Formation Mechanism of Spherical TiC in Ni-Ti-C System during Combustion Synthesis
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
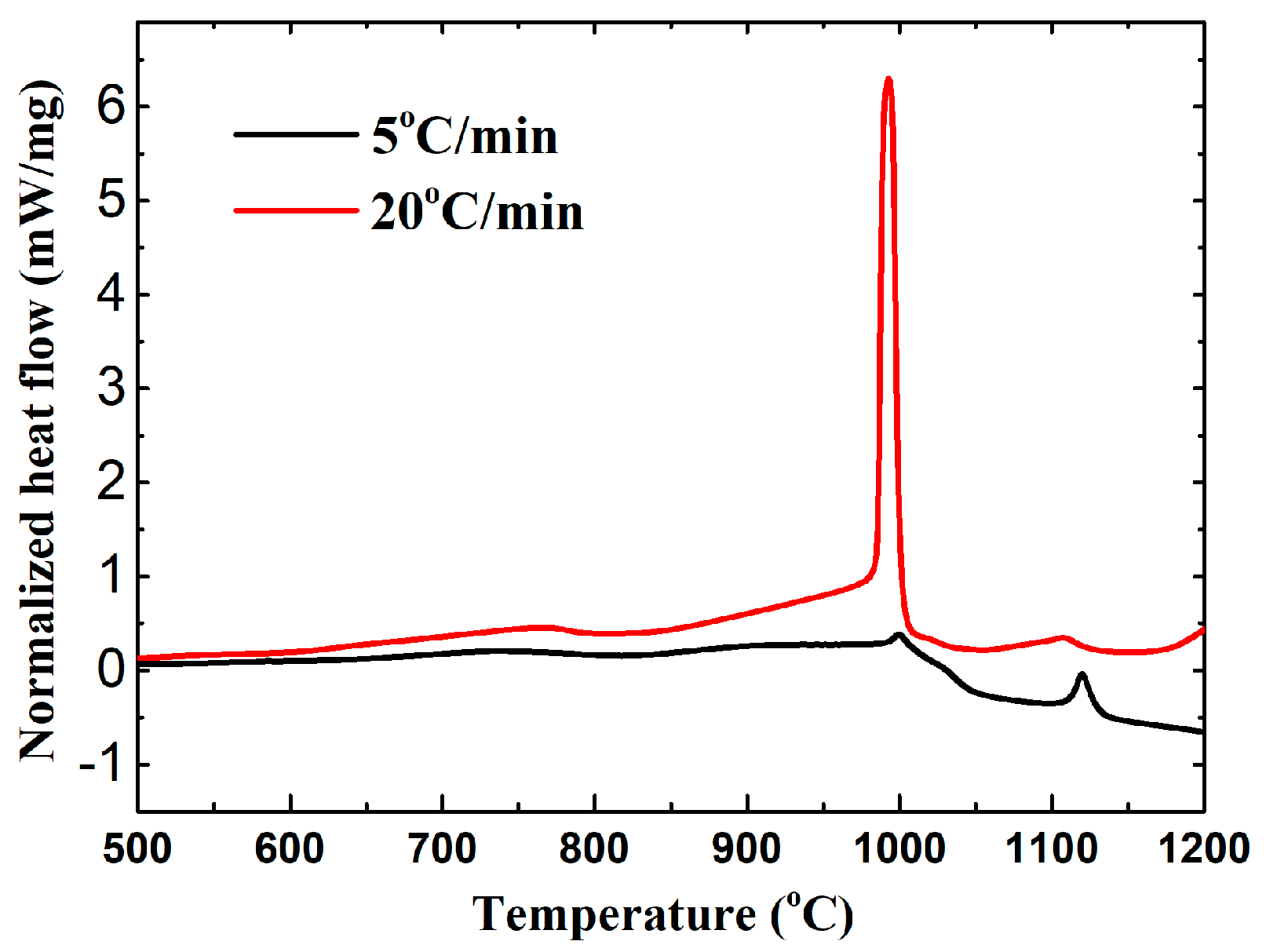
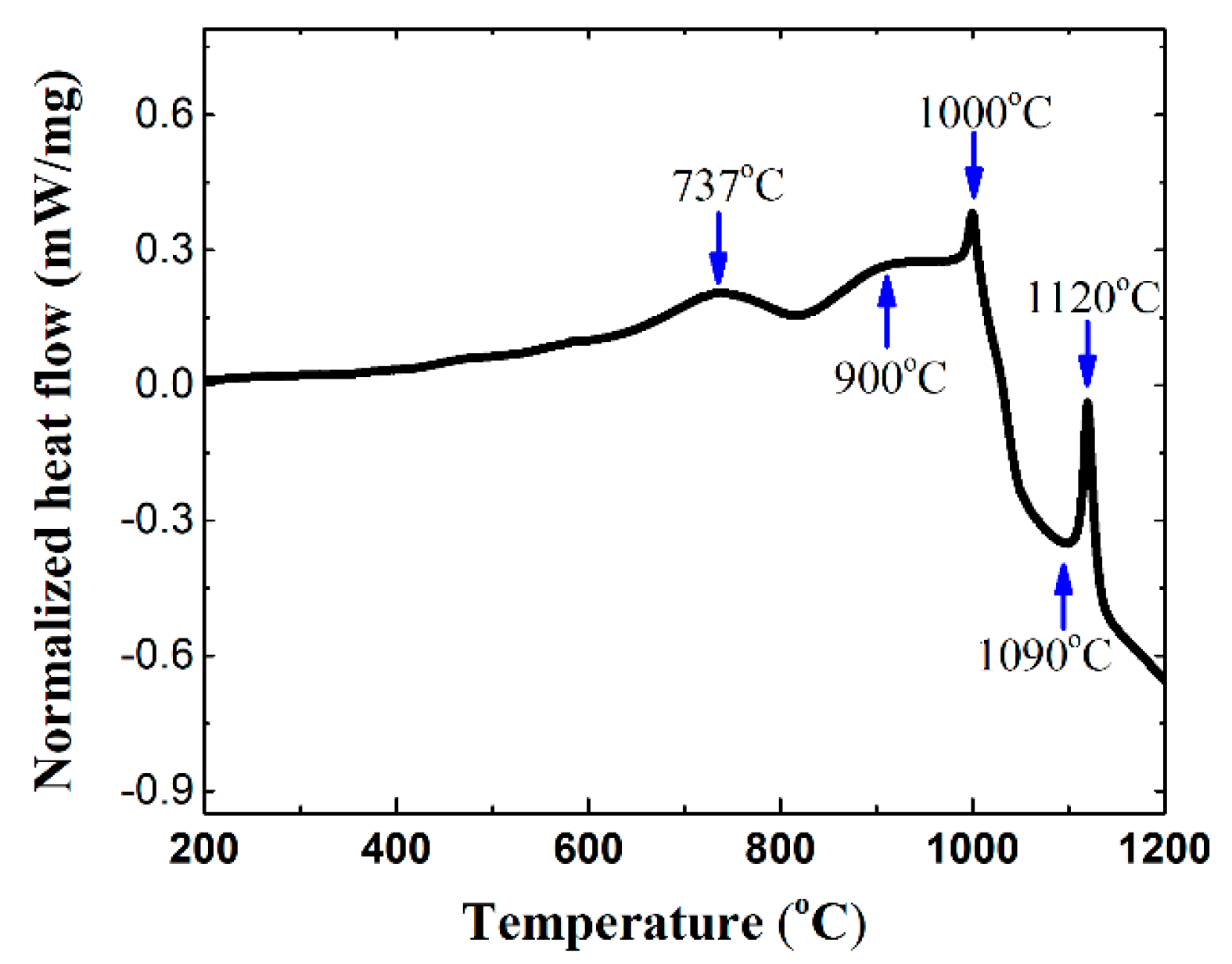
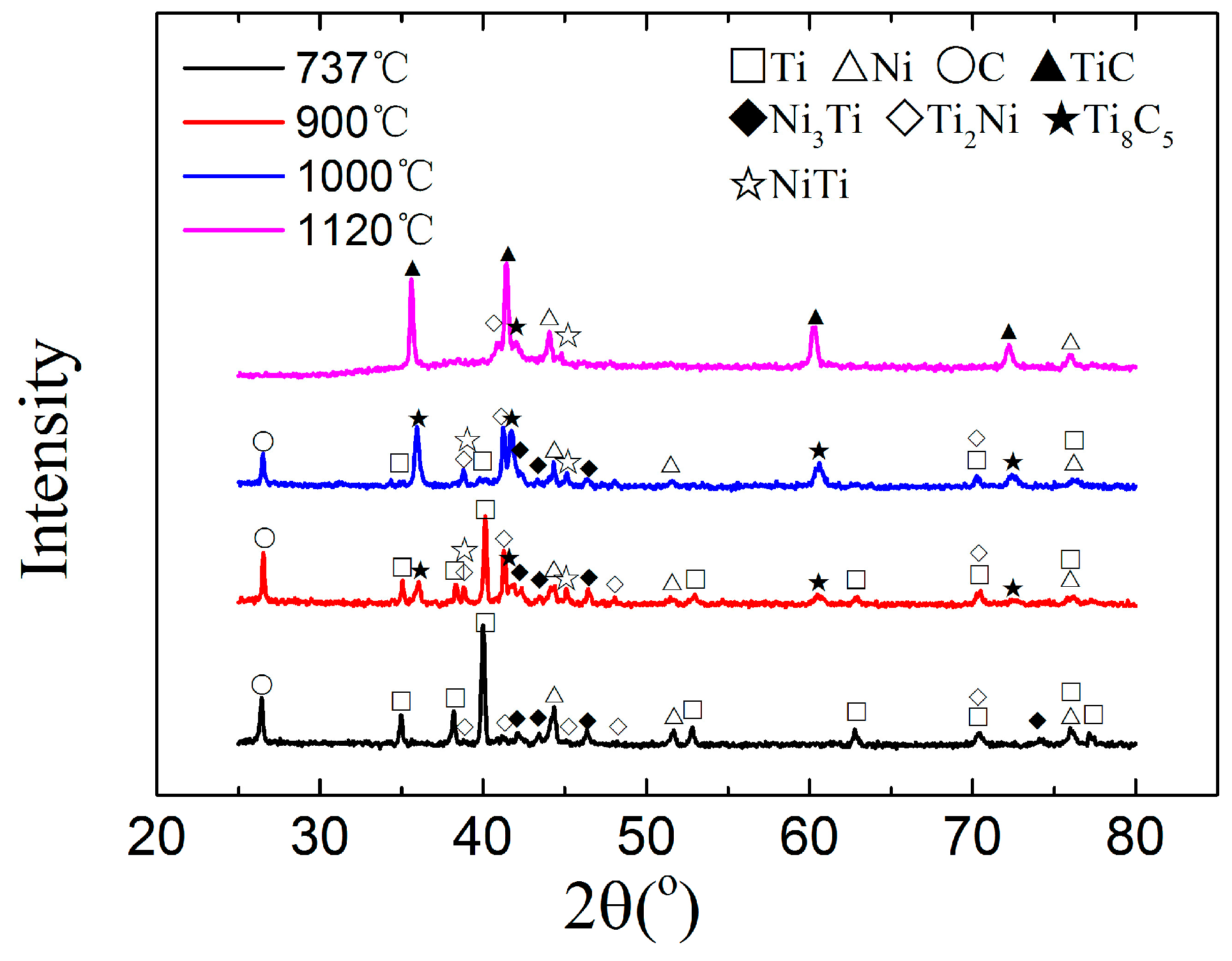
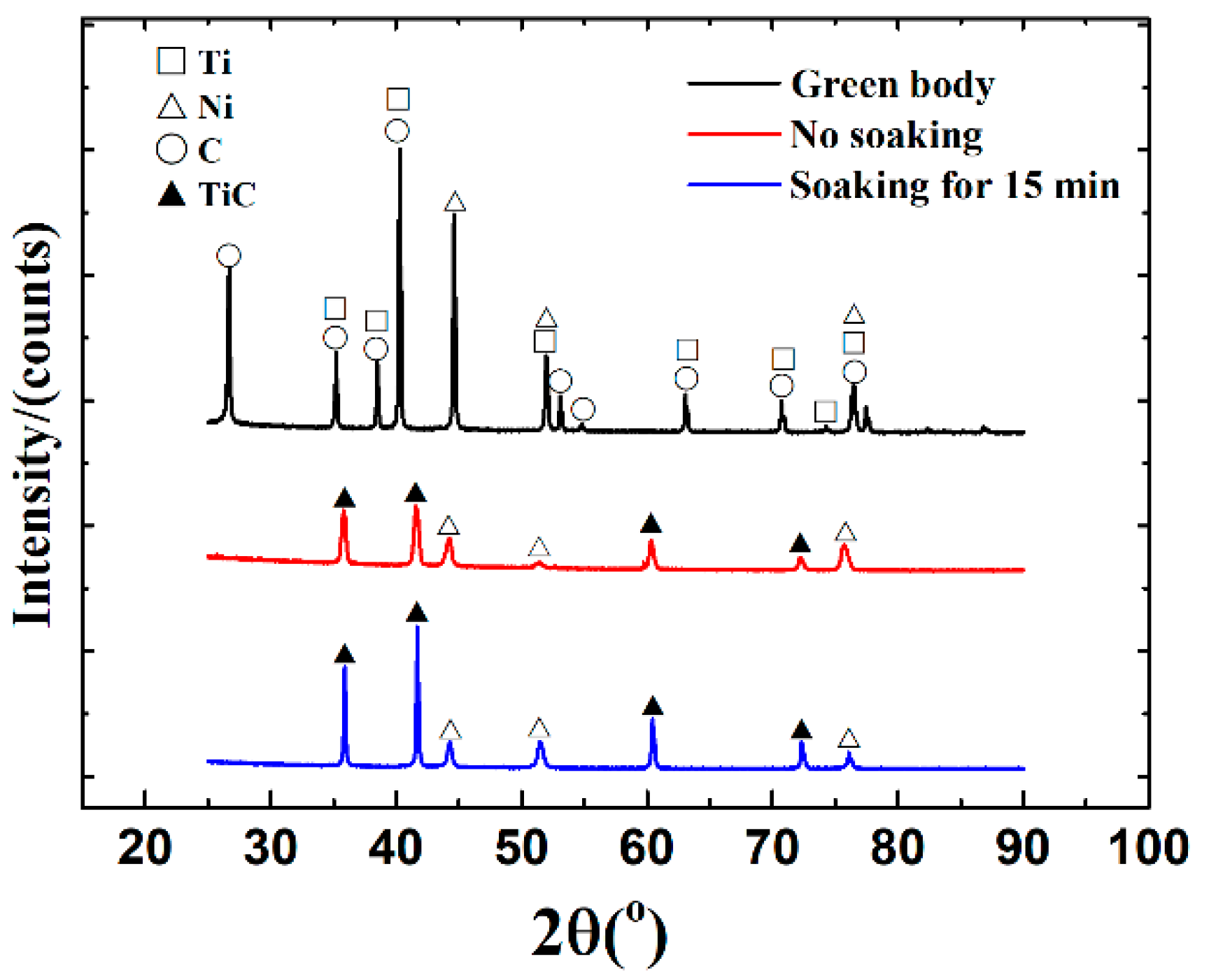
3.1. Synthesis Mechanism
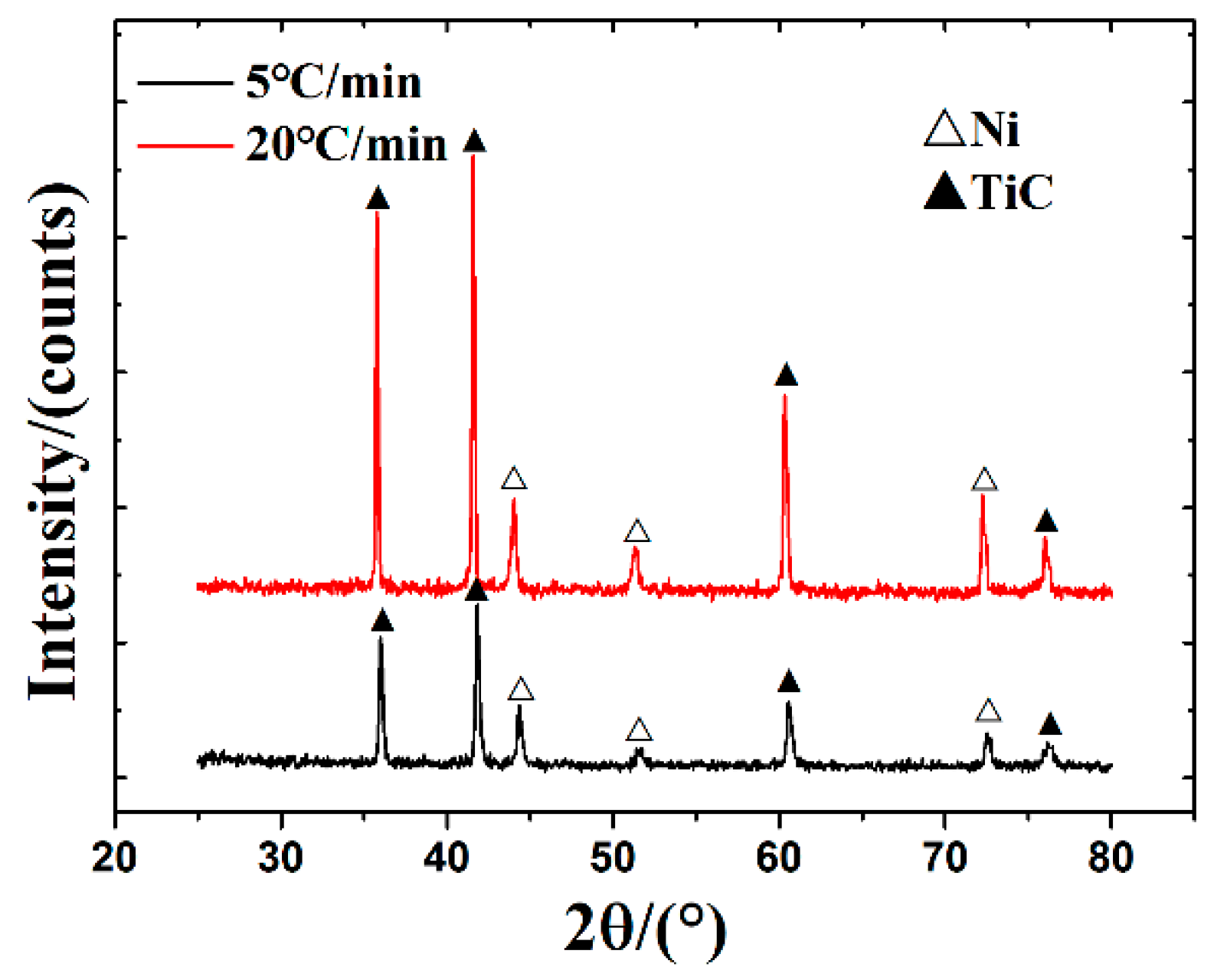
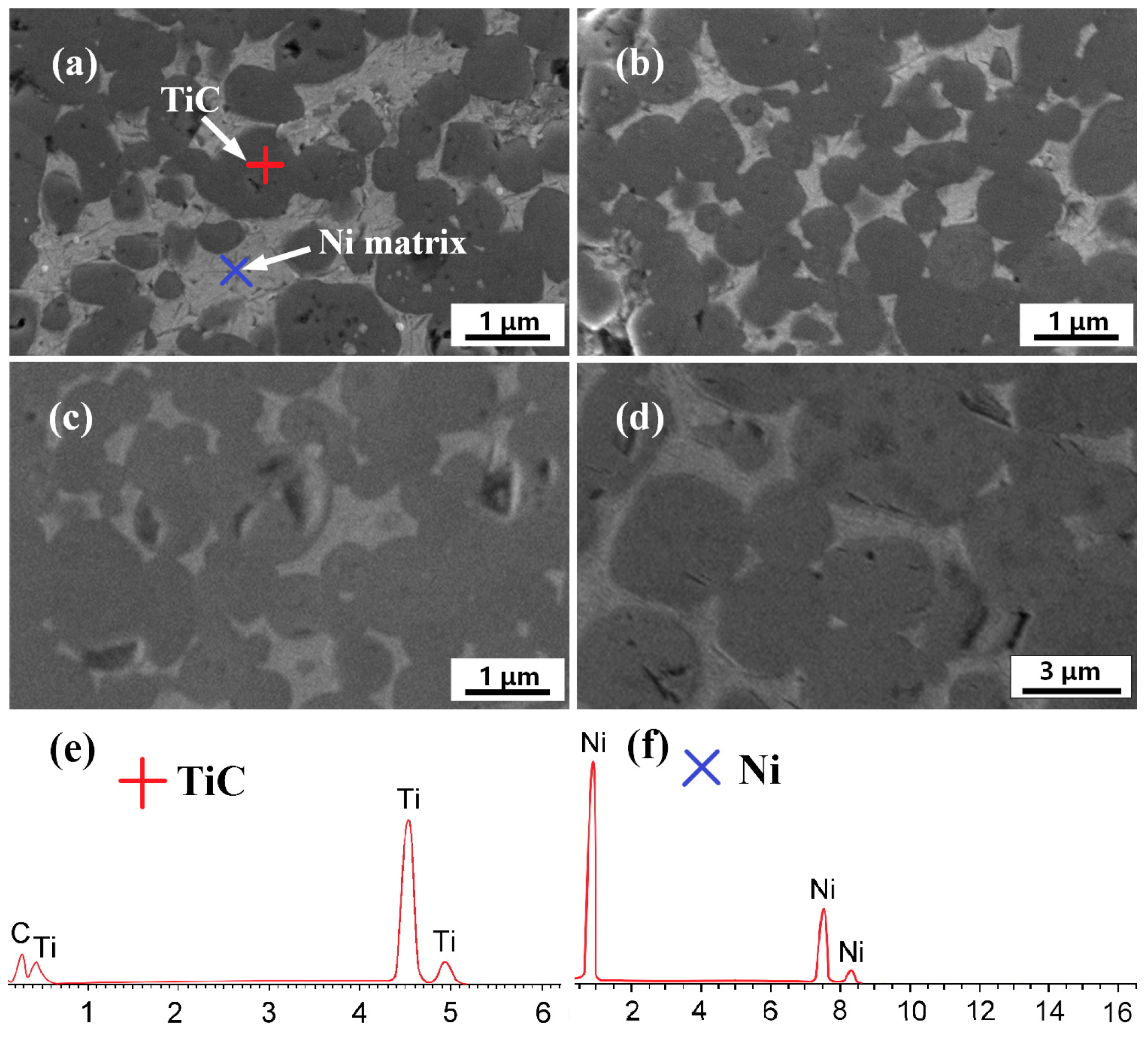
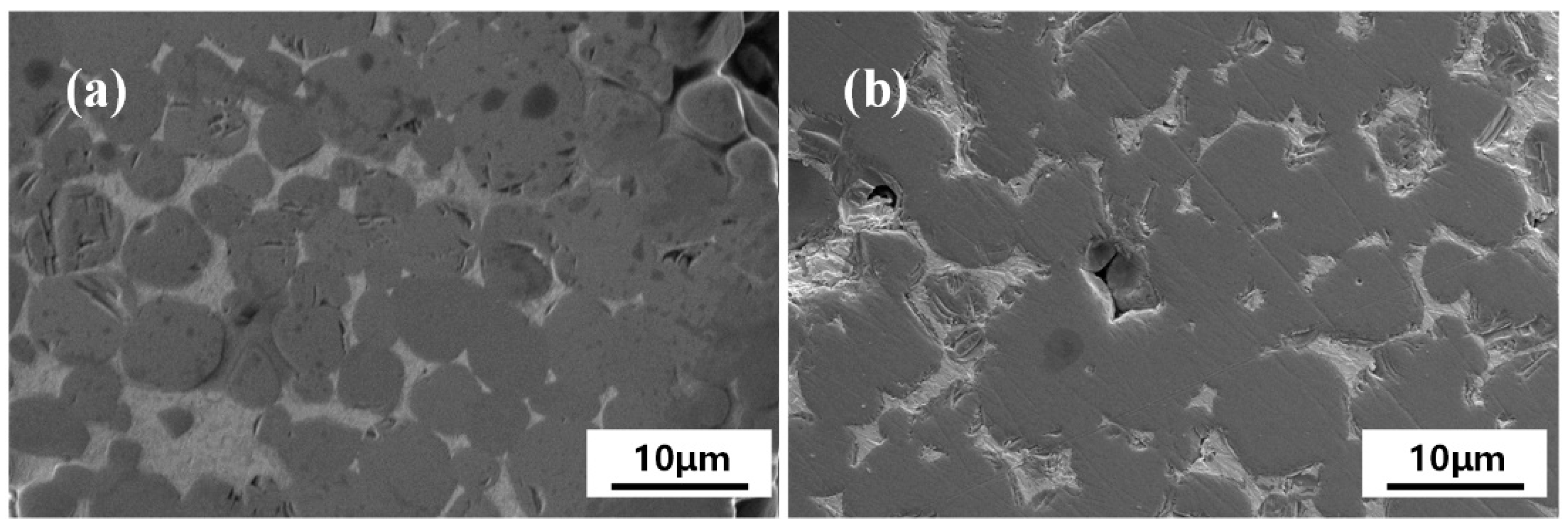
3.2. Microstructure
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tjong, S.C.; Ma, Z.Y. Microstructural and mechanical characteristics of in situ metal matrix composites. Mater. Sci. Eng. R 2000, 29, 49–113. [Google Scholar] [CrossRef]
- Yang, Y.F.; Jin, S.B.; Jiang, Q.C. Effect of reactant C/Ti ratio on the stoichiometry, morphology of TiCx and mechanical properties of TiCx-Ni composite. CrystEngComm 2013, 15, 852–855. [Google Scholar] [CrossRef]
- Yang, Y.F.; Wang, H.Y.; Liang, Y.H.; Zhao, R.Y.; Jiang, Q.C. Fabrication of steel matrix composites locally reinforced with different ratios of TiC/TiB2 particulates using SHS reactions of Ni-Ti-B4C and Ni-Ti-B4C-C systems during casting. Mater. Sci. Eng. A 2007, 445–446, 398–404. [Google Scholar] [CrossRef]
- Azadmehr, A.; Taheri-nassaj, E. An in situ (W,Ti)C-Ni composite fabricated by SHS method. J. Non-Cryst. Solids 2008, 354, 3225–3234. [Google Scholar] [CrossRef]
- Zohari, S.; Sadeghian, Z.; Lotfi, B.; Broeckmann, C. Application of spark plasma sintering (SPS) for the fabrication of in situ Ni-TiC nanocomposite clad layer. J. Alloys Compd. 2015, 633, 479–483. [Google Scholar] [CrossRef]
- Manukyan, K.V.; Lin, Y.C.; Rouvimov, S.; McGinn, P.J.; Mukasyan, A.S. Microstructure-reactivity relationship of Ti + C reactive nanomaterials. J. Appl. Phys. 2013, 113, 024302. [Google Scholar] [CrossRef]
- Huang, X.; Yin, C.; Zhao, Z.; Zhang, L.; Wu, J. Microstructures and Toughening of TiC-TiB2 Ceramic Composites with Cr-Based Alloy Phase Prepared by Combustion Synthesis in High-Gravity Field. Adv. Mater. Sci. Eng. 2015, 2015, 358746. [Google Scholar] [CrossRef]
- Gotman, I.; Travitzky, N.A.; Gutmanas, E.Y. Dense in situ TiB2/TiN and TiB2/TiC ceramic matrix composites: Reactive synthesis and properties. Mater. Sci. Eng. A 1998, 244, 127–137. [Google Scholar] [CrossRef]
- Dunmead, S.D.; Readey, D.W.; Semler, C.E.; Hol, J.B. Kinetics of combustion synthesis in the Ti-C and Ti-C-Ni systems. J. Am. Ceram. Soc. 1989, 72, 2318–2324. [Google Scholar] [CrossRef]
- Lasalvia, J.C.; Kim, D.K.; Lipsett, R.A.; Meyers, M.A. Combustion synthesis in the Ti-C-Ni-Mo system: Part I. Micromechanisms. Metall. Mater. Trans. A 1995, 26, 3001–3009. [Google Scholar] [CrossRef]
- Klinger, L.; Gotman, I.; Horvitz, D. In situ processing of TiB2/TiC ceramic composites by thermal explosion under pressure: Experimental study and modeling. Mater. Sci. Eng. A 2001, 302, 92–99. [Google Scholar] [CrossRef]
- Yang, Y.F.; Wang, H.Y.; Liang, Y.H.; Zhao, R.Y.; Jiang, Q.C. Effect of nickel addition on the exothermic reaction of titanium and boron carbide. J. Mater. Res. 2007, 22, 169–174. [Google Scholar] [CrossRef]
- Saidia, A.; Chrysanthou, A.; Wood, J.V.; Kellie, J.L.F. Preparation of Fe-TiC composites by the thermal-explosion mode of combustion synthesis. Ceram. Int. 1997, 23, 185–189. [Google Scholar] [CrossRef]
- Zarrinfar, N.; Shipway, P.; Kennedy, A.; Saidi, A. Carbide stoichiometry in TiCx and Cu-TiCx produced by self-propagating high-temperature synthesis. Scr. Mater. 2002, 46, 121–126. [Google Scholar] [CrossRef]
- Li, Y.X.; Hu, J.D.; Wang, H.Y.; Guo, Z.X.; Chumakov, A.N. Thermodynamic and lattice parameter calculation of TiCx produced from Al-Ti-C powders by laser igniting self-propagating high-temperature synthesis. Mater. Sci. Eng. A 2007, 458, 235–239. [Google Scholar] [CrossRef]
- Xiao, G.; Fan, Q.; Gu, M.; Wang, Z.; Jin, Z. Dissolution-precipitation mechanism of self-propagating high-temperature synthesis of TiC-Ni cermet. Mater. Sci. Eng. A 2004, 382, 132–140. [Google Scholar] [CrossRef]
- Yang, Y.F.; Wang, H.Y.; Zhao, R.Y.; Liang, Y.H.; Zhan, L.; Jiang, Q.C. Effects of C particle size on the ignition and combustion characteristics of the SHS reaction in the 20 wt % Ni-Ti-C system. J. Alloys Compd. 2008, 460, 276–282. [Google Scholar] [CrossRef]
- Wong, J.; Larson, E.M.; Holt, J.B.; Waide, P.A.; Rupp, B.; Frahm, R. Time-resolved X-ray diffraction study of solid combustion Reactions. Science 1990, 249, 1406–1409. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.; Okamoto, H. ASM Handbook (Vol. 3): Alloy Phase Diagram; ASM International: Geauga County, OH, USA, 1992; ISBN 9780871703811. [Google Scholar]
- Backerud, L.; Carlsson, B.; Oskarsson, R.; Mikus, M. Study of the Ni-Rich and Co-Rich Corners of the Systems Ni-Ti-C and Co-Ti-C. Scand. J. Metall. 1974, 3, 225–235. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, G.; Wang, W.; Wang, R.; Zhao, C.; Pan, W.; Huang, H.; Du, D.; Wang, D.; Shu, D.; Dong, A.; et al. Formation Mechanism of Spherical TiC in Ni-Ti-C System during Combustion Synthesis. Materials 2017, 10, 1007. https://doi.org/10.3390/ma10091007
Zhu G, Wang W, Wang R, Zhao C, Pan W, Huang H, Du D, Wang D, Shu D, Dong A, et al. Formation Mechanism of Spherical TiC in Ni-Ti-C System during Combustion Synthesis. Materials. 2017; 10(9):1007. https://doi.org/10.3390/ma10091007
Chicago/Turabian StyleZhu, Guoliang, Wei Wang, Rui Wang, Chuanbao Zhao, Weitao Pan, Haijun Huang, Dafan Du, Donghong Wang, Da Shu, Anping Dong, and et al. 2017. "Formation Mechanism of Spherical TiC in Ni-Ti-C System during Combustion Synthesis" Materials 10, no. 9: 1007. https://doi.org/10.3390/ma10091007



