Poly(Neopentyl Glycol Furanoate): A Member of the Furan-Based Polyester Family with Smart Barrier Performances for Sustainable Food Packaging Applications
Abstract
:1. Introduction
2. Results and Discussion
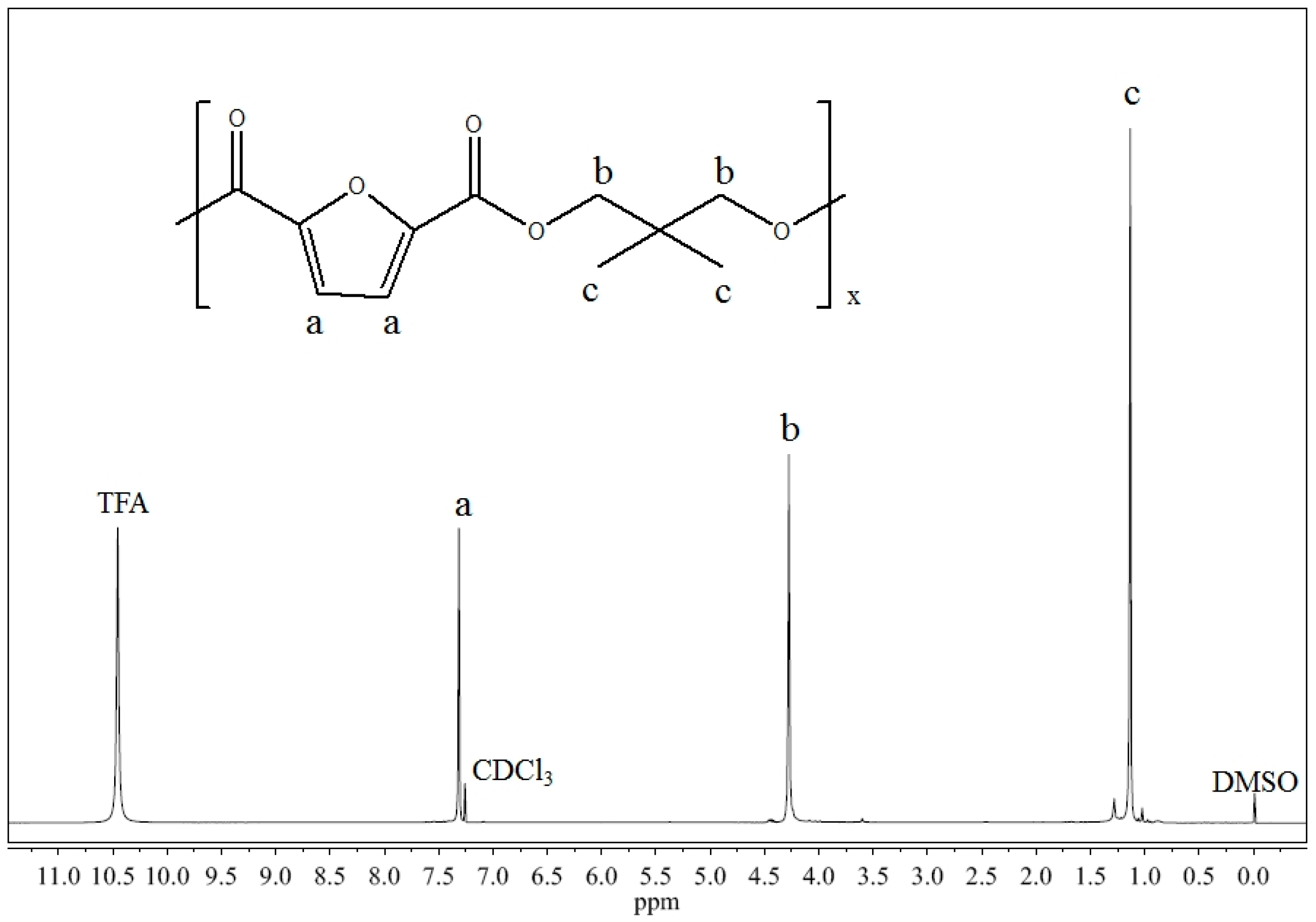
2.1. Molecular Characterization
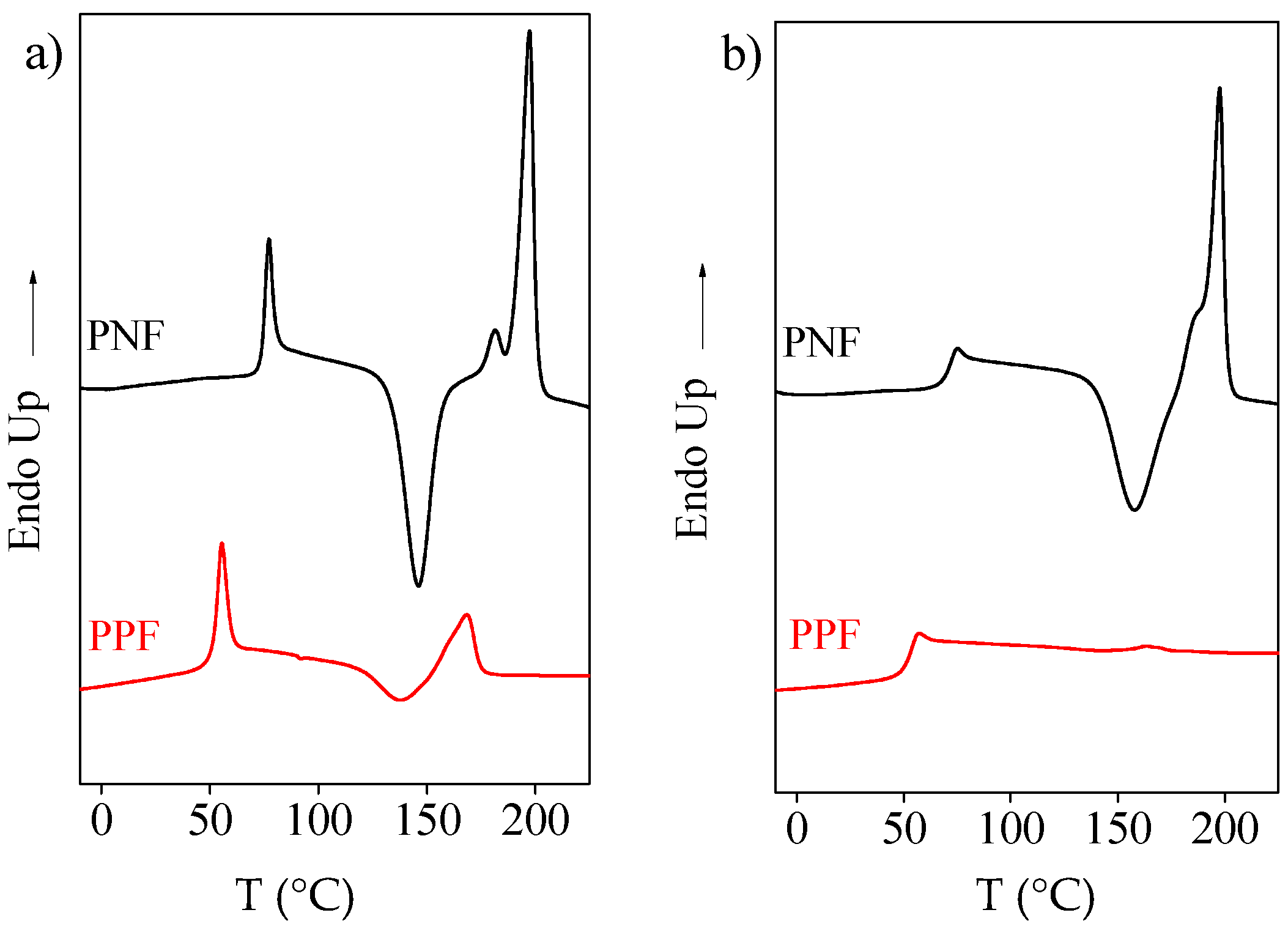
2.2. Thermal Characterization
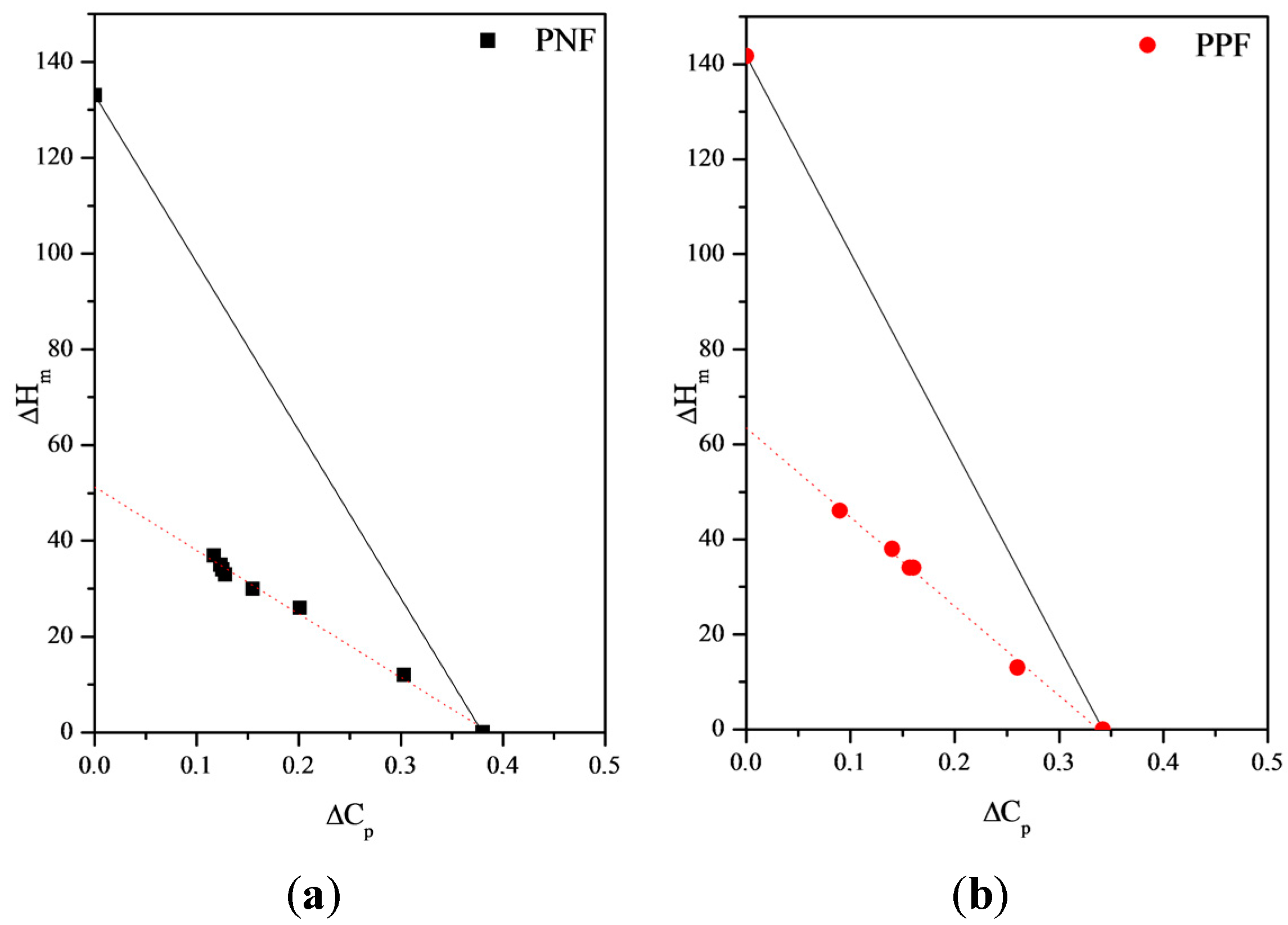
Rigid-Amorphous Phase
2.3. Permeability Behavior
2.3.1. Barrier Properties
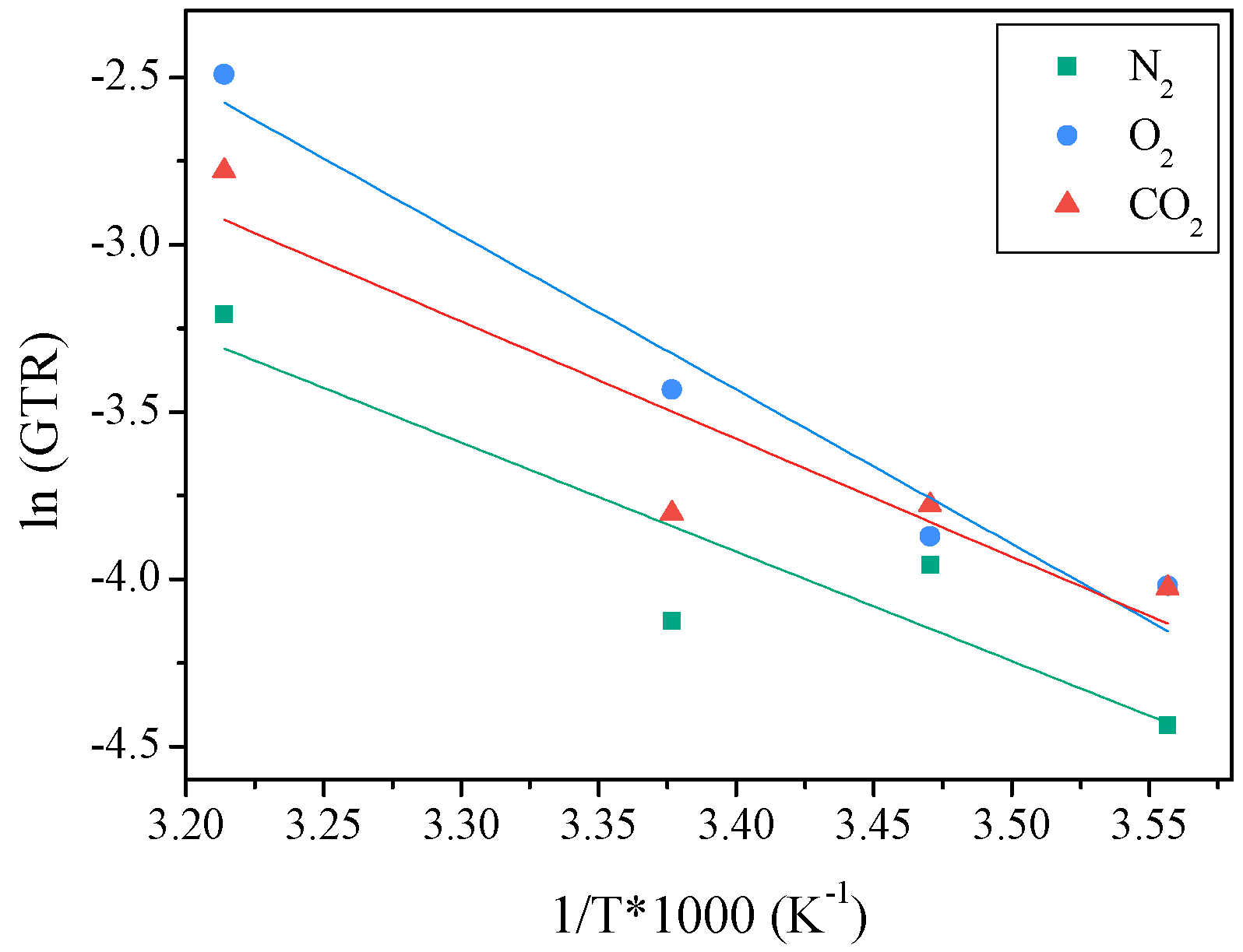
2.3.2. Activation Energy of Gas Transport Process
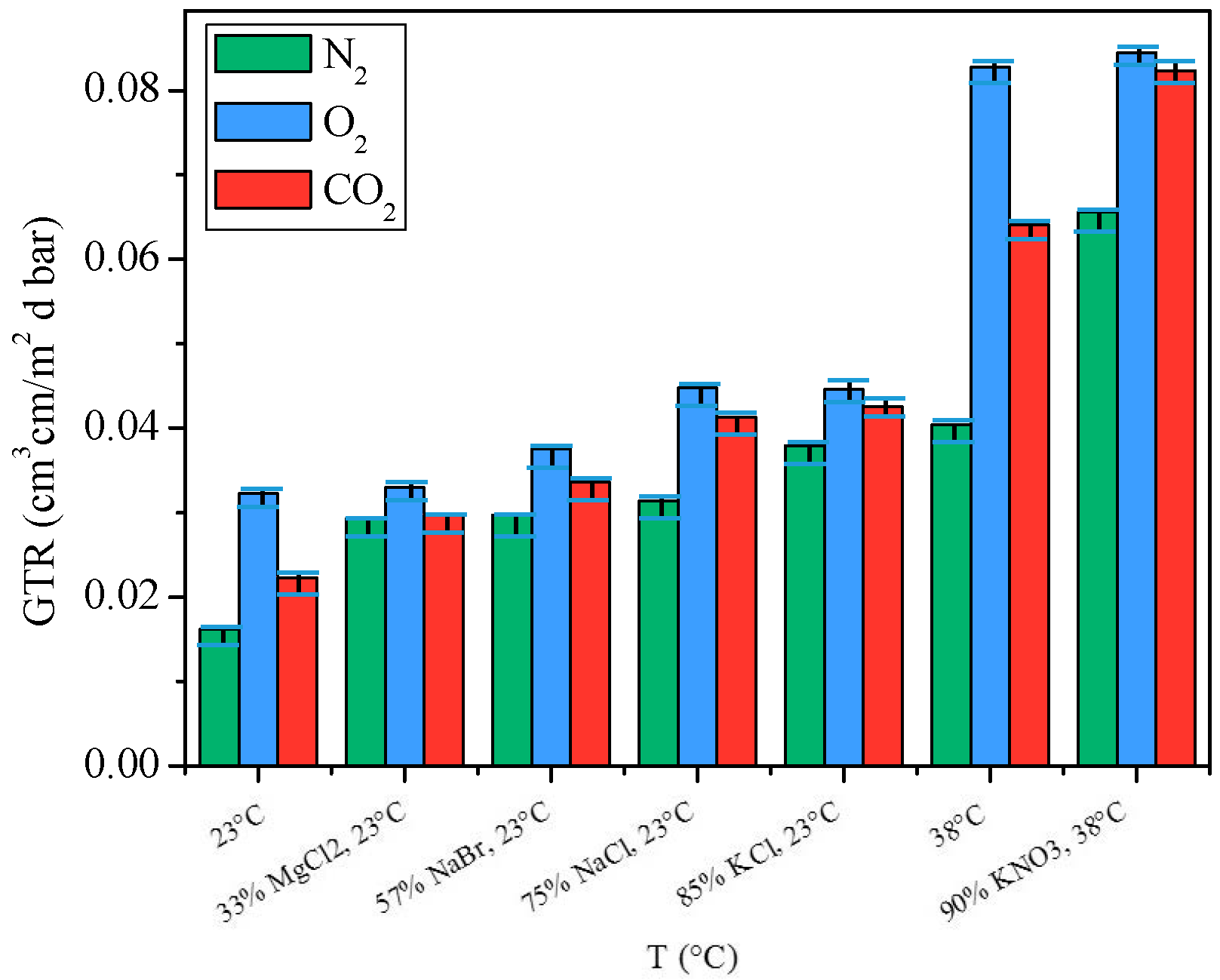
2.3.3. Gas Barrier Behavior at Different Relative Humidity
- -
- for N2 an increment of 82% was recorded at 23 °C from 0% of RH to 85% of RH and of 63% at 38 °C from 0% RH to 90% RH;
- -
- for O2 gas an increment of 2.5% was recorded at 23 °C from 0% of RH to 85% of RH and of 2% at 38 °C from 0% RH to 90% RH;
- -
- for CO2 an increment of 33% was recorded at 23 °C from 0% of RH to 85% of RH and of 29% at 38 °C from 0% RH to 90% RH.
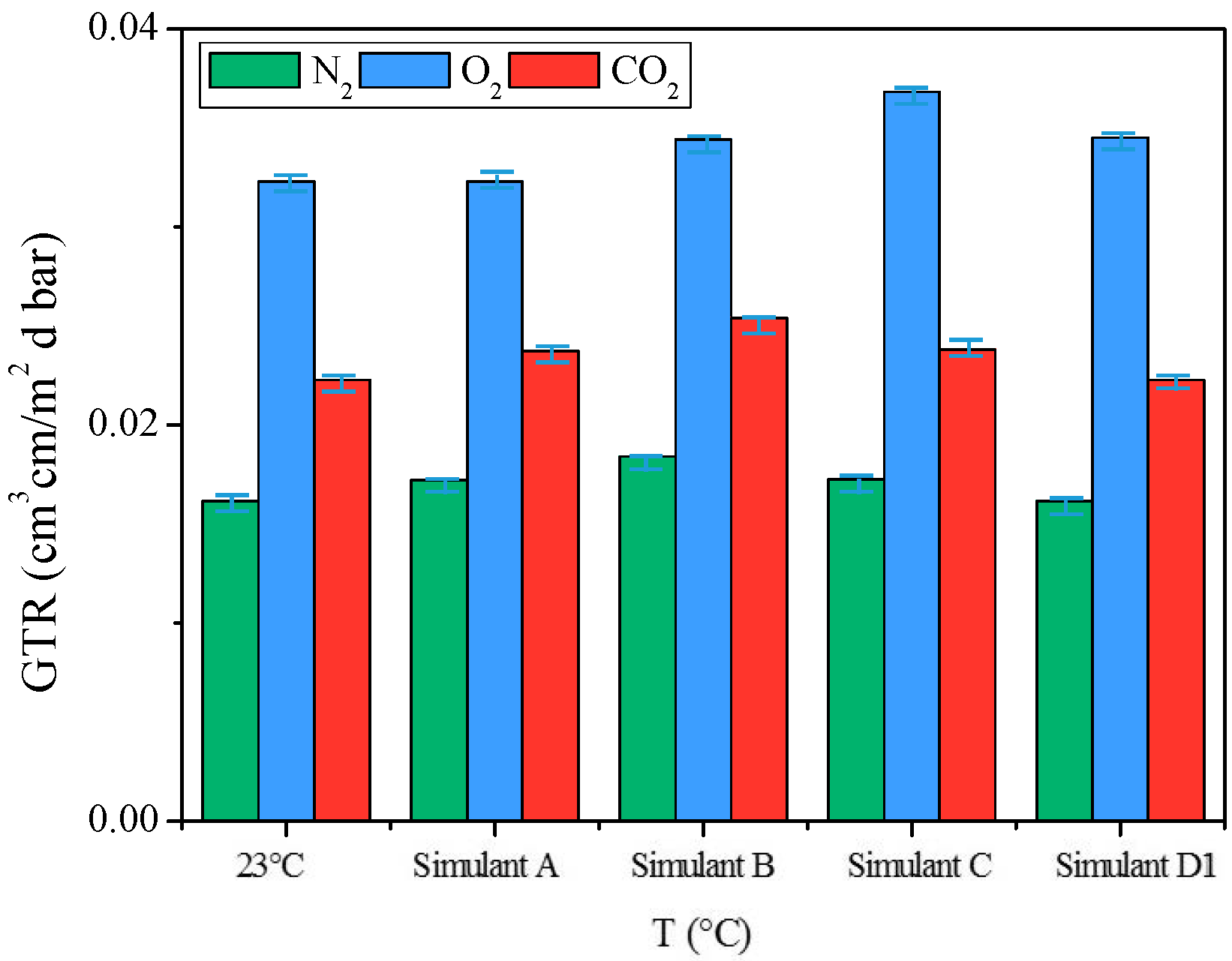
2.3.4. Gas Barrier Behavior after Food Simulants Contact
2.4. Mechanical Characterization
3. Materials and Methods
3.1. Materials
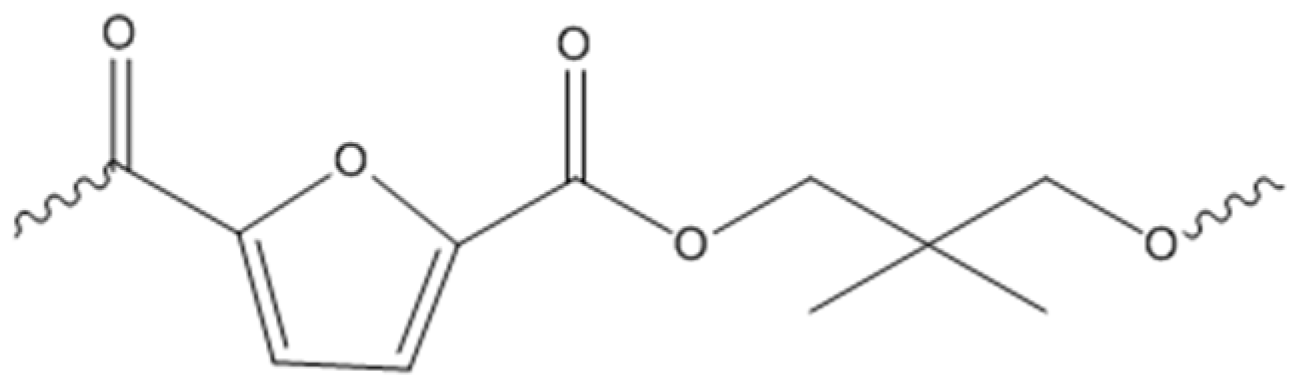
3.2. Polymer Synthesis
3.3. Molecular, and Thermal Characterization
3.4. Water Contact Angle Measurements
3.5. Tensile Tests
3.6. Gas Transport Measurements
3.7. Simulant Liquids
- -
- Simulant A, Ethanol 10% (v/v), 10 days, 40 °C
- -
- Simulant B, Acetic acid 3% (v/v), 10 days, 40 °C
- -
- Simulant C, Ethanol 20% (v/v), 10 days, 40 °C
- -
- Simulant D1, Ethanol 50% (v/v), 10 days, 40 °C
3.8. Relative Humidity Solution
- -
- Standard Environment, 23 °C, 85% of RH, with saturated KCl solution;
- -
- Tropical Climate, 38 °C, 90% RH, with saturated KNO3 solution;
- -
- 33% RH, 23 °C, with saturated MgCl2 solution;
- -
- 57% RH, 23 °C, with saturated NaBr solution;
- -
- 75% RH, 23 °C, with saturated NaCl solution;
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Plastic Europe: Plastics—The Facts 2016, An Analysis of European Plastics Production, Demand and Waste Data 2016. Available online: www.plasticseurope.org (accessed on 28 July 2017).
- European Bioplastics—Bioplastics, Facts and Figures 2017. Available online: http://en.european-bioplastics.org (accessed on 28 July 2017).
- Tadahisa, I. Biodegradable and Bio-based Polymers: Future prospects of eco-friendly plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef]
- Burgess, S.K.; Leisen, J.E.; Kraftschik, B.E.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Chain Mobility, Thermal, and Mechanical Properties of Poly(ethylene furanoate) Compared to Poly(ethylene terephthalate). Macromolecules 2014, 47, 1383–1391. [Google Scholar] [CrossRef]
- Burgess, S.K.; Karvan, O.; Johnson, J.R.; Kriegel, R.M.; Koros, W.J. Oxygen sorption and transport in amorphous poly(ethylene furanoate). Polymer 2014, 55, 4748–4756. [Google Scholar] [CrossRef]
- Burgess, S.K.; Kriegel, R.M.; Koros, W.J. Carbon Dioxide Sorption and Transport in Amorphous Poly(ethylene furanoate). Macromolecules 2015, 48, 2184–2193. [Google Scholar] [CrossRef]
- Burgess, S.K.; Mikkilineni, D.S.; Yu, D.B.; Kim, D.J.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Water sorption in poly(ethylene furanoate) compared to poly(ethylene terephthalate). Part 2: Kinetic sorption. Polymer 2014, 55, 6870–6882. [Google Scholar] [CrossRef]
- Eerhart, A.J.J.E.; Faaij, A.P.C.; Patel, M.K. Replacing fossil based PET with biobased PEF; proess analysis, energy and GHG balance. Energy Environ. Sci. 2012, 5, 6407–6422. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Terzopoulou, Z.; Bikiaris, D.N. Production of bio-based 2,5-furan dicarboxylate polyesters: Recent progress and critical aspects in their synthesis and thermal properties. Eur. Polym. J. 2016, 83, 202–229. [Google Scholar] [CrossRef]
- Soccio, M.; Costa, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Salatelli, E.; Manaresi, P.; Munari, A. Novel fully biobased poly(butylene 2,5-furanoate/diglycolate) copolymers containing ether linkages: Structure-property relationships. Eur. Polym. J. 2016, 81, 397–412. [Google Scholar] [CrossRef]
- Vannini, M.; Marchese, P.; Celli, A.; Lorenzetti, C. Fully biobased poly(propylene 2,5-furandicarboxylate) for packaging applications: Excellent barrier properties. Green Chem. 2015, 17, 4162–4166. [Google Scholar] [CrossRef]
- Guidotti, G.; Lotti, N.; Soccio, M.; Gigli, M.; Genovese, L.; Siracusa, V.; Munari, A. Novel biobased copolymers of poly(propylene 2,5-furanoate) for sustainable food packaging: Synergistic effect of aromatic furan and aliphatic cyclohexane rings. Green Chem. 2017. submitted. [Google Scholar]
- Tsanaktsis, V.; Terzopoulou, Z.; Exarhopoulos, S.; Bikiaris, D.N.; Achilias, D.S.; Papageorgiou, D.G.; Papageorgiou, G.Z. Sustainable, eco-friendly polyesters synthesized from renewable resources: Preparation and thermal characteristics of poly(dimethyl-propylene furanoate). Polym. Chem. 2015, 6, 8284–8296. [Google Scholar] [CrossRef]
- Soccio, M.; Lotti, N.; Finelli, L.; Gazzano, M.; Munari, A. Neopentyl glycol containing poly(propylene azelate)s: Synthesis and thermal properties. Eur. Polym. J. 2007, 43, 3301–3313. [Google Scholar] [CrossRef]
- Soccio, M.; Lotti, N.; Finelli, L.; Gazzano, M.; Munari, A. Neopentyl glycol containing poly(propylene terephthalate)s: Structure-properties relationships. J. Polym. Sci. Part B: Polym. Phys. 2008, 46, 170–181. [Google Scholar] [CrossRef]
- Dimitriadis, T.; Bikiaris, D.N.; Papageorgiou, G.Z.; Floudas, G. Molecular Dynamics of Poly(ethylene-2,5-furanoate) (PEF) as a Function of the Degree of Crystallinity by Dielectric Spectroscopy and Calorimetry. Macromol. Chem. Phys. 2016, 2017, 2056–2062. [Google Scholar] [CrossRef]
- Soccio, M.; Martinez-Tong, D.E.; Alegría, A.; Munari, A.; Lotti, N. Molecular dynamics of fully biobased poly(butylene 2,5-furanoate) as revealed by broadband dielectric spectroscopy. Polymer 2017, in press. [Google Scholar]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Tsanaktsis, V.; Bikiaris, D.N. Synthesis of the bio-based polyester poly(propylene 2,5-furan dicarboxylate). Comparison of thermal behavior and solid state structure with its terephthalate and naphthalate homologues. Polymer 2015, 62, 28–38. [Google Scholar] [CrossRef]
- Robertson, G.L. Food Packaging—Principle and Practice, 2nd ed.; Taylor & Francis Group, CRC Press: Boca Raton, FL, USA, 2013; Chapter 4; p. 107. [Google Scholar]
- Hu, Y.S.; Prattipati, V.; Mehta, S.; Schiraldi, D.A.; Hiltner, A.; Baer, E. Improving gas barrier of PET by blending with aromatic polyamides. Polymer 2005, 46, 2685–2698. [Google Scholar] [CrossRef]
- Siracusa, V.; Blanco, I.; Romano, S.; Tylewicz, U.; Rocculi, P.; Dalla Rosa, M. Poly(lactic acid)-Modified Films for Food Packaging Application: Physical, Mechanical, and Barrier Behavior. J. Appl. Polym. Sci. 2012, 125, 390–401. [Google Scholar] [CrossRef]
- Shmid, M.; Zillinger, W.; Muller, K.; Sangerlaub, S. Permeation of water vapour, nitrogen, oxygen and carbon dioxide through whey protein isolate based films and coatings—Permselectivity and activation energy. Food Packag. Shelf Life 2015, 6, 21–29. [Google Scholar] [CrossRef]
- Atkins, P.; Jones, L. Chemical Principles: The Quest for Insight, 5th ed.; Freeman WH & Co.: New York, NY, USA, 2012. [Google Scholar]
- Siracusa, V.; Ingrao, C. Correlation among gas barrier behaviour, temperature and thickness in BOPP films for food packaging usage: A lab-scale testing experience. Polym. Test. 2017, 59, 277–289. [Google Scholar] [CrossRef]
- Abenojar, J.; Pantoja, M.; Matìnez, M.A.; Del Real, J.C. Aging by mixture and/or temperature of epoxy/SiC composites: Thermal and mechanical properties. J. Comp. Mater. 2015, 49, 2963–2975. [Google Scholar] [CrossRef]
- Meiser, A.; Willstrand, K.; Possart, W. Influence of composition, humidity, and temperature on chemical aging in epoxies: A local study of the interphase with air. J. Adhes. 2010, 86, 222–243. [Google Scholar] [CrossRef]
- Lawton, J.W.; Doane, W.M.; Willett, J.L. Aging and Moisture Effects on the Tensile Properties of Starch/Poly(hydroxyester ether) Composites. J. Appl. Polym. Sci. 2006, 100, 3332–3339. [Google Scholar] [CrossRef]
- Tomé, L.C.; Gonçalves, C.M.B.; Boaventura, M.; Brandão, L.; Mendes, A.M.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A.; Freire, C.S.R.; Marrucho, I.M. Preparation and evaluation of the barrier properties of cellophane membranes modified with fatty acids. Carbohydr. Polym. 2011, 83, 836–842. [Google Scholar] [CrossRef]
- Siracusa, V.; Lotti, N.; Munari, A.; Dalla Rosa, M. Poly(butylene succinate) and poly(butylene succinate-co-adipate) for food packaging applications: Gas barrier properties after stressed treatments. Polym. Degrad. Stab. 2015, 119, 35–45. [Google Scholar] [CrossRef]
- European Union (EU) Regulations No. 10/2011 on Plastic Materials and Articles Intended to Come into Contact with Food. Available online: http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32011R0010 (accessed on 4 February 2011).
- Knoop, R.J.I.; Vogelzang, W.; van Haveren, J.; van Es, D.S. High molecular weight poly(ethylene-2,5-furanoate); critical aspects in synthesis and mechanical property determination. J. Polym. Sci. Polym. Chem. 2013, 51, 4191–4199. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, Q.; Zhang, Q.; Ye, C.; Zhou, G. A series of furan-aromatic polyesters synthesized via direct esterification method based on renewable resources. J. Polym. Sci. Polym. Chem. 2012, 50, 1026–1036. [Google Scholar] [CrossRef]
- Drelich, J. Guidelines to measurements of reproducible contact angle using a sessile-drop technique. Surf. Innov. 2013, 1, 1–5. [Google Scholar] [CrossRef]
- Siracusa, V. Food packaging permeability behaviour: A report. Int. J. Polym. Sci. 2012, 1, 1–11. [Google Scholar] [CrossRef]
- Gas Permeability Testing Manual, Registergericht Munchen HRB 77020; Brugger Feinmechanik GmbH: Munchen, Germany, 2008.

| PNF | PPF * | |
|---|---|---|
| MOLECULAR CHARACTERIZATION | ||
| Mn (g/mol) | 34,000 | 30,000 |
| D | 4.0 | 2.3 |
| WCA (°) | 112 ± 2 | 101 ± 3 |
| THERMAL CHARACTERIZATION | ||
| Thermogravimetric analysis | ||
| Tonset (°C) | 364 ± 1 | 360 ± 1 |
| Tmax (°C) | 395 ± 1 | 387 ± 1 |
| Differential scanning calorimetry | ||
| 1st scan | ||
| Tm (°C) | 197 ± 1 | 168 ± 1 |
| ΔHm (J/g) | 30 ± 3 | 7 ± 4 |
| Tg (°C) | 73 ± 1 | 50 ± 1 |
| ΔCp (J/g°C) | 0.350 ± 0.002 | 0.194 ± 0.003 |
| Tcc (°C) | 146 ± 1 | 137 ± 1 |
| ΔHcc (J/g) | 30 ± 3 | 7 ± 3 |
| 2st scan | ||
| Tm (°C) | 197 ± 1 | - |
| ΔHm (J/g) | 27 ± 4 | - |
| Tg (°C) | 70 ± 1 | 50 ± 1 |
| ΔCp (J/g°C) | 0.304 ± 0.001 | 0.19 ± 0.001 |
| Tcc (°C) | 158 ± 1 | - |
| ΔHcc (J/g) | 27 ± 3 | - |
| MECHANICAL CHARACTERIZATION | ||
| E (MPa) | 1648 ± 100 | 1363 ± 158 |
| σB (MPa) | 45 ± 5 | 31 ± 3 |
| εB (%) | 4 ± 1 | 3 ± 1 |
| T (°C) | N2-GTR (cm3/m2 d bar) | O2-GTR (cm3/m2 d bar) | CO2-GTR (cm3/m2 d bar) | CO2/O2 | CO2/N2 | EGTRN2 (KJ/mol K) | EGTRO2 (KJ/mol K) | EGTRCO2 (KJ/mol K) |
|---|---|---|---|---|---|---|---|---|
| 8 | 0.012 ± 1.2 × 10−4 | 0.018 ± 5.0 × 10−5 | 0.018 ± 4.3 × 10−5 | 0.99 | 1.51 | 27 (0.8) | 38 (1) | 29 (0.9) |
| 15 | 0.019 ± 2.3 × 10−4 | 0.021 ± 1.9 × 10−4 | 0.023 ± 3.2 × 10−4 | 1.10 | 1.20 | |||
| 23 | 0.016 ± 1.6 × 10−4 | 0.032 ± 9.4 × 10−5 | 0.022 ± 3.2 × 10−4 | 0.69 | 1.38 | |||
| 38 | 0.040 ± 3.2 × 10−4 | 0.083 ± 4.3 × 10−4 | 0.062 ± 3.2 × 10−4 | 0.75 | 1.54 |
| Sample | O2-GTR cm3cm m−2 day−1 atm−1 | CO2-GTR cm3cm m−2 day−1 atm−1 | BIF O2 | BIF CO2 | References |
|---|---|---|---|---|---|
| PNF 1 | 0.0323 | 0.0223 | 11 | 61 | This work |
| PPF 1 | 0.0224 | 0.0288 | 16 | 48 | [12] |
| PPF 2 | 0.0472 | n.a. | 8 | - | [11] |
| PEF 3 | 0.0702 | 0.1710 | 2 | 8 | [5,6] |
| PET 3 | 0.7480 | 3.2237 | 0.5 | 0.4 | [5,6] |
| PET 4 | n.a | 3.4868 | - | 0.4 | [5,6] |
| PET 5 | 0.3630 | 1.37 | 1 | 1 | [20] |
| PLA 6 | 1.3349 | 3.2854 | 0.3 | 0.4 | [21] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genovese, L.; Lotti, N.; Siracusa, V.; Munari, A. Poly(Neopentyl Glycol Furanoate): A Member of the Furan-Based Polyester Family with Smart Barrier Performances for Sustainable Food Packaging Applications. Materials 2017, 10, 1028. https://doi.org/10.3390/ma10091028
Genovese L, Lotti N, Siracusa V, Munari A. Poly(Neopentyl Glycol Furanoate): A Member of the Furan-Based Polyester Family with Smart Barrier Performances for Sustainable Food Packaging Applications. Materials. 2017; 10(9):1028. https://doi.org/10.3390/ma10091028
Chicago/Turabian StyleGenovese, Laura, Nadia Lotti, Valentina Siracusa, and Andrea Munari. 2017. "Poly(Neopentyl Glycol Furanoate): A Member of the Furan-Based Polyester Family with Smart Barrier Performances for Sustainable Food Packaging Applications" Materials 10, no. 9: 1028. https://doi.org/10.3390/ma10091028






