Improvement of Early Strength of Cement Mortar Containing Granulated Blast Furnace Slag Using Industrial Byproducts
Abstract
:1. Introduction
2. Experimental
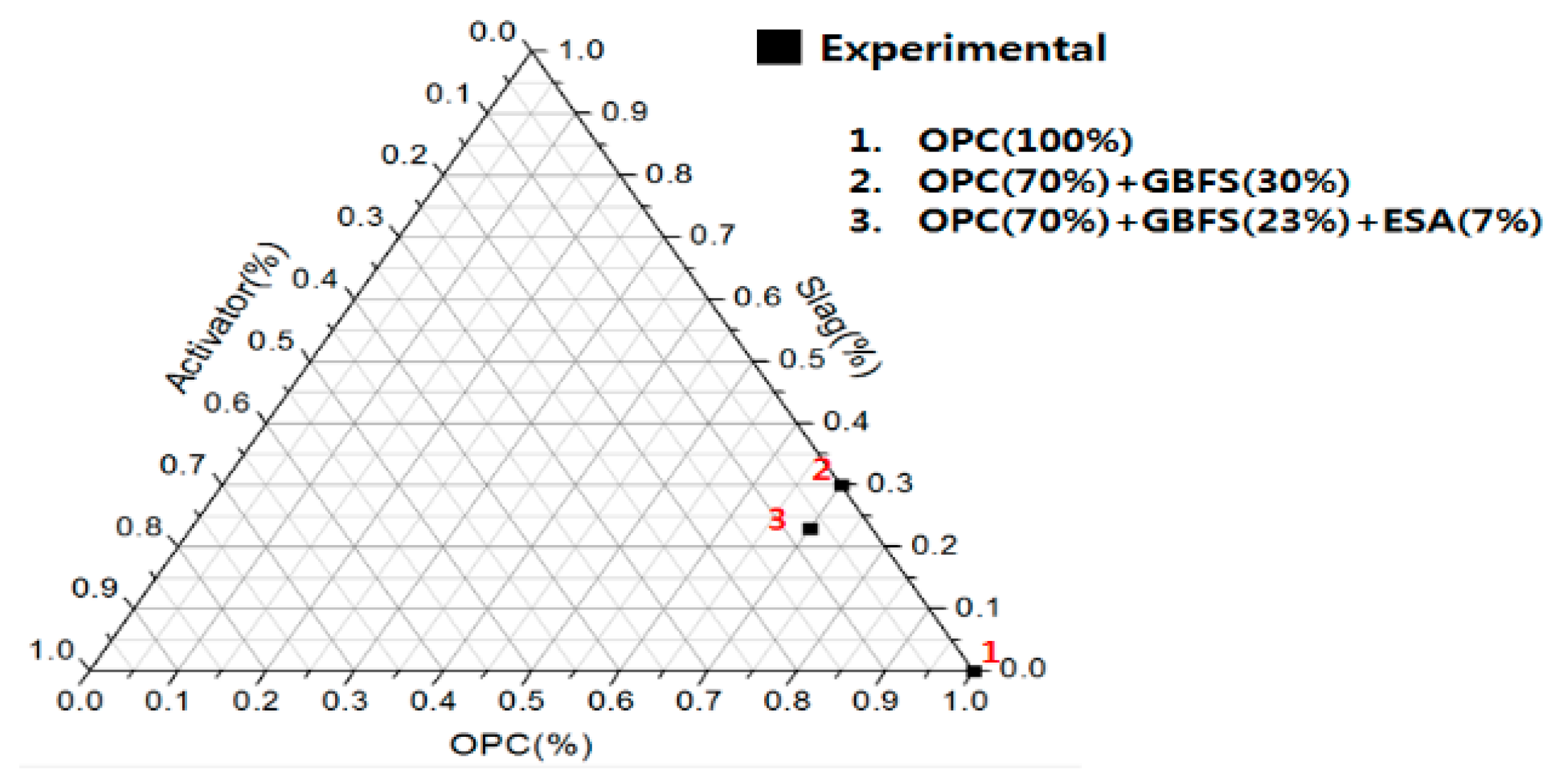
2.1. Experimental Plan and Mortar Mix
2.2. Materials for Experiment (Raw Materials)
2.3. Method of Experiment and Items for Measurement
3. Result of Experiment and Discussion
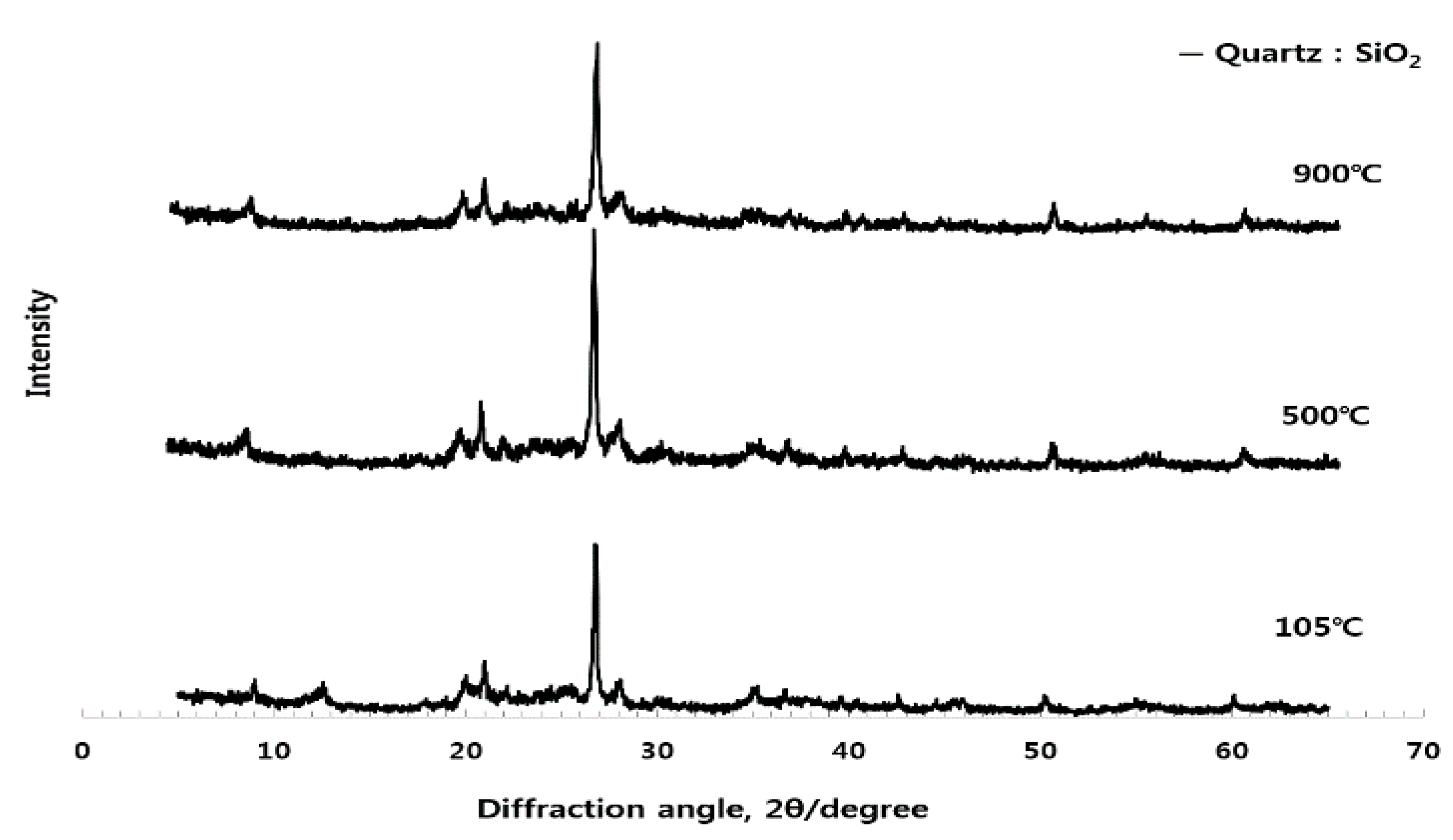
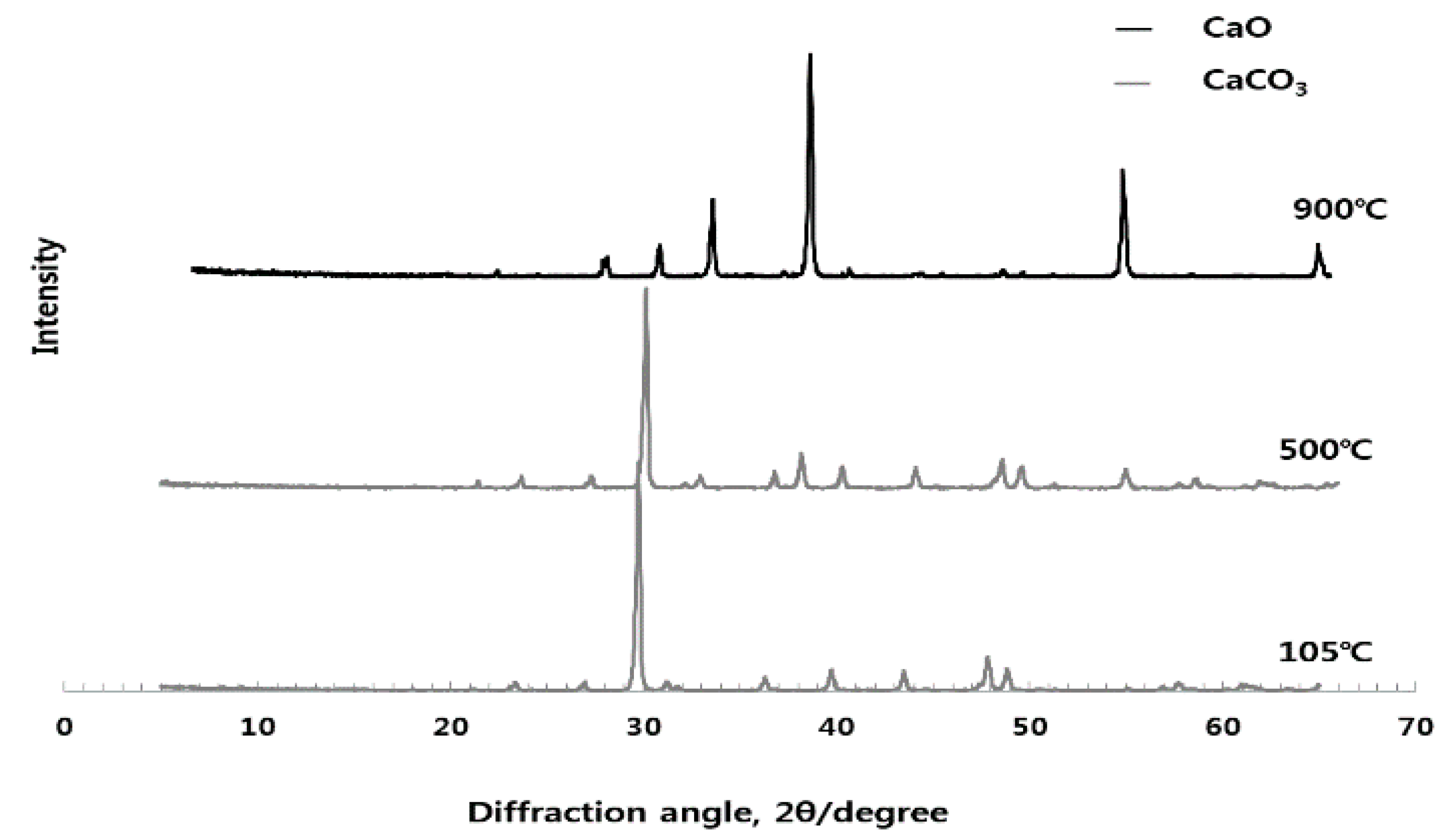
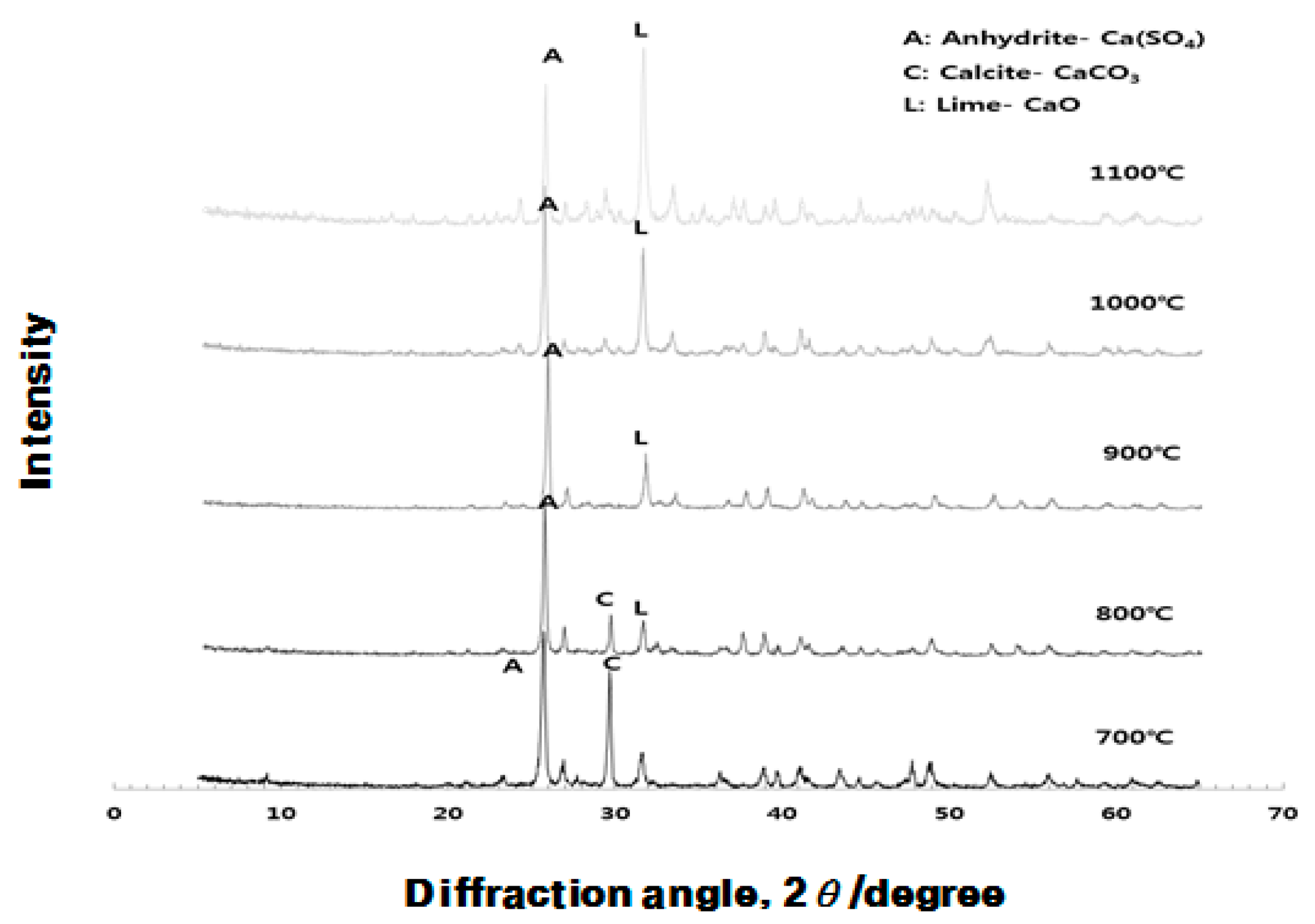
3.1. Analysis of Crystal Structure for Different Burning Temperature of Raw Material (ESA) (XRD)
3.2. Mortar Experiment
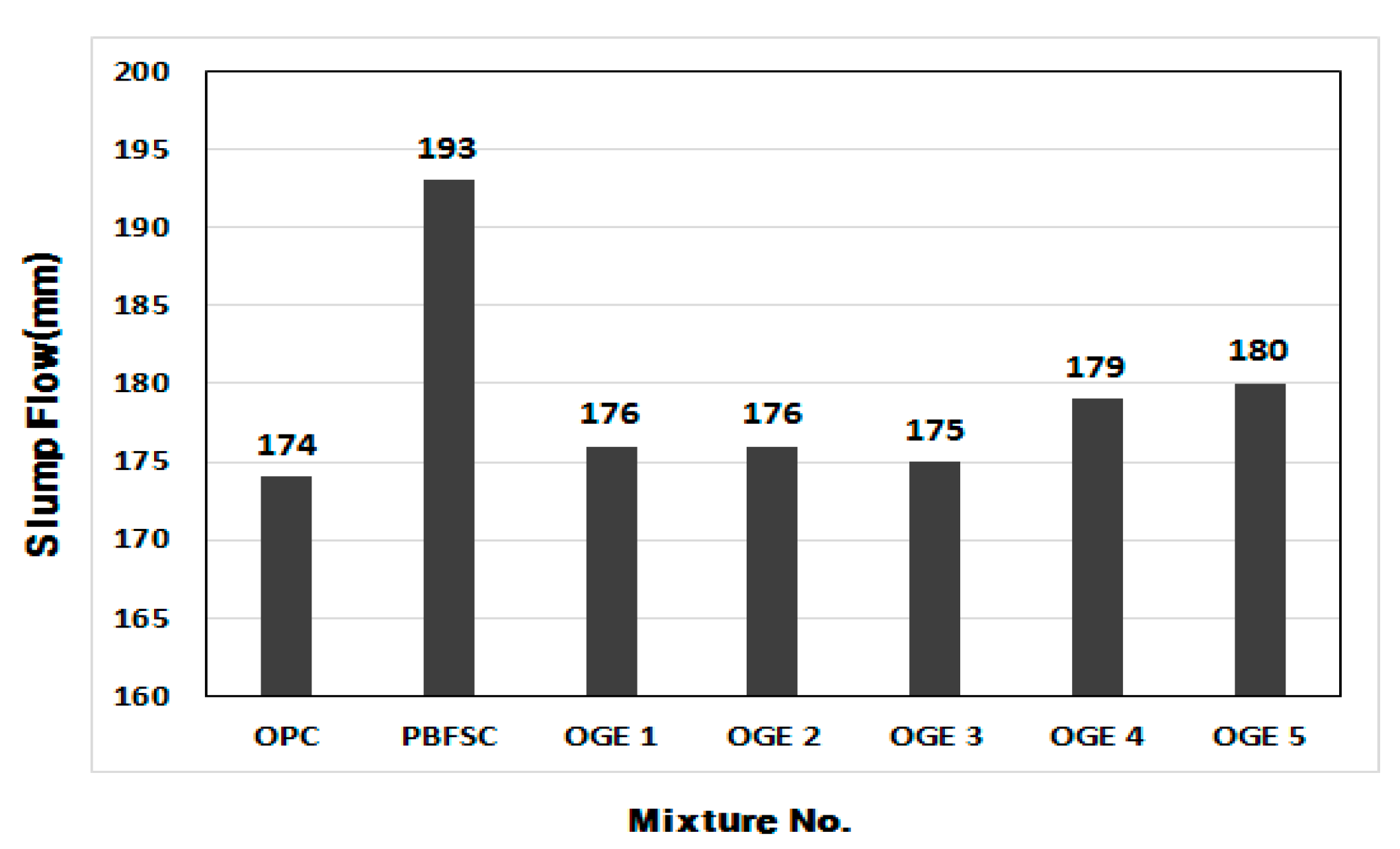
3.2.1. Flow of Mortar
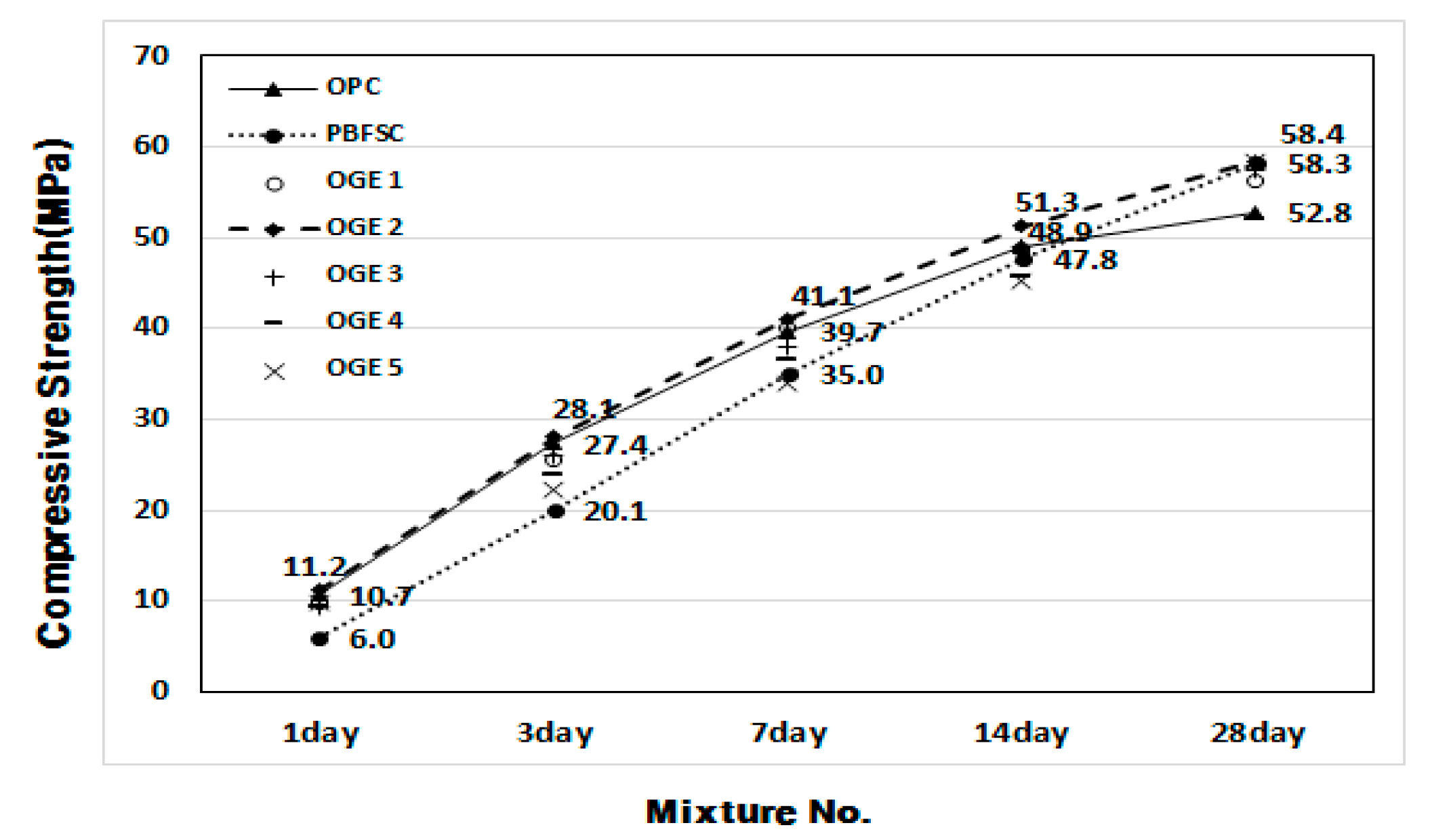
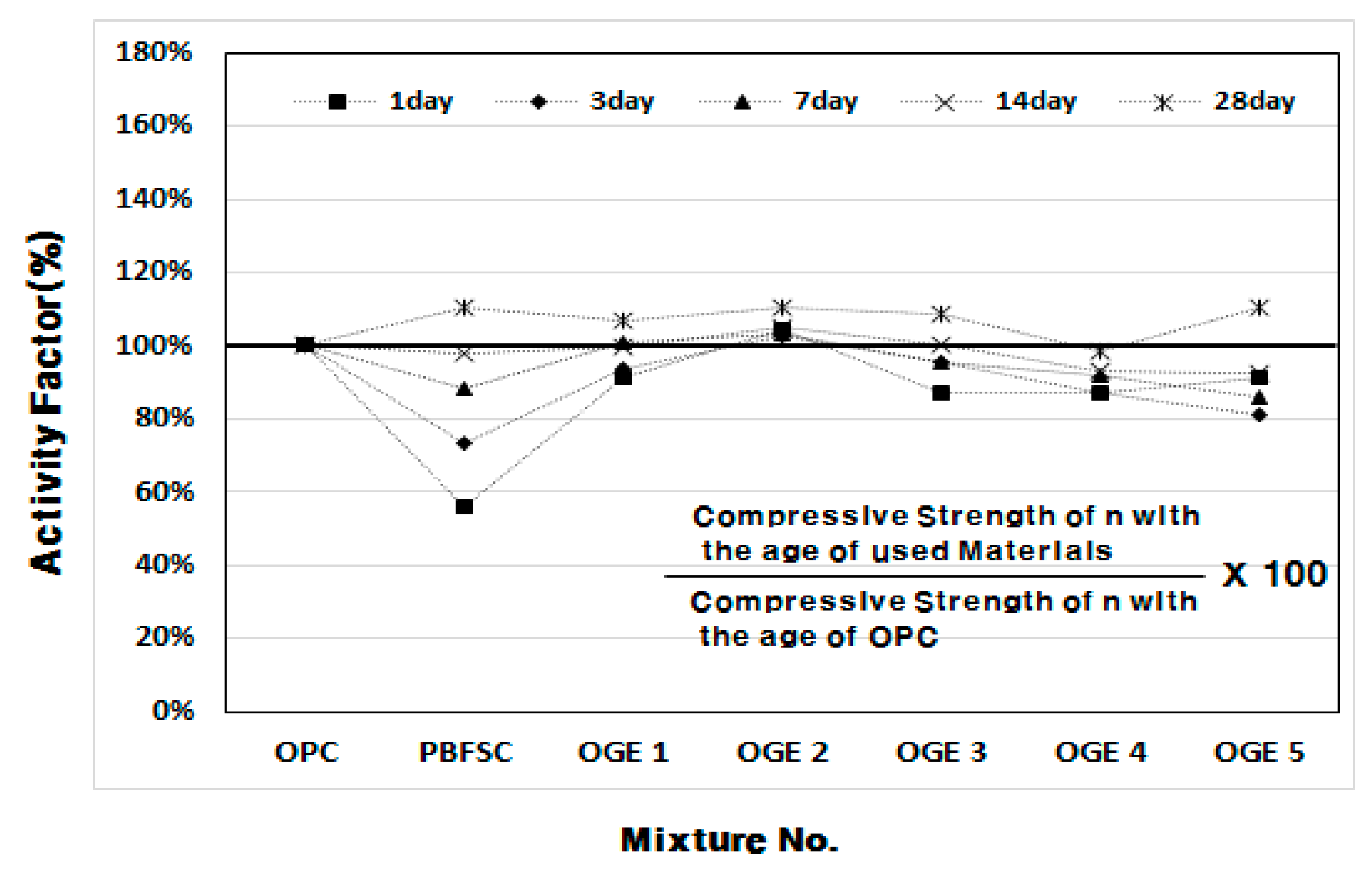
3.2.2. Compressive Strength (MPa) and Activity Factor (%) of OGE at Different ESA Burning Temperatures
3.3. Cement Paste Experiment
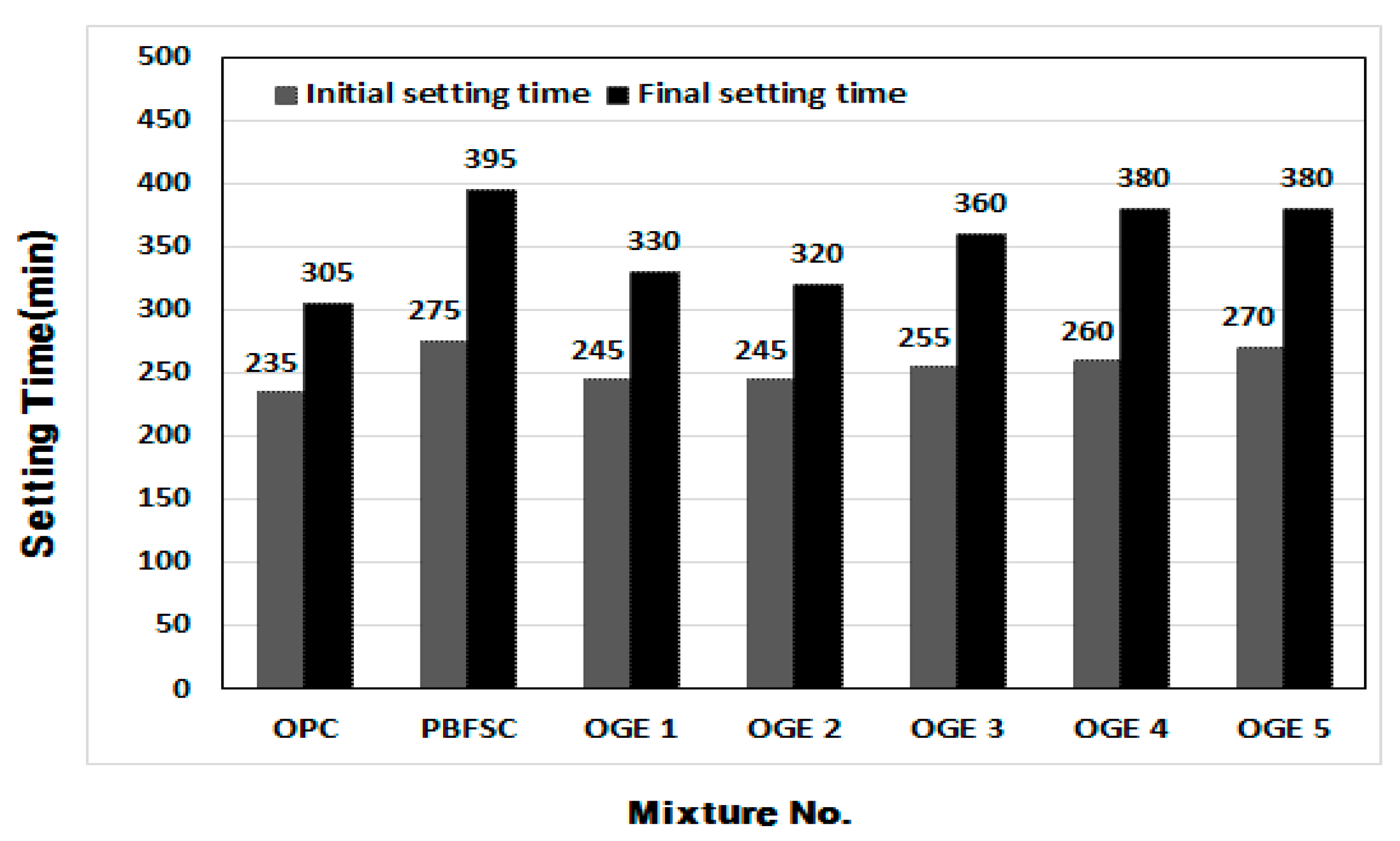
3.3.1. Setting Time of Cement Paste
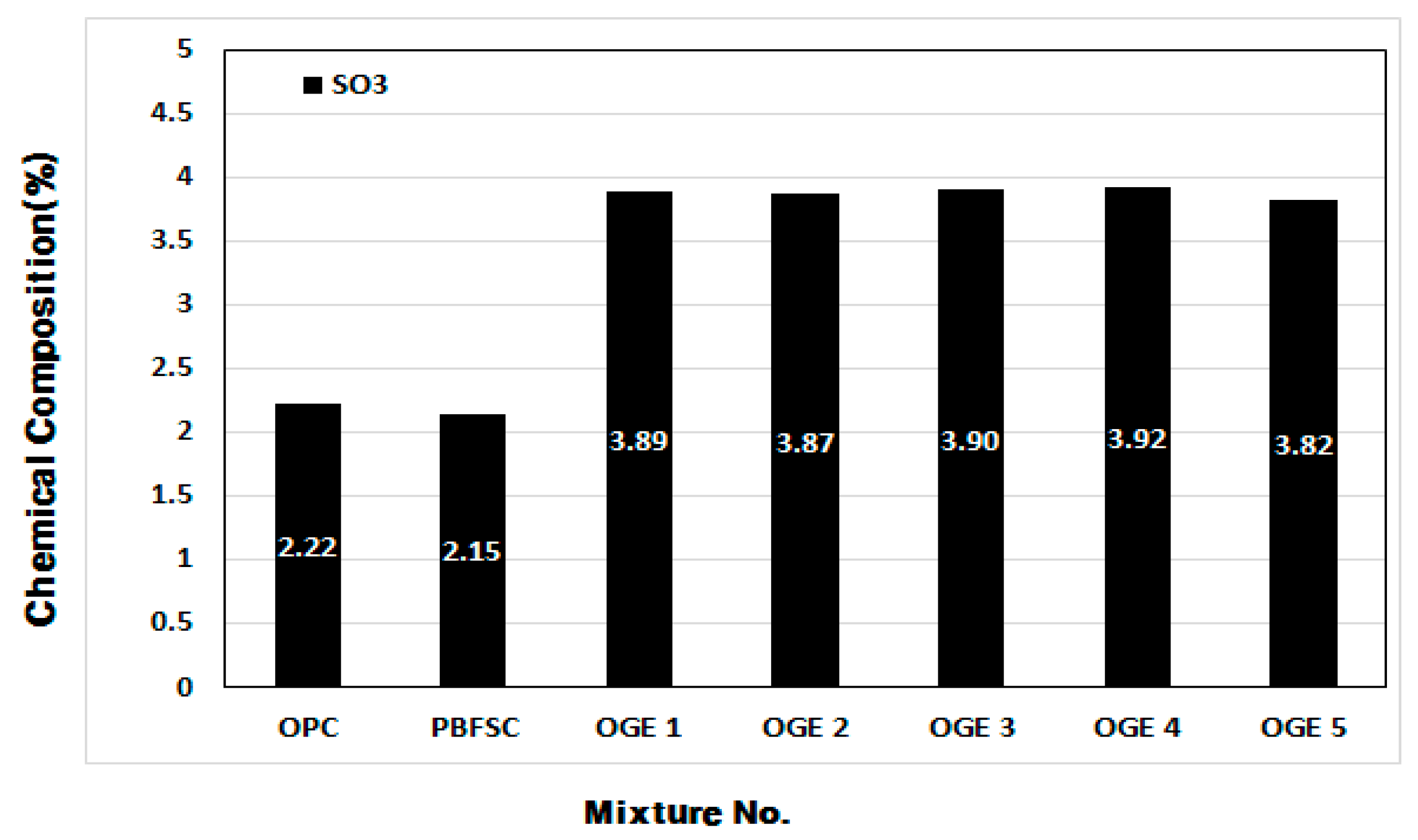
3.3.2. Change in Chemical Composition of ESA and OGE according to Burning Temperature
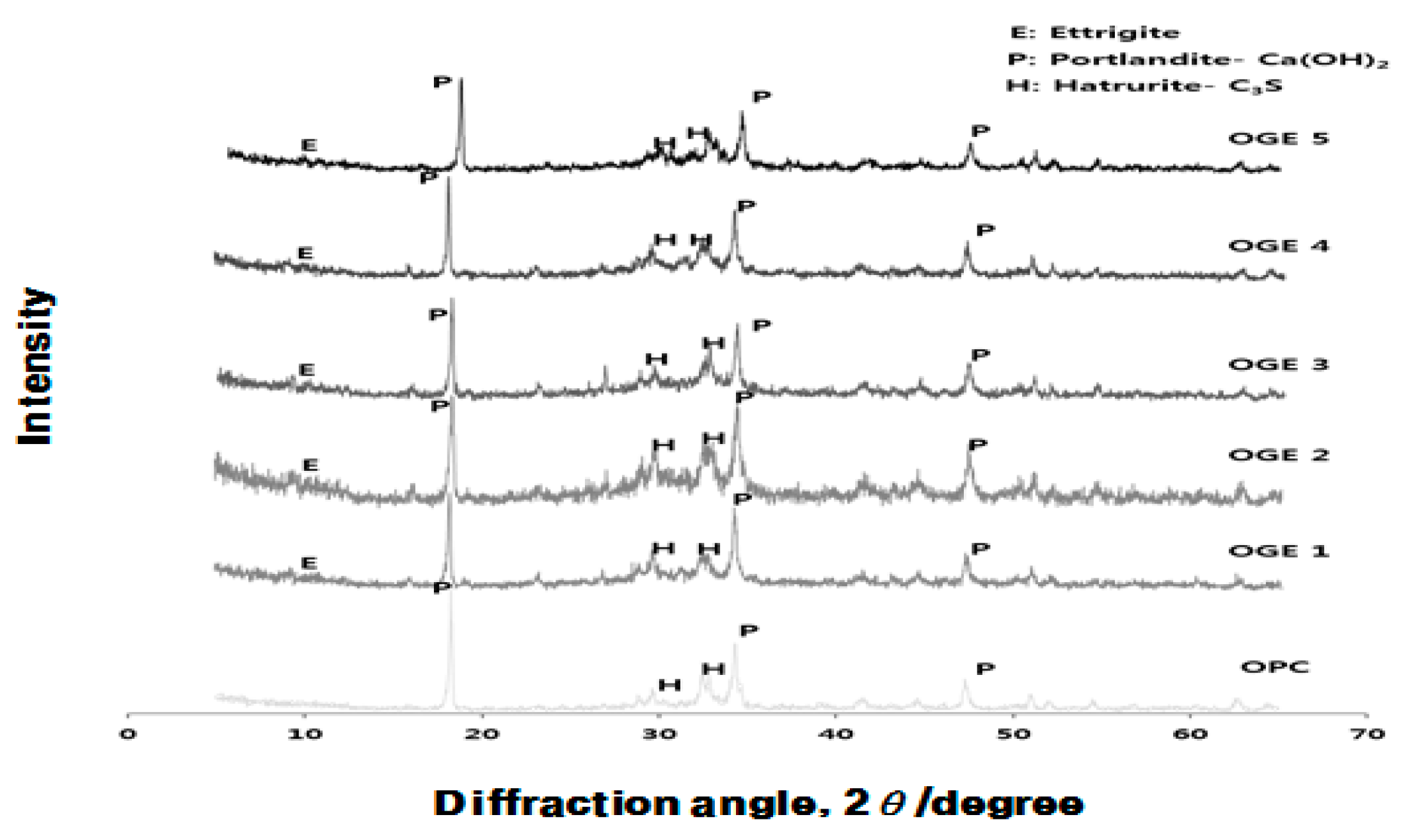
3.3.3. Analysis of Crystal Structure (XRD) according to Use of ESA
3.3.4. Stability Test (Free-lime Quantitative Analysis)
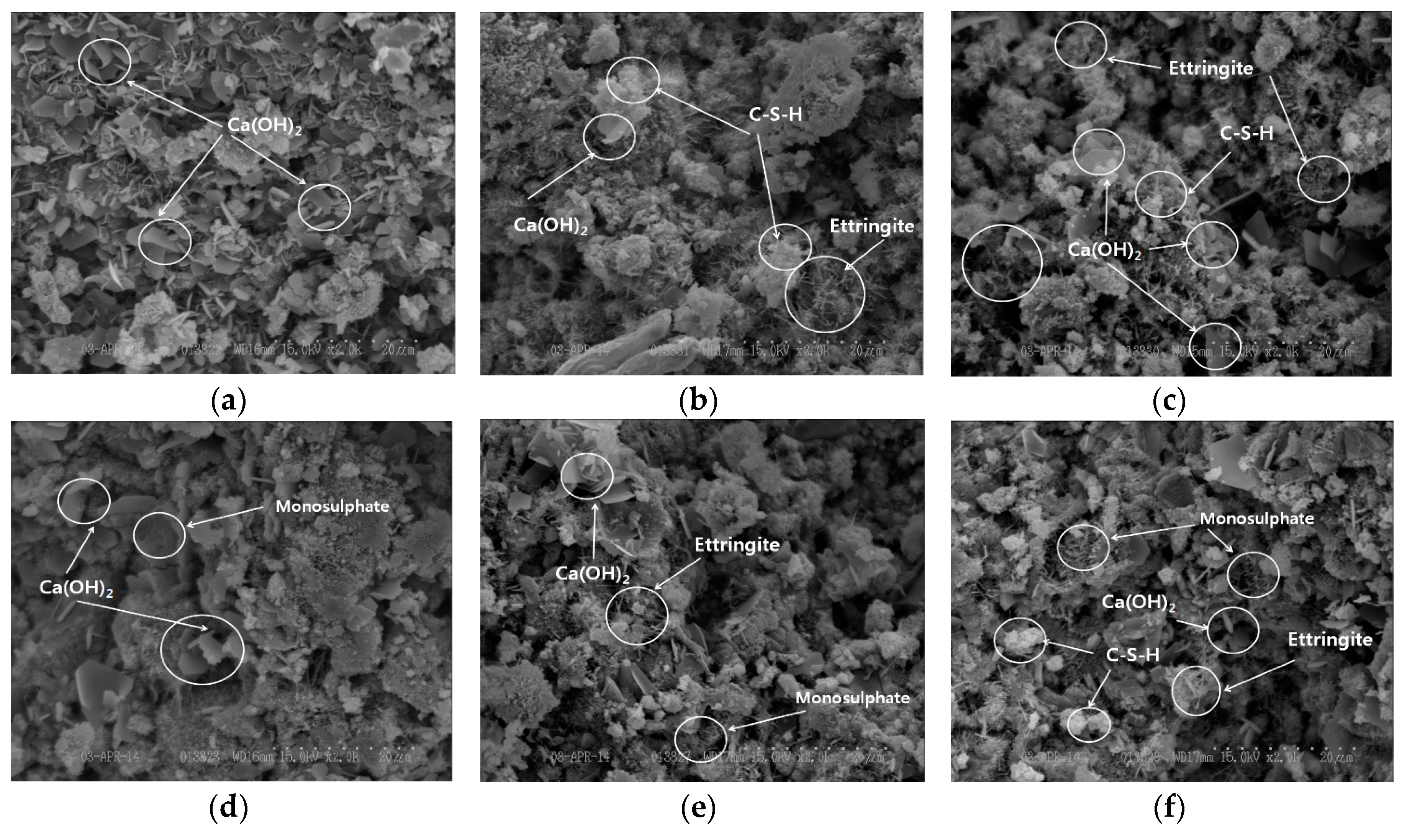
3.3.5. Result of SEM Measurement of OGE for Different ESA Burning Temperatures
4. Conclusions
- In the mortar experiment, the compressive strength decreased as the sintering temperature of ESA increased. The use of OGE 2 showed an equivalent compressive strength in OPC and early age. In addition, the compressive strength at 28 days was also excellent.
- When the setting time was measured in the cement paste experiment, the OPC reaction was the fastest. As the burning temperature rises, the content of SO3 decreases, leading to a rise in the content of CaO and SiO2.
- In the XRD and XRF analysis, an increase in the early compressive strength development of OGE was caused by the continuous generation of Ca(OH)2 from the pH of the strongly alkaline ESA. In addition, the early strength development was also attributable to the generation and filling effect of C-S-H and Ettringite from the supply of SO3.
- In the stability test, ESA-mixed GBFS was found to be stable in terms of excessive expansion and cracks were generated because of the existence of free-lime when applying a construction technology such as steam curing. As the content of free-lime in it is lower than that in OPC, ESA-mixed GBFS is thought to be available when applying the steam curing method such as Precast and when manufacturing special products.
- In conclusion, ESA developed by using an industrial by-product and industrial waste could be used to supply SO3 and apply alkaline-activation to improve the compressive strength at the mortar level, as a way of improving the early strength of GBFS-mixed concrete, which usually has the problem of a low early-strength.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bilim, C.; Atiş, C.D. Alkali activation of mortars containing different replacement levels of ground granulated blast furnace slag. Constr. Build. Mater. 2012, 28, 708–712. [Google Scholar] [CrossRef]
- Hannesson, G.; Kuder, K.; Shogren, R.; Lehman, D. The influence of high volume of fly ash and slag on the compressive strength of self-consolidating concrete. Constr. Build. Mater. 2012, 30, 161–168. [Google Scholar] [CrossRef]
- Özodabaş, A.; Yılmaz, K. Improvement of the performance of alkali activated blast furnace slag mortars with very finely ground pumice. Constr. Build. Mater. 2013, 48, 26–34. [Google Scholar] [CrossRef]
- Rashad, A.M. A comprehensive overview about the influence of different additives on the properties of alkali-activated slag—A guide for Civil Engineer. Constr. Build. Mater. 2013, 47, 29–55. [Google Scholar] [CrossRef]
- Yüksel, İ.; Bilir, T.; Özkan, Ö. Durability of concrete incorporating non-ground blast furnace slag and bottom ash as fine aggregate. Build. Environ. 2007, 42, 2651–2659. [Google Scholar]
- Chen, H.-J.; Huang, S.-S.; Tang, C.-W.; Malek, M.A.; Ean, L.-W. Effect of curing environments on strength, porosity and chloride ingress resistance of blast furnace slag cement concretes: A construction site study. Constr. Build. Mater. 2012, 35, 1063–1070. [Google Scholar] [CrossRef]
- Garg, M.; Jain, N. Waste gypsum from intermediate dye industries for production of building materials. Constr. Build. Mater. 2010, 24, 1632–1637. [Google Scholar] [CrossRef]
- Boháč, M.; Palou, M.; Novotný, R.; Másilko, J.; Všianský, D.; Staněk, T. Investigation on early hydration of ternary Portland cement-blast-furnace slag—Metakaolin blends. Constr. Build. Mater. 2014, 64, 333–341. [Google Scholar] [CrossRef]
- Barnett, S.J.; Soutsos, M.N.; Millard, S.G.; Bungey, J.H. Strength development of martars containing ground blast-furnace slag: Effect of curing temperature and determination of apparent activation energies. Cem. Concr. Res. 2006, 36, 434–440. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Bandopadhyay, A.; Alex, T.C.; Kumar, B.R.; Das, S.K.; Mehrotra, S.P. Mechanical activation of granulated blast furnace slag and its effect on theproperties and structure of portland slag cement. Cem. Concr. Compos. 2008, 30, 679–685. [Google Scholar] [CrossRef]
- Miyazawa, S.; Yokomuro, T.; Sakai, E.; Yatagai, A.; Nito, N.; Koibuchi, K. Properties of concrete using high C3S cement with ground granulated blast-furnace slag. Constr. Build. Mater. 2014, 61, 90–96. [Google Scholar] [CrossRef]
- Bellmann, F.; Stark, J. Activation of blast furnace slag by a new method. Cem. Concr. Res. 2009, 39, 644–650. [Google Scholar] [CrossRef]
- Zhu, J.; Zhong, Q.; Chen, G.; Li, D. Effect of particlesize of blast furnace slag on properties of portland cement. Procedia Eng. 2012, 27, 231–236. [Google Scholar] [CrossRef]
- Mun, K.J.; So, S.Y.; Soh, Y.S. The effect of slaked lime, anhydrous gypsum and limestone powder on properties of blast furnace slag cement mortar and concrete. Constr. Build. Mater. 2007, 21, 1576–1582. [Google Scholar] [CrossRef]
- Binici, H.; Temiz, H.; Köse, M.M. The effect of fineness on the properties of the blended cements incorporating ground granulated blast furnace slag and ground basaltic pumice. Constr. Build. Mater. 2007, 21, 1122–1128. [Google Scholar] [CrossRef]
- Lim, N.-G.; Her, J.-W. A Foundational Study on Properties of High-Strength Concrete using Nanoslag by Silica Fume Replacement. Archit. Inst. Korea 2008, 12, 23–29. [Google Scholar]
- Yang, K.-H.; Cho, A.-R.; Song, J.-K.; Nam, S.-H. Hydration products and strength development of calcium hydroxide-based alkali-activated slag mortars. Constr. Build. Mater. 2012, 29, 410–419. [Google Scholar] [CrossRef]
- Thomas, J.J.; Allen, A.J.; Jennings, H.M. Density and water content of nanoscale solid C–S–H formed in alkali-activated slag (AAS) paste and implications for chemical shrinkage. Cem. Concr. Res. 2012, 42, 377–383. [Google Scholar] [CrossRef]
- Xu, H.; Gong, W.; Syltebo, L.; Izzo, K.; Lutze, W.; Pegg, I.L. Effect of blast furnace slag grades on fly ash based geopolymer waste forms. Fuel 2014, 133, 332–340. [Google Scholar] [CrossRef]
- Gwak, B.-H.; Lee, H.-S.; Kim, J.-H.; Kim, J.-S. The study on the development of mortar’s early compressive strength with blast furnace slag powder using firing powders. Korea Concr. Inst. 2012, 24, 265–266. [Google Scholar]
- Kim, J.-H.; Lee, H.-S.; Lee, K.-J. An experimental study on the early compressive strength improvement of cement mortar mixing Ground Granulated Blast Furnace Slag using Industrial byproducts developed in Mock-up rotary kiln. Archit. Inst. Korea 2013, 33, 585–586. [Google Scholar]
- Zhong, S.; Ni, K.; Li, J. Properties of mortars made by uncalcined FGD gypsum-fly ash-ground granulated blast furnace slag composite binder. Waste Manag. 2012, 32, 1468–1472. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Georgin, J.; Ambroise, J.; Péra, J. The influence of gypsum ratio on the mechanical performance of slag cement accelerated by calcium sulfoaluminate cement. Constr. Build. Mater. 2011, 25, 1298–1304. [Google Scholar] [CrossRef]
- Menéndez, G.; Bonavetti, V.; Irassar, E.F. Strength development of ternary blended cement with limestone filler and blast-furnace slag. Cem. Concr. Compos. 2003, 25, 61–67. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Li, L. A review: The comparison between alkali-activated slag (Si+Ca) and metakaolin (Si+Al) cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Kim, T.H.; Tae, S.H.; Chae, C.U.; Choi, W.Y. The Environmental Impact and Cost Analysis of Concrete Mixing Blast Furnace Slag Containing Titanium Gypsum and Sludge in South Korea. Sustainability 2016, 8, 502. [Google Scholar] [CrossRef]




| Raw Materials | Blaine (cm2/g) | Ig. Loss | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | SO3 |
|---|---|---|---|---|---|---|---|---|---|---|
| OPC | 3450 | 1.54 | 21.58 | 4.77 | 3.43 | 62.59 | 2.59 | 0.11 | 0.85 | 2.22 |
| GBFS1 1 | 4330 | 0.13 | 34.56 | 15.30 | 0.33 | 42.71 | 3.90 | 0.19 | 0.36 | 1.98 |
| GBFS2 2 | 4325 | 0.12 | 36.1 | 15.24 | 0.45 | 43.33 | 4.16 | 0.21 | 0.49 | 0.01 |
| Materials 1 | Ig. Loss | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | SO3 | pH | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sludge | 105 °C | 19.42 | 43.16 | 23.92 | 6.07 | 0.85 | 1.60 | 0.58 | 2.27 | 0.55 | 7.6 |
| 600 °C | 4.25 | 50.72 | 29.71 | 6.76 | 0.96 | 1.96 | 0.80 | 2.61 | 0.51 | 7.7 | |
| 900 °C | 1.29 | 52.35 | 31.22 | 6.72 | 0.94 | 2.02 | 0.76 | 2.65 | 0.33 | 7.7 | |
| 1100 °C | 0.00 | 53.38 | 30.73 | 7.20 | 1.03 | 2.06 | 1.00 | 2.76 | 0.10 | 7.7 | |
| CG | 105 °C | 11.66 | 1.89 | 0.49 | 0.84 | 31.72 | 1.33 | 0.11 | 0.38 | 47.84 | 7.1 |
| 600 °C | 4.14 | 2.20 | 0.60 | 0.92 | 34.28 | 1.64 | 0.13 | 0.42 | 51.69 | 7.4 | |
| 900 °C | 1.09 | 2.51 | 0.66 | 0.99 | 35.57 | 2.04 | 0.15 | 0.55 | 52.12 | 8.4 | |
| 1100 °C | 0.00 | 2.34 | 0.56 | 1.01 | 37.40 | 1.84 | 0.14 | 0.51 | 51.95 | 7.9 | |
| LP | 105 °C | 40.68 | 2.15 | 0.34 | 0.24 | 55.31 | 1.02 | 0.00 | 0.06 | 0.03 | 7.2 |
| 600 °C | 40.86 | 2.16 | 0.35 | 0.23 | 55.12 | 1.02 | 0.01 | 0.06 | 0.04 | 8.1 | |
| 900 °C | 34.00 | 2.39 | 0.39 | 0.26 | 61.19 | 1.37 | 0.02 | 0.08 | 0.15 | 12.3 | |
| 1100 °C | 0.00 | 4.49 | 0.49 | 0.37 | 91.04 | 2.55 | 0.03 | 0.05 | 0.77 | 12.3 | |
| ESA | Ig. Loss | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | SO3 | pH |
|---|---|---|---|---|---|---|---|---|---|---|
| ESA 700 °C | 12.11 | 13.23 | 7.79 | 2.50 | 33.08 | 0.90 | 0.24 | 0.73 | 25.87 | 11.7 |
| ESA 800 °C | 6.86 | 14.00 | 8.41 | 2.65 | 35.06 | 0.95 | 0.25 | 0.78 | 27.20 | 12.3 |
| ESA 900 °C | 2.04 | 13.65 | 8.07 | 2.86 | 38.78 | 1.02 | 0.23 | 0.74 | 28.63 | 12.4 |
| ESA 1000 °C | 0.00 | 16.86 | 9.86 | 3.06 | 39.82 | 1.25 | 0.30 | 0.81 | 24.17 | 11.8 |
| ESA 1100 °C | 0.00 | 16.98 | 9.30 | 3.28 | 44.42 | 1.24 | 0.33 | 0.91 | 19.48 | 11.2 |
| OGE | Ig. Loss | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | SO3 |
|---|---|---|---|---|---|---|---|---|---|
| PBFSC 1 | 1.07 | 24.67 | 7.49 | 2.12 | 56.59 | 3.69 | 0.18 | 0.86 | 2.15 |
| OGE 1 2 (ESA 700 °C) | 1.59 | 22.81 | 6.73 | 2.27 | 57.07 | 3.14 | 0.16 | 0.94 | 3.89 |
| OGE 2 2 (ESA 800 °C) | 1.19 | 23.05 | 6.76 | 2.27 | 57.49 | 3.19 | 0.15 | 0.93 | 3.87 |
| OGE 3 2 (ESA 900 °C) | 0.73 | 22.99 | 6.79 | 2.27 | 57.58 | 3.18 | 0.15 | 0.94 | 3.90 |
| OGE 4 2 (ESA 1000 °C) | 0.00 | 23.26 | 7.01 | 2.32 | 57.63 | 3.17 | 0.17 | 0.96 | 3.92 |
| OGE 5 2 (ESA 1100 °C) | 0.00 | 23.42 | 6.95 | 2.33 | 58.10 | 3.21 | 0.15 | 0.95 | 3.82 |
| NO | Specimen | W/B (%) | Dosage (Mass Ratio) | Flow (mm) | Compressive Strength (MPa) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OPC | GBFS | ESA | 1 day | 3 day | 7 day | 14 day | 28 day | ||||
| 1 | OPC | 50 | 100 | 0 | 0 | 174 | 10.7 | 27.4 | 39.7 | 48.9 | 52.8 |
| 2 | PBFSC | 50 | 70 | 30 | 0 | 193 | 6.0 | 20.1 | 35.0 | 47.8 | 58.3 |
| 3 | OGE 1 | 50 | 70 | 23 | 7 | 176 | 9.8 | 25.6 | 40.0 | 48.7 | 56.3 |
| 4 | OGE 2 | 50 | 70 | 23 | 7 | 176 | 11.2 | 28.1 | 41.1 | 51.3 | 58.4 |
| 5 | OGE 3 | 50 | 70 | 23 | 7 | 175 | 9.3 | 26.1 | 38.0 | 48.9 | 57.5 |
| 6 | OGE 4 | 50 | 70 | 23 | 7 | 179 | 9.3 | 23.9 | 36.5 | 45.6 | 52.1 |
| 7 | OGE 5 | 50 | 70 | 23 | 7 | 180 | 9.8 | 22.3 | 34.0 | 45.2 | 58.3 |
| NO | Specimen | W/B (%) | Dosage (Mass Ratio) | Curing Temperature (°C) | ||
|---|---|---|---|---|---|---|
| OPC | GBFS | ESA | ||||
| 1 | OPC | 50 | 100 | 0 | 0 | 20 |
| 2 | PBFSC | 50 | 70 | 30 | 0 | 20 |
| 3 | OGE 1~5 | 50 | 70 | 23 | 7 | 20 |
| Experiments | Measurement Items | Standard |
|---|---|---|
| Material (ESA) | XRD analysis | ASTM C457 |
| Mortar | Slump flow | ASTM 1437-15 |
| Compressive strength | ASTM C109 | |
| Cement Paste | setting time by Vicat needle | ISO 9587 |
| Hydrates by XRD analysis | ASTM C457 | |
| Hydrates by SEM analysis | - | |
| Free-lime | - |
| NO | 0.1 N HCl Consumption (mL) | Free Lime (%) |
|---|---|---|
| OPC | 2.20 | 0.62 |
| PBFSC | 1.15 | 0.32 |
| OGE 1 | 1.20 | 0.34 |
| OGE 2 | 1.20 | 0.34 |
| OGE 3 | 1.15 | 0.32 |
| OGE 4 | 1.20 | 0.34 |
| OGE 5 | 1.20 | 0.31 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Lee, H.-S. Improvement of Early Strength of Cement Mortar Containing Granulated Blast Furnace Slag Using Industrial Byproducts. Materials 2017, 10, 1050. https://doi.org/10.3390/ma10091050
Kim J-H, Lee H-S. Improvement of Early Strength of Cement Mortar Containing Granulated Blast Furnace Slag Using Industrial Byproducts. Materials. 2017; 10(9):1050. https://doi.org/10.3390/ma10091050
Chicago/Turabian StyleKim, Jin-Hyoung, and Han-Seung Lee. 2017. "Improvement of Early Strength of Cement Mortar Containing Granulated Blast Furnace Slag Using Industrial Byproducts" Materials 10, no. 9: 1050. https://doi.org/10.3390/ma10091050




