Evaluation of the Healing Potential of Demineralized Dentin Matrix Fixed with Recombinant Human Bone Morphogenetic Protein-2 in Bone Grafts
Abstract
:1. Introduction
2. Materials and Methods
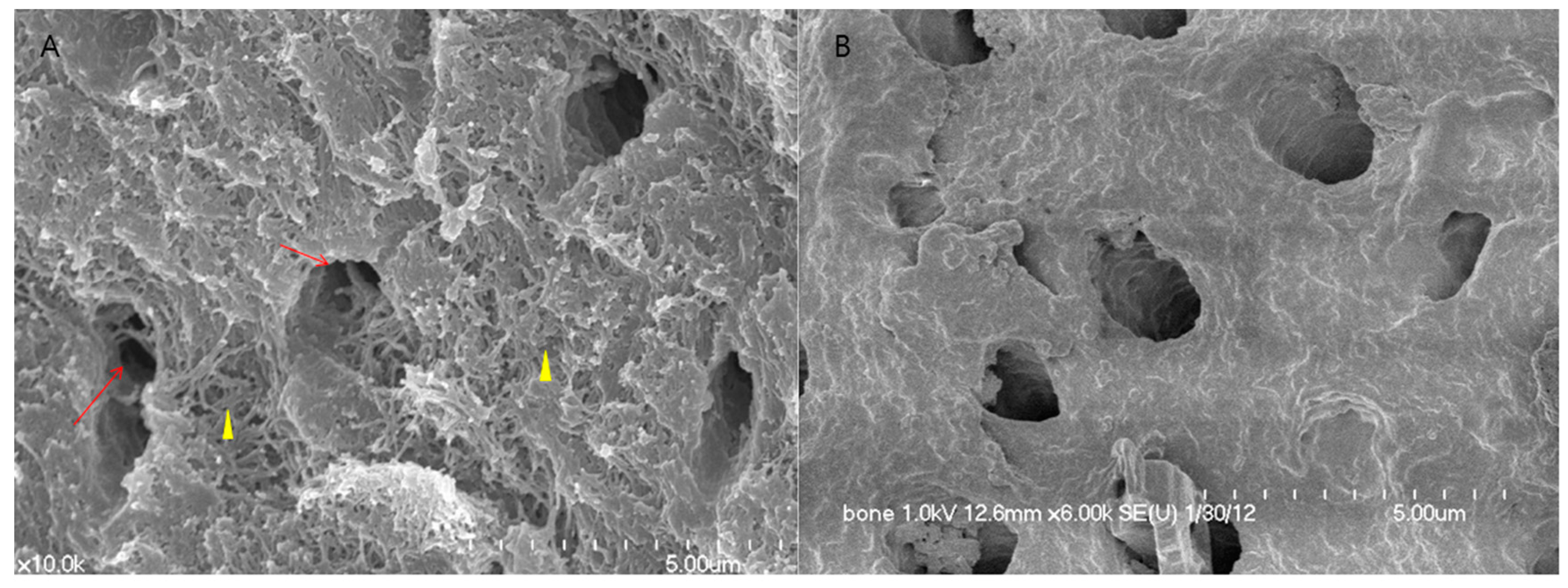
2.1. Human DDM Fixed with rhBMP-2 Fabrication (Dip-Dry Method)
2.2. Animal Experimental Study: Assessment of Bone Recovery after Placement of Human DDM Fixed with rhBMP-2 and Autogenous Bone Grafts in Beagles
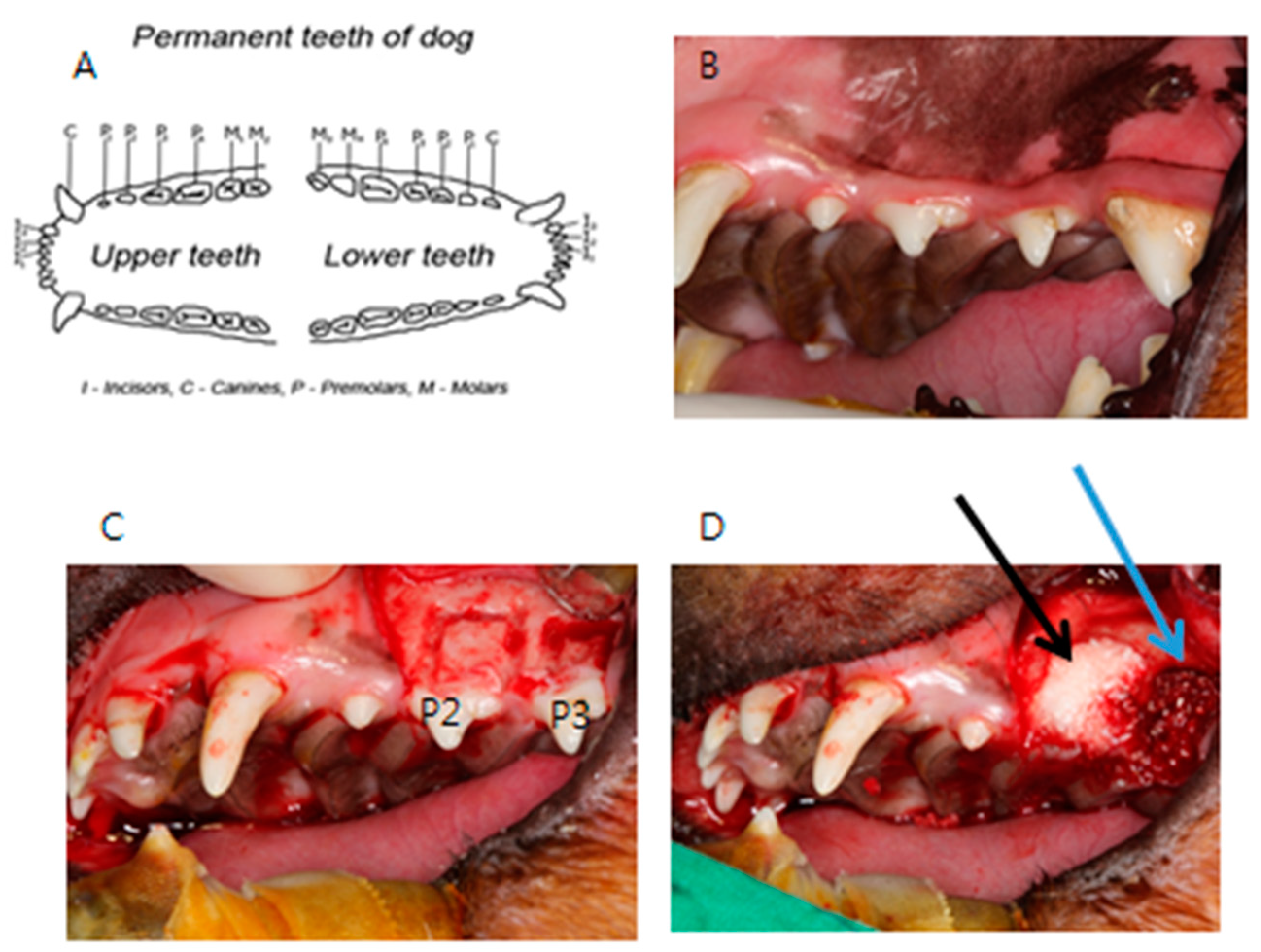
2.3. Human Clinical Study and a Case Report: Clinical Study of a Variety of Bone Graft Using ADDM Fixed with rhBMP-2
- Patients requiring a bone graft at the site of implant placement;
- Patients whose teeth could be extracted and used to make ADDM fixed with rhBMP-2 for use as bone graft material;
- Patients who consented to the clinical study;
- Patients who were healthy or had controlled systemic disease; and
- Non-smokers.
3. Results
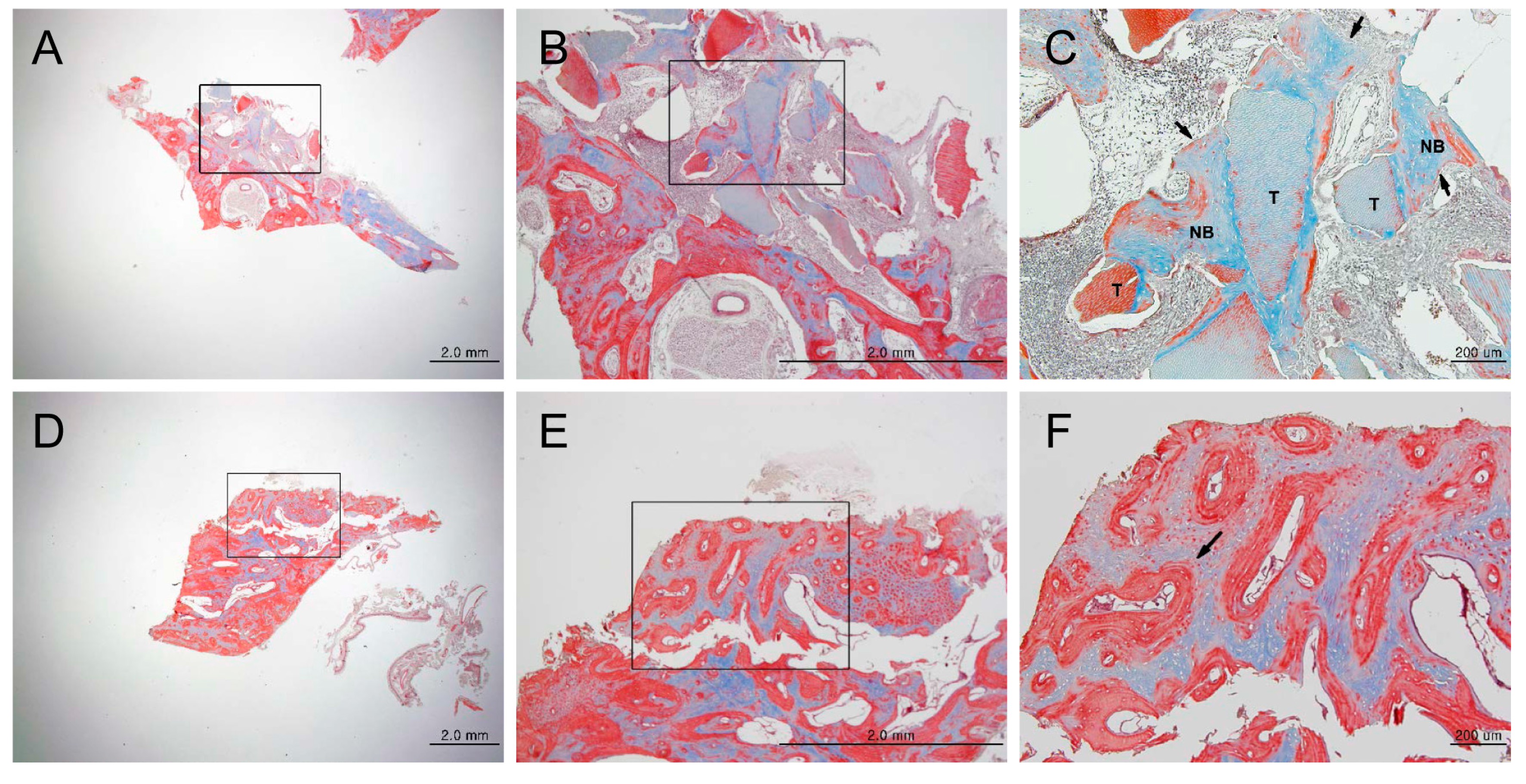
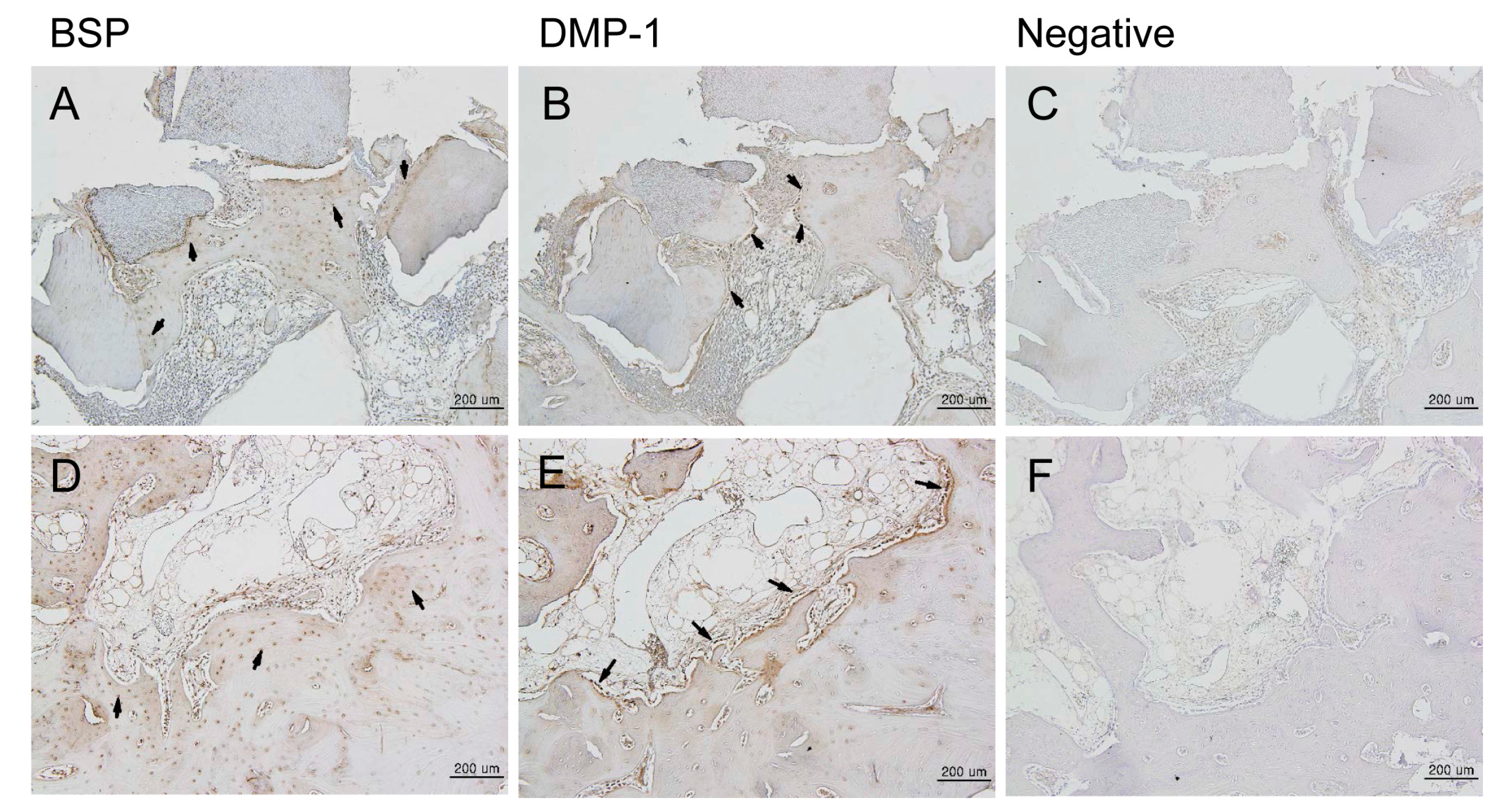
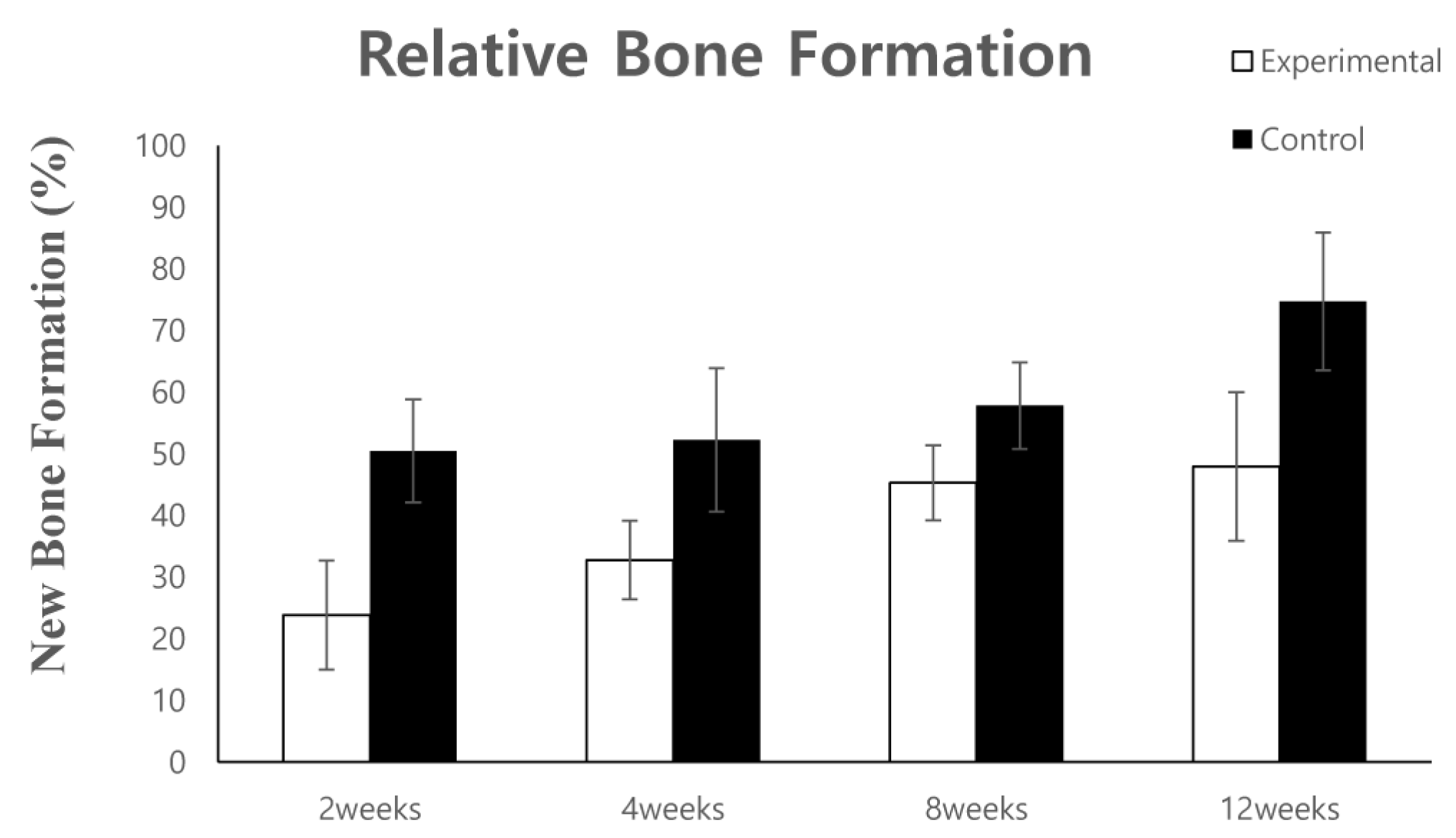
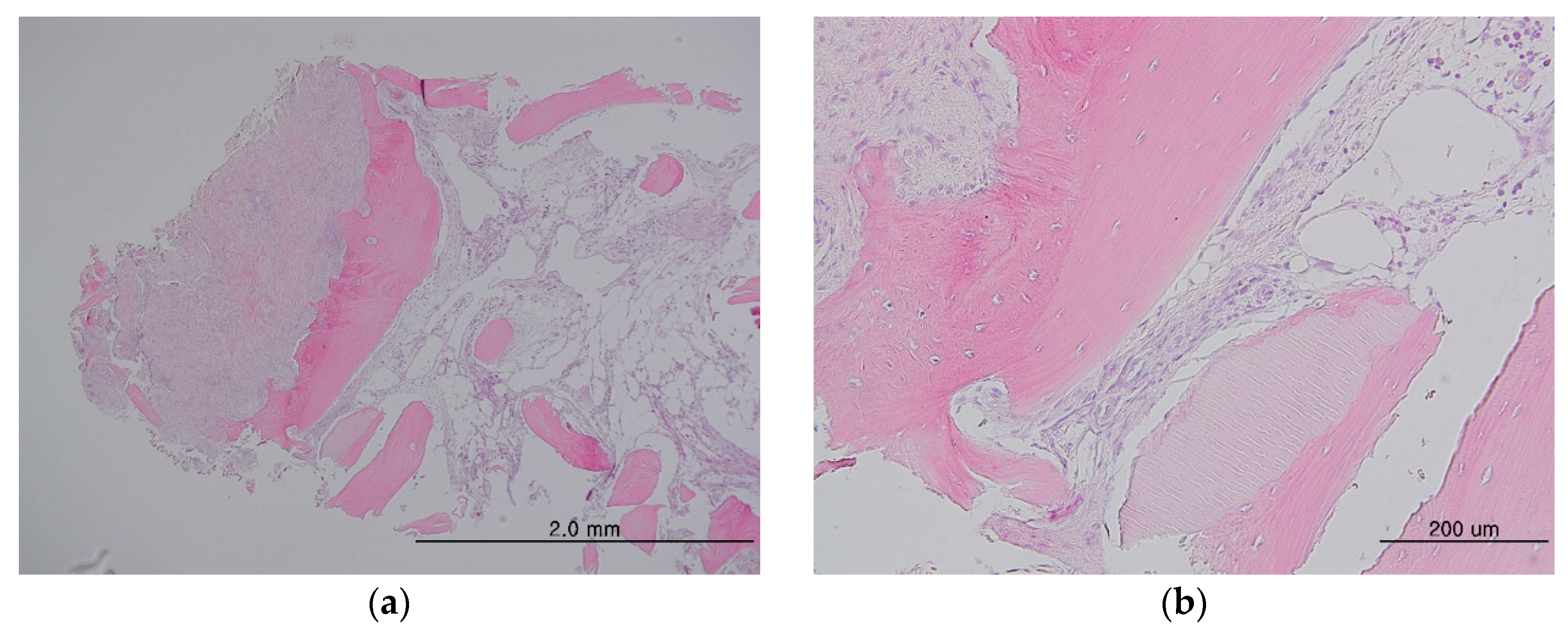
3.1. Animal Experimental Study
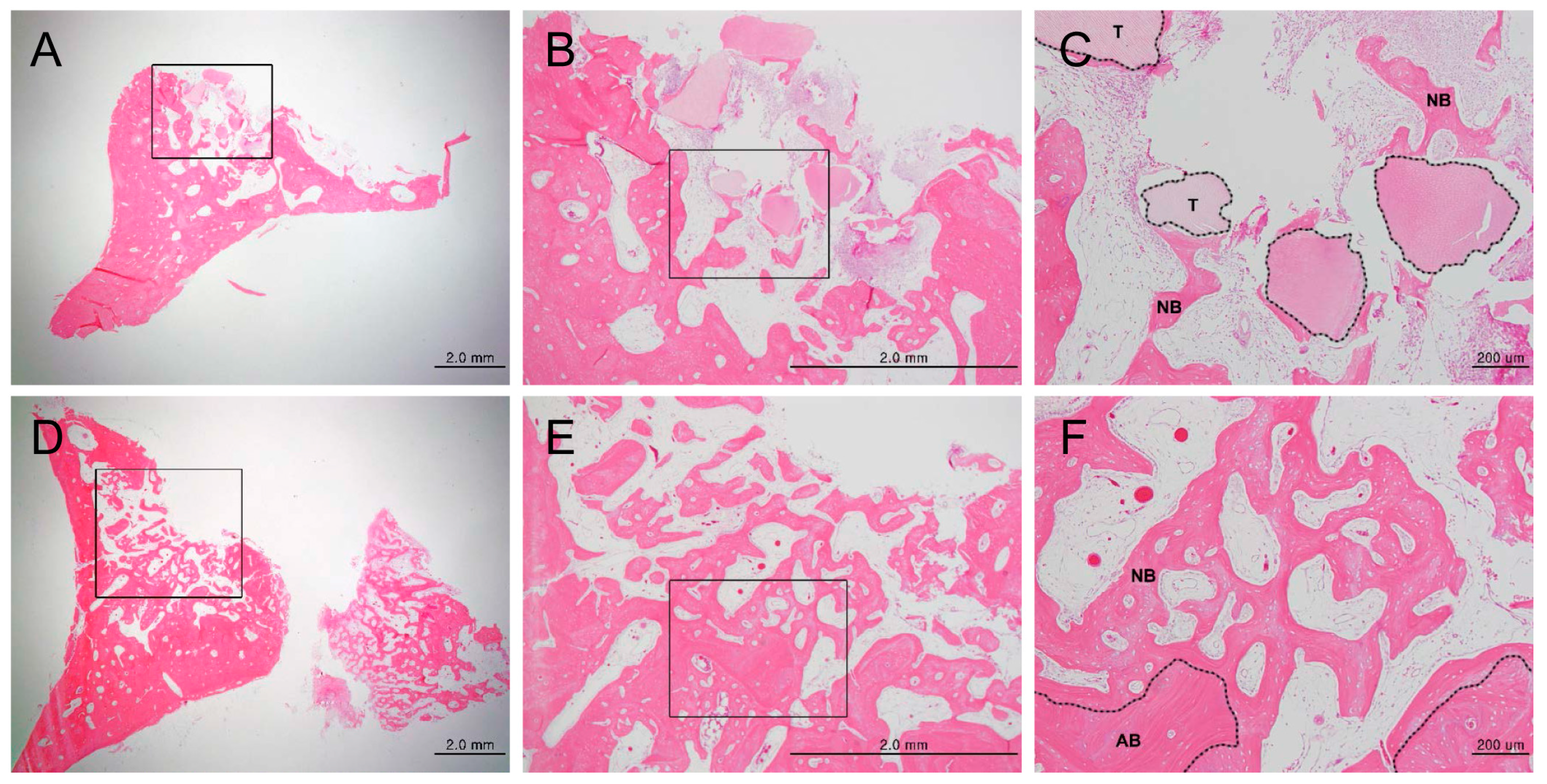
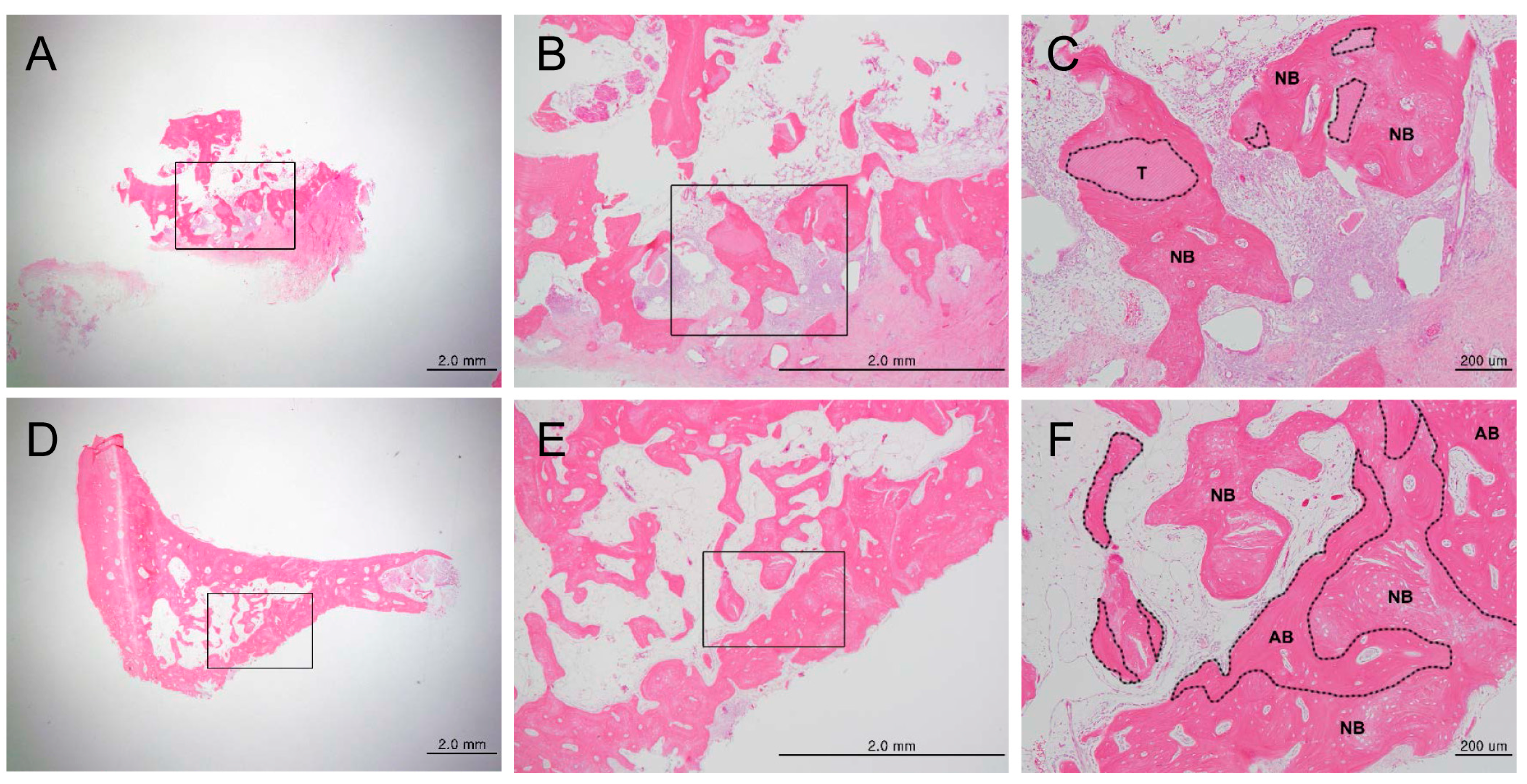
3.1.1. Histologic Findings
3.1.2. Histomorphometric Analysis
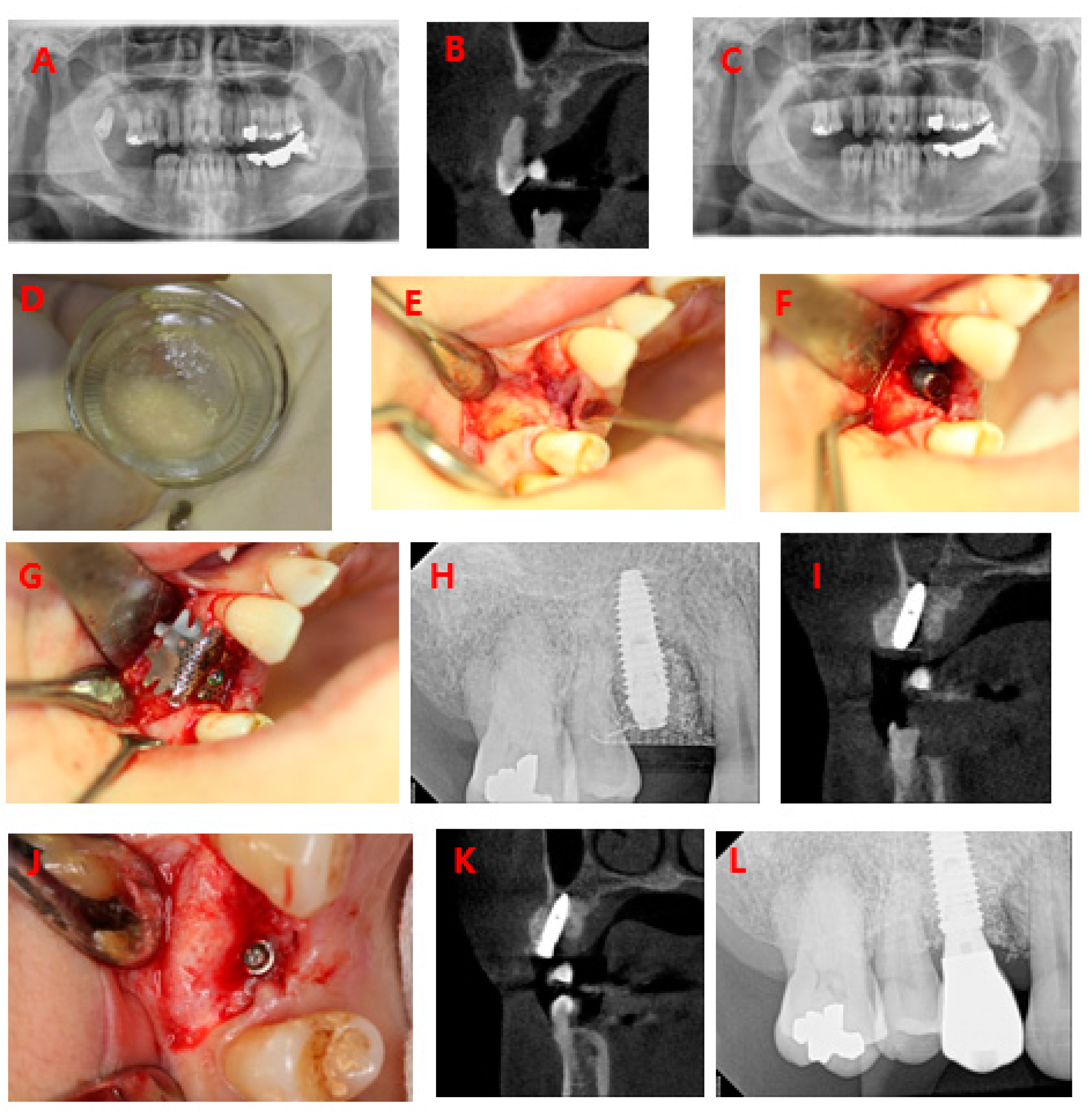
3.2. Human Clinical Study and Case Report
3.2.1. Clinical Study
3.2.2. Case Report
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, Y.K. Bone graft material using teeth. J. Korean Assoc. Oral Maxillofac. Surg. 2012, 38, 134–138. [Google Scholar] [CrossRef]
- Lee, S.H. Low Crystalline hydroxyl carbonate apatite. J. Korean Dent. Assoc. 2006, 44, 524–533. [Google Scholar]
- Bessho, K.; Tanaka, N.; Matsumoto, J.; Tagawa, T.; Murata, M. Human dentin-matrix-derived bone morphogenetic protein. J. Dent. Res. 1991, 70, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Kim, H.K.; Lim, S.C. Combined implantation of particulate dentine, plaster of Paris, and a bone xenograft (Bio-Oss) for bone regeneration in rats. J. Craniomaxillofac. Surg. 2001, 29, 282–288. [Google Scholar]
- Kim, Y.K.; Kim, S.G.; Byeon, J.H.; Lee, H.J.; Um, I.U.; Lim, S.C.; Kim, S.Y. Development of a novel bone grafting material using autogenous teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, Y.K.; Yi, Y.J.; Choi, J.H. Clinical evaluation of ridge augmentation using autogenous tooth bone graft material: Case series study. J. Korean Assoc. Oral Maxillofac. Surg. 2013, 39, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Yun, P.Y.; Um, I.W.; Lee, H.J.; Yi, Y.J.; Bae, J.H.; Lee, J. Alveolar ridge preservation of an extraction socket using autogenous tooth bone graft material for implant site development: Prospective case series. J. Adv. Prosthodont. 2014, 6, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.H.; Ahn, J.S.; Lee, J.I.; Ahn, K.J.; Yun, P.Y.; Kim, Y.K. A prospective study on the effectiveness of newly developed autogenous tooth bone graft material for sinus bone graft procedure. J. Adv. Prosthodont. 2014, 6, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.T.; Han, I.H.; Huh, J.B.; Kang, J.K.; Ryu, J.J. Review of the developmental trend of implant surface modification using organic biomaterials. J. Korean Acad. Prosthodont. 2011, 49, 254–262. [Google Scholar] [CrossRef]
- Cheng, H.; Jiang, W.; Phillips, F.M.; Haydon, R.C.; Peng, Y.; Zhou, L.; Luu, H.H.; An, N.; Breyer, B.; Vanichakarn, P.; et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J. Bone Jt. Surg. Am. 2003, 85, 1544–1552. [Google Scholar] [CrossRef]
- Boyne, P.J.; Marx, R.E.; Nevins, M.; Triplett, G.; Lazaro, E.; Lilly, L.C.; Alder, M.; Nummikoski, P. A feasibility study evaluating rhBMP-2/absorbable collagen sponge for maxillary sinus floor augmentation. Int. J. Periodont. Restor. Dent. 1997, 17, 11–25. [Google Scholar]
- Hanisch, O.; Tatakis, D.N.; Boskovic, M.M.; Rohrer, M.D.; Wikesjö, U.M. Bone formation and reosseointegration in peri-implantitis defects following surgical implantation of rhBMP-2. Int. J. Oral Maxillofac. Implants 1997, 12, 604–610. [Google Scholar] [PubMed]
- Howell, T.H.; Fiorellini, J.; Jones, A.; Alder, M.; Nummikoski, P.; Lazaro, M.; Lilly, L.; Cochran, D. A feasibility study evaluating rhBMP-2/absorbable collagen sponge device for local alveolar ridge preservation or augmentation. Int. J. Periodont. Restor. Dent. 1997, 17, 124–139. [Google Scholar]
- Sigurdsson, T.J.; Nygaard, L.; Tatakis, D.N.; Fu, E.; Turek, T.J.; Jin, L.; Wozney, J.M.; Wikesjö, U.M. Periodontal repair in dogs: Evaluation of rhBMP-2 carriers. Int. J. Periodont. Restor. Dent. 1996, 16, 524–537. [Google Scholar]
- Ahn, K.J.; Park, J.C.; Kim, Y.K. Experimental study on healing procedure after combined grafting of recombinant human bone morphogenetic protein-2 and anorganic bovine bone. Oral Biol. Res. 2014, 38, 83–91. [Google Scholar]
- Kim, Y.K.; Um, I.W.; An, H.J.; Kim, K.W.; Hong, K.S.; Murata, M. Effects of demineralized dentin matrix used as an rhBMP-2 carrier for bone regeneration. J. Hard Tissue Biol. 2014, 23, 415–422. [Google Scholar] [CrossRef]
- Nam, J.H.; Park, J.C.; Yu, S.B.; Chung, Y.I.; Tae, G.Y.; Kim, J.J. Bone regeneration with MMP sensitive hyaluronic acid-based hydrogel, rhBMP-2 and nanoparticles in rat calvarial critical size defect (CSD) model. J. Korean Assoc. Oral Maxillofac. Surg. 2009, 35, 137–145. [Google Scholar]
- Kim, S.J.; Kim, M.R.; Oh, J.S.; Han, I.; Shin, S.W. Effects of polycaprolactone-tricalcium phosphate, recombinant human bone morphogenetic protein-2 and dog mesenchymal stem cells on bone formation: Pilot study in dogs. Yonsei Med. J. 2009, 50, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, C.S.; Choi, K.H.; Jung, U.W.; Yun, J.H.; Choi, S.H. The induction of bone formation in rat calvarial defects and subcutaneous tissues by recombinant human BMP-2, produced in Escherichia coli. Biomaterials 2010, 31, 3512–3519. [Google Scholar] [CrossRef] [PubMed]
- Barr, T.; McNamara, A.J.; Sándor, G.K.; Clokie, C.M.; Peel, S.A. Comparison of the osteoinductivity of bioimplants containing recombinant human bone morphogenetic proteins 2 (Infuse) and 7 (OP-1). Oral Surg. Oral Med. Oral Pathol. Oral Radiol.Endodontol. 2010, 109, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, E.; Thompson, E.M.; Matsiko, A.; O’Brien, F.J.; López-Noriega, A. Long-term controlled delivery of rhBMP-2 from collagen-hydroxyapatite scaffolds for superior bone tissue regeneration. J Control. Release 2015, 207, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Ike, K.; Urist, M.R. Recycled dentin root matrix for a carrier of recombinant human bone morphogenetic protein. J. Oral Implantol. 1998, 24, 124–132. [Google Scholar] [CrossRef]
- Murata, M. Bone engineering using human demineralized dentin matrix and recombinant human BMP-2. J. Hard Tissue Biol. 2005, 14, 80–81. [Google Scholar] [CrossRef]
- Murata, M.; Sato, D.; Hino, J.; Akazawa, T.; Tazaki, J.; Ito, K.; Arisue, M. Acid-insoluble human dentin as carrier material for recombinant human BMP-2. J. Biomed. Mater. Res. A 2012, 100, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Akazawa, T.; Hino, J.; Tazaki, J.; Ito, K.; Arisue, M. Biochemical and histo-morphometrical analyses of bone and cartilage induced by human decalcified dentin matrix and BMP-2. Oral Biol. Res. 2011, 35, 9–14. [Google Scholar] [CrossRef]
- Burg, K.J.; Porter, S.; Kellam, J.F. Biomaterial developments for bone tissue engineering. Biomaterials 2000, 21, 2347–2359. [Google Scholar] [CrossRef]
- Kirker-Head, C.A. Potential applications and delivery strategies for bone morphogenetic proteins. Adv. Drug Deliv. Rev. 2000, 43, 65–92. [Google Scholar] [CrossRef]
- Li, R.H.; Wozney, J.M. Delivering on the promise of bone morphogenetic proteins. Trends Biotechnol. 2001, 19, 255–265. [Google Scholar] [CrossRef]
- Li, J.; Yang, J.; Zhong, X.; He, F.; Wu, X.; Shen, G. Demineralized dentin matrix composite collagen material for bone tissue regeneration. J Biomater. Sci. Polym. Ed. 2013, 24, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Samandari, M.H.; Haghighat, A.; Torabinia, N.; Taghian, M.; Sadri, L.; Naemy, V. Socket preservation using freeze-dried bone allograft with and without plasma rich in growth factors in dogs. Dent. Res. J. 2016, 13, 432–439. [Google Scholar]
| F/U Period (Months) | Primary Stability (ISQ) | Secondary Stability (ISQ) | Marginal Bone Loss (mm) | |
|---|---|---|---|---|
| Minimum | 3.1 | 49 | 45 | 0 |
| Maximum | 17.8 | 85 | 87 | 0.8 |
| Average | 10.5 | 71.8 | 78.0 | 0.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-Y.; Kim, Y.-K.; Park, Y.-H.; Park, J.-C.; Ku, J.-K.; Um, I.-W.; Kim, J.-Y. Evaluation of the Healing Potential of Demineralized Dentin Matrix Fixed with Recombinant Human Bone Morphogenetic Protein-2 in Bone Grafts. Materials 2017, 10, 1049. https://doi.org/10.3390/ma10091049
Kim S-Y, Kim Y-K, Park Y-H, Park J-C, Ku J-K, Um I-W, Kim J-Y. Evaluation of the Healing Potential of Demineralized Dentin Matrix Fixed with Recombinant Human Bone Morphogenetic Protein-2 in Bone Grafts. Materials. 2017; 10(9):1049. https://doi.org/10.3390/ma10091049
Chicago/Turabian StyleKim, Sang-Yun, Young-Kyun Kim, Yeoung-Hyun Park, Joo-Cheol Park, Jeong-Kui Ku, In-Woong Um, and Ji-Yun Kim. 2017. "Evaluation of the Healing Potential of Demineralized Dentin Matrix Fixed with Recombinant Human Bone Morphogenetic Protein-2 in Bone Grafts" Materials 10, no. 9: 1049. https://doi.org/10.3390/ma10091049





