of Zinc(II) from Aqueous Solutions Using a Mesoporous Activated Carbon Prepared from Agricultural Waste" Materials 10, no. 9: 1002. https://doi.org/10.3390/ma10091002





