Nitrogen-Doped Carbon Nanoparticles for Oxygen Reduction Prepared via a Crushing Method Involving a High Shear Mixer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphological Characterization
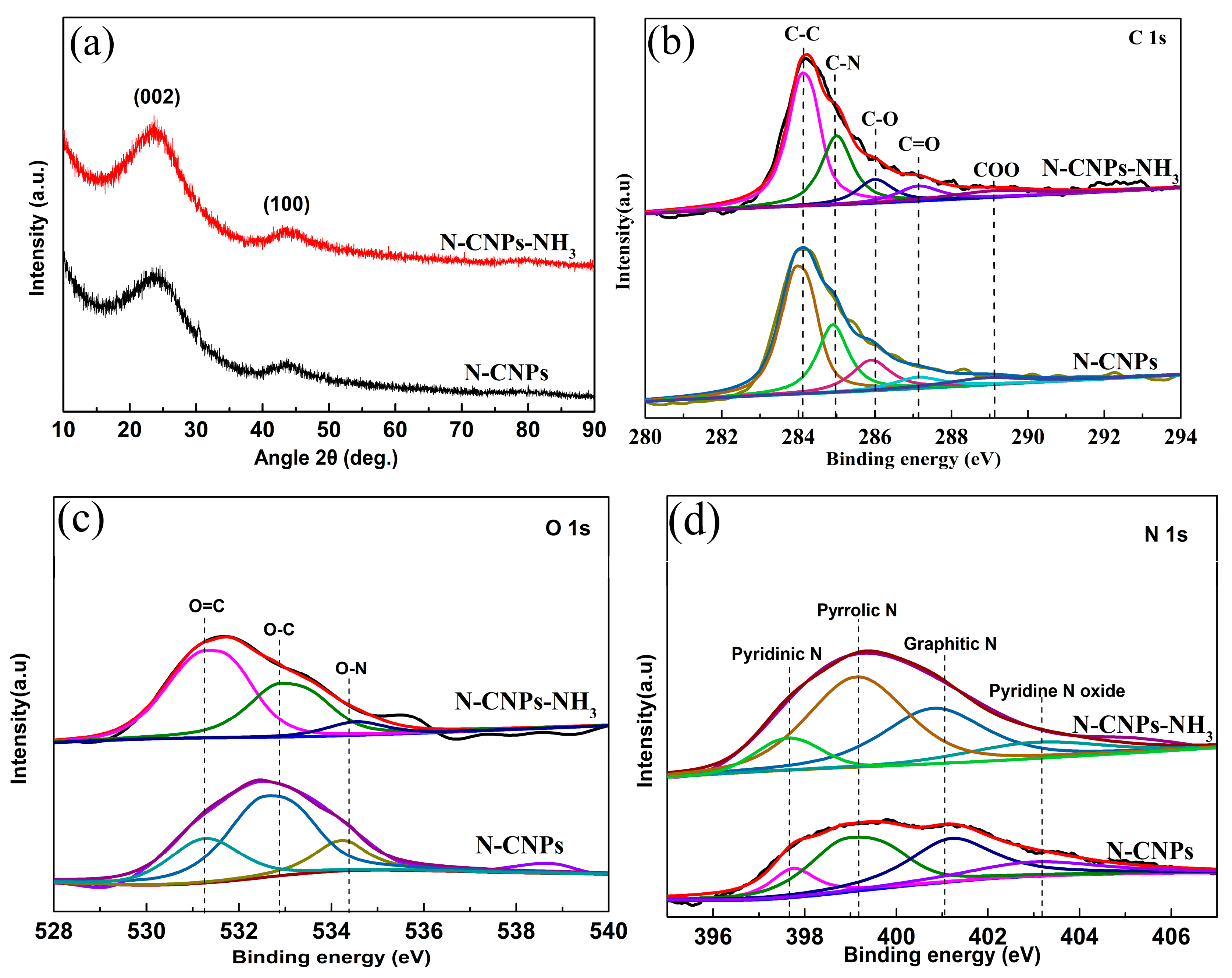
2.2. Structure and Composition Characterization
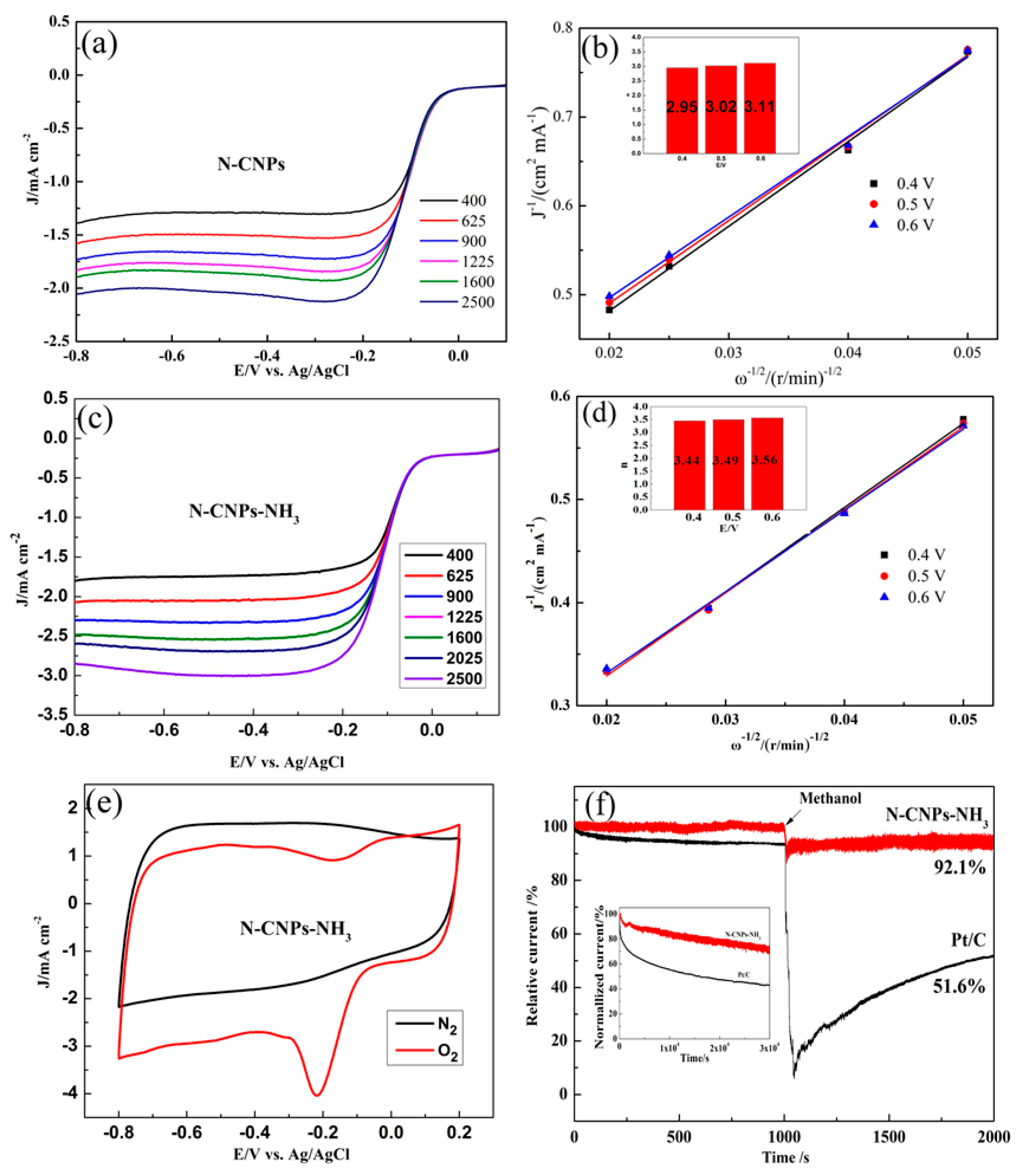
2.3. Electrochemical Consequences
3. Materials and Methods
3.1. Preparation of BP-Derived Carbon Materials
3.2. Physical Characterization of the Samples
3.3. Electrochemical Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, J.; Zhang, C.; Zhao, Y.; Amiinu, I.S.; Zhou, H.; Liu, X.; Tang, Y.; Mu, S. Three dimensional few-layer porous carbon nanosheets towards oxygen reduction. Appl. Catal. B Environ. 2017, 211, 148–156. [Google Scholar] [CrossRef]
- Castro, R.S.D.; Caetano, L.R.; Ferreira, G.; Padilha, P.M.; Saeki, M.J.; Zara, L.F.; Martines, M.A.U.; Castro, G.R. Banana Peel Applied to the Solid Phase Extraction of Copper and Lead from River Water: Preconcentration of Metal Ions with a Fruit Waste. Ind. Eng. Chem. Res. 2011, 50, 3446–3451. [Google Scholar] [CrossRef]
- Ma, M.; You, S.; Wang, W.; Liu, G.; Qi, D.; Chen, X.; Qu, J.; Ren, N. Biomass-Derived Porous Fe3C/Tungsten Carbide/Graphitic Carbon Nanocomposite for Efficient Electrocatalysis of Oxygen Reduction. ACS Appl. Mater. Interfaces 2016, 8, 32307–32316. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Li, X.; Li, L.; Wei, X. 4-A versatile biomass derived carbon material for oxygen reduction reaction, supercapacitors and oil/water separation. Nano Energy 2017, 33, 334–342. [Google Scholar] [CrossRef]
- Borghei, M.; Laocharoen, N.; Kibena-Põldsepp, E.; Johansson, L.-S.; Campbell, J.; Kauppinen, E.; Tammeveski, K.; Rojas, O.J. 5-Porous N,P-doped carbon from coconut shells with high electrocatalytic activity for oxygen reduction: Alternative to Pt-C for alkaline fuel cells. Appl. Catal. B Environ. 2017, 204, 394–402. [Google Scholar] [CrossRef]
- Guo, Z.; Xiao, Z.; Ren, G.; Xiao, G.; Zhu, Y.; Dai, L.; Jiang, L. 6-Natural tea-leaf-derived, ternary-doped 3D porous carbon as a high-performance electrocatalyst for the oxygen reduction reaction. Nano Res. 2016, 9, 1244–1255. [Google Scholar] [CrossRef]
- Song, S.; Ma, F.; Wu, G.; Ma, D.; Geng, W.; Wan, J. 7-Facile self-templating large scale preparation of biomass-derived 3D hierarchical porous carbon for advanced supercapacitors. J. Mater. Chem. A 2015, 3, 18154–18162. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.; Mei, C.; Xu, J.; Zhou, S.; Wong, C.-P. 8-Evaluating biomass-derived hierarchically porous carbon as the positive electrode material for hybrid Na-ion capacitors. J. Power Sources 2017, 342, 48–55. [Google Scholar] [CrossRef]
- Liu, L.; Yang, X.; Ma, N.; Liu, H.; Xia, Y.; Chen, C.; Yang, D.; Yao, X. Scalable and Cost-Effective Synthesis of Highly Efficient Fe2N-Based Oxygen Reduction Catalyst Derived from Seaweed Biomass. Small 2016, 12, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Wang, S.; Li, L.; Dou, S. Bio-Nanotechnology in High-Performance Supercapacitors. Adv. Energy Mater. 2017, 1700592. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Yuan, Q.; Tang, H.; Yu, F.; Lv, X. A green adsorbent derived from banana peel for highly effective removal of heavy metal ions from water. RSC Adv. 2016, 6, 45041–45048. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, L.; Qi, P.; Zhu, M.; Wang, G.; Ma, Y.; Guo, X.; Chen, H.; Zhang, B.; Zhao, Z.; et al. Nitrogen-Doped Banana Peel-Derived Porous Carbon Foam as Binder-Free Electrode for Supercapacitors. Nanomaterials 2016, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, M.; Li, Y.; Zhang, M.; Xue, X.; Shi, Y.; Dai, B.; Guo, X.; Yu, F. Heteroatom-doped porous carbon from methyl orange dye wastewater for oxygen reduction. Green Energy Environ. 2017. [Google Scholar] [CrossRef]
- Karunagaran, R.; Tung, T.; Shearer, C.; Tran, D.; Coghlan, C.; Doonan, C.; Losic, D. A Unique 3D Nitrogen-Doped Carbon Composite as High-Performance Oxygen Reduction Catalyst. Materials 2017, 10, 921. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Li, Y.; Huo, J.; Chen, R.; Dai, L.; Wang, S. Defect Chemistry of Nonprecious-Metal Electrocatalysts for Oxygen Reactions. Adv. Mater. 2017, 1606459. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zhang, Q. Nanocarbon for Oxygen Reduction Electrocatalysis: Dopants, Edges, and Defects. Adv. Mater. 2017, 29, 1604103. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, L.; Hu, R.; Liao, W.; Li, Z.; Li, Y.; Guo, C. Surface Modification of Multi-Walled Carbon Nanotubes via Hemoglobin-Derived Iron and Nitrogen-Rich Carbon Nanolayers for the Electrocatalysis of Oxygen Reduction. Materials 2017, 10, 564. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, J.; Ye, D.; Zhu, X.; Liao, Q. Bamboo charcoal as a cost-effective catalyst for an air-cathode of microbial fuel cells. Electrochim. Acta 2017, 224, 585–592. [Google Scholar] [CrossRef]
- Wan, K.; Tan, A.-D.; Yu, Z.-P.; Liang, Z.-X.; Piao, J.-H.; Tsiakaras, P. 2D nitrogen-doped hierarchically porous carbon: Key role of low dimensional structure in favoring electrocatalysis and mass transfer for oxygen reduction reaction. Appl. Catal. B Environ. 2017, 209, 447–454. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, L.; Shi, G.; Liu, J.; Chen, J.; Dai, L. N,P-Codoped Carbon Networks as Efficient Metal-free Bifunctional Catalysts for Oxygen Reduction and Hydrogen Evolution Reactions. Angew. Chem. Int. Ed. 2016, 55, 2230–2234. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Piao, J.; Liang, Z. Synthesis of 2D Nitrogen-Doped Mesoporous Carbon Catalyst for Oxygen Reduction Reaction. Materials 2017, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yin, J.; Wang, X.; Wang, H.; Yang, X. Microorganism-Derived Heteroatom-Doped Carbon Materials for Oxygen Reduction and Supercapacitors. Adv. Funct. Mater. 2013, 23, 1305–1312. [Google Scholar] [CrossRef]
- Yuan, W.; Feng, Y.; Xie, A.; Zhang, X.; Huang, F.; Li, S.; Zhang, X.; Shen, Y. Nitrogen-doped nanoporous carbon derived from waste pomelo peel as a metal-free electrocatalyst for the oxygen reduction reaction. Nanoscale 2016, 8, 8704–8711. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shi, Y.; Li, M.; Wang, Z.; Wu, S.; Lyu, F.; Shang, C.; Lu, Z. Ultrafine N-doped carbon nanoparticles with controllable size to enhance electrocatalytic activity for oxygen reduction reaction. RSC Adv. 2016, 6, 110758–110764. [Google Scholar] [CrossRef]
- Li, Q.; Xu, D.; Ou, X.; Yan, F. Nitrogen-Doped Graphitic Porous Carbon Nanosheets Derived from In Situ Formed g-C3N4 Templates for the Oxygen Reduction Reaction. Chem. Asian J. 2017, 12, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiang, J.; Mu, C.; Wen, F.; Yuan, S.; Zhao, J.; Xu, D.; Su, C.; Liu, Z. SnS2 Nanoflakes Anchored Graphene obtained by Liquid Phase Exfoliation and MoS2 Nanosheet Composites as Lithium and Sodium Battery Anodes. Electrochim. Acta 2017, 227, 203–209. [Google Scholar] [CrossRef]
- Fu, S.; Zhu, C.; Song, J.; Du, D.; Lin, Y. Metal-Organic Framework-Derived Non-Precious Metal Nanocatalysts for Oxygen Reduction Reaction. Adv. Energy Mater. 2017, 1700363. [Google Scholar] [CrossRef]
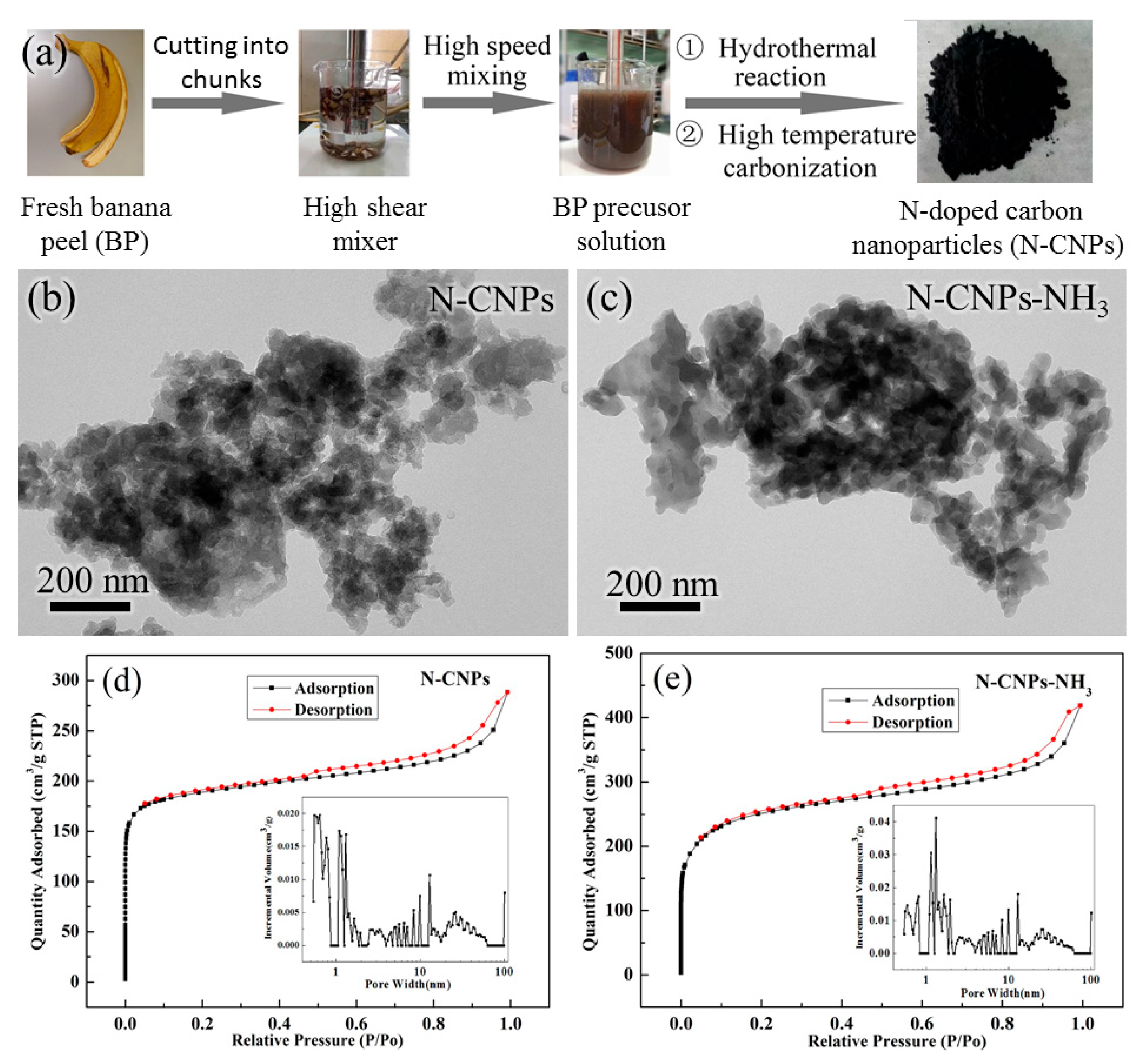
| Sample | SBET (m2/g) | DBJH (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| N-CNPs | 734.8 | 2.4 | 0.23 |
| N-CNPs-NH3 | 941.2 | 2.7 | 0.45 |
| Sample | Content (%) | Content of N Species (%) | |||||
|---|---|---|---|---|---|---|---|
| C (%) | N (%) | O (%) | Pyridinic | Pyrrolic | Graphitic | Oxidized | |
| N-CNPs | 89.65 | 1.02 | 9.33 | 0.11 | 0.40 | 0.41 | 0.08 |
| N-CNPs-NH3 | 88.89 | 2.43 | 8.68 | 0.23 | 1.14 | 0.79 | 0.27 |
| Catalysts | Electrolyte | Onset Potential (V) | Main Precursors Materials | Ref. |
|---|---|---|---|---|
| N-doped carbon | 0.1 M KOH | −0.05 | Bacillus subtilis | [22] |
| N-doped nanoporous carbon | 0.1 M KOH | 0.01 | Pomelo peel | [23] |
| N-doped carbon nanoparticles | 0.1 M KOH | −0.02 | Sterculia scaphigera | [24] |
| Oxygen-containing N-doped carbon | 0.1 M KOH | −0.04 | Glucose and dicyandiamide | [25] |
| N-CNPs-NH3 | 0.1 M KOH | −0.033 | Fresh banana peel | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Wu, T.; Wang, Y.; Zhang, J.; Wang, G.; Zhang, J.; Dai, B.; Yu, F. Nitrogen-Doped Carbon Nanoparticles for Oxygen Reduction Prepared via a Crushing Method Involving a High Shear Mixer. Materials 2017, 10, 1030. https://doi.org/10.3390/ma10091030
Shi L, Wu T, Wang Y, Zhang J, Wang G, Zhang J, Dai B, Yu F. Nitrogen-Doped Carbon Nanoparticles for Oxygen Reduction Prepared via a Crushing Method Involving a High Shear Mixer. Materials. 2017; 10(9):1030. https://doi.org/10.3390/ma10091030
Chicago/Turabian StyleShi, Lei, Tao Wu, Yiqing Wang, Jie Zhang, Gang Wang, Jinli Zhang, Bin Dai, and Feng Yu. 2017. "Nitrogen-Doped Carbon Nanoparticles for Oxygen Reduction Prepared via a Crushing Method Involving a High Shear Mixer" Materials 10, no. 9: 1030. https://doi.org/10.3390/ma10091030