Optimal Structure of a Plasmonic Chip for Sensitive Bio-Detection with the Grating-Coupled Surface Plasmon-Field Enhanced Fluorescence (GC-SPF)
Abstract
:1. Introduction
2. Results
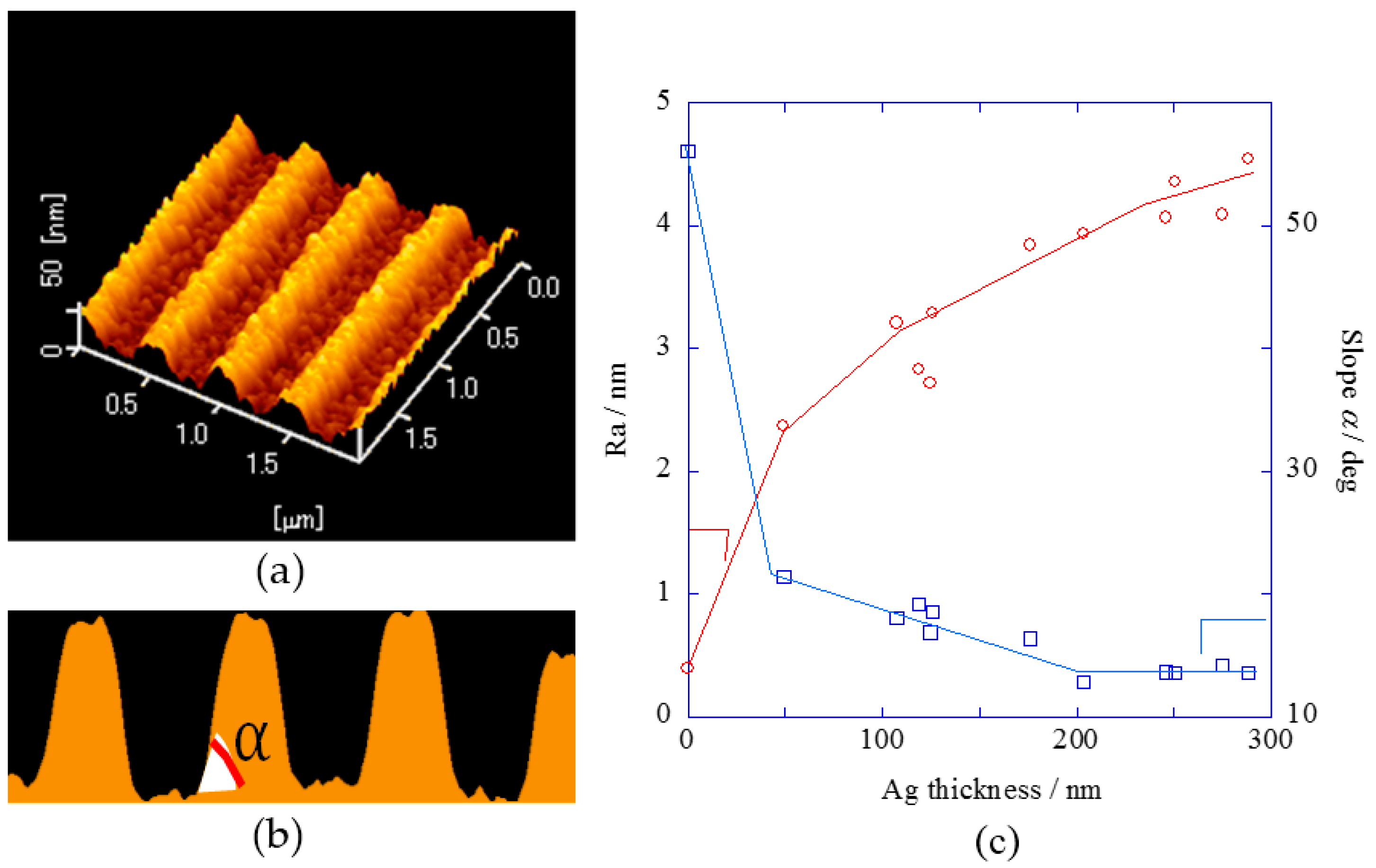
2.1. Surface Analysis
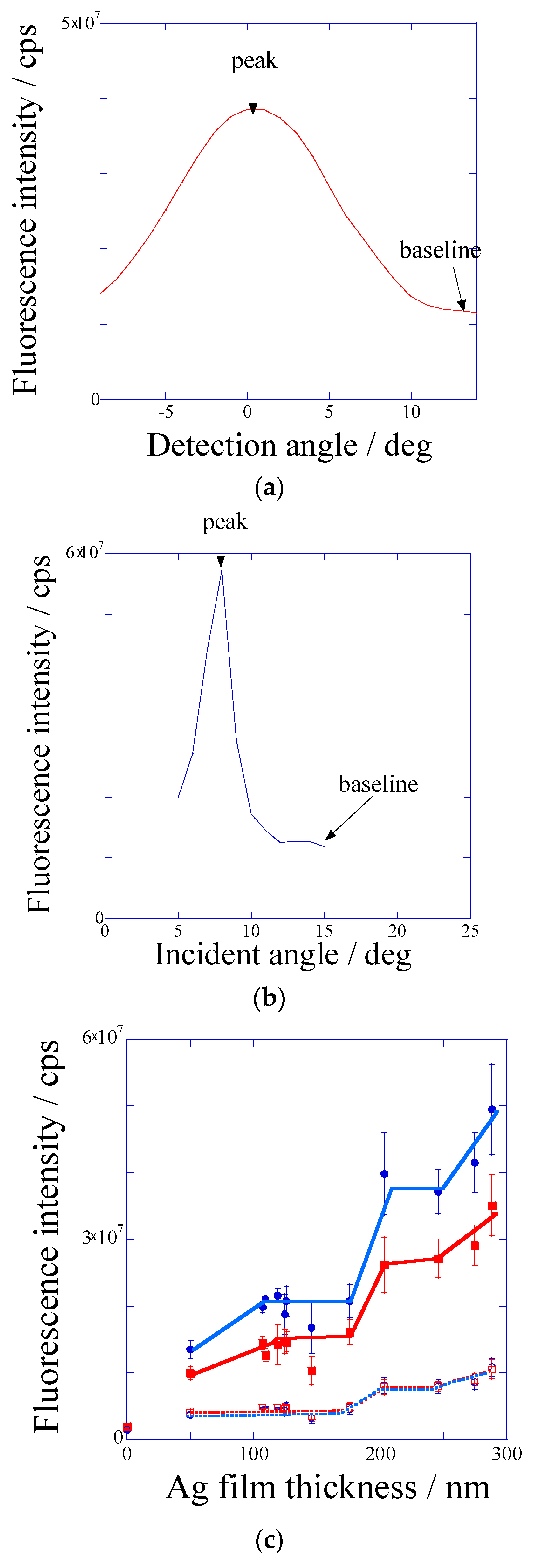
2.2. SPF Measurement
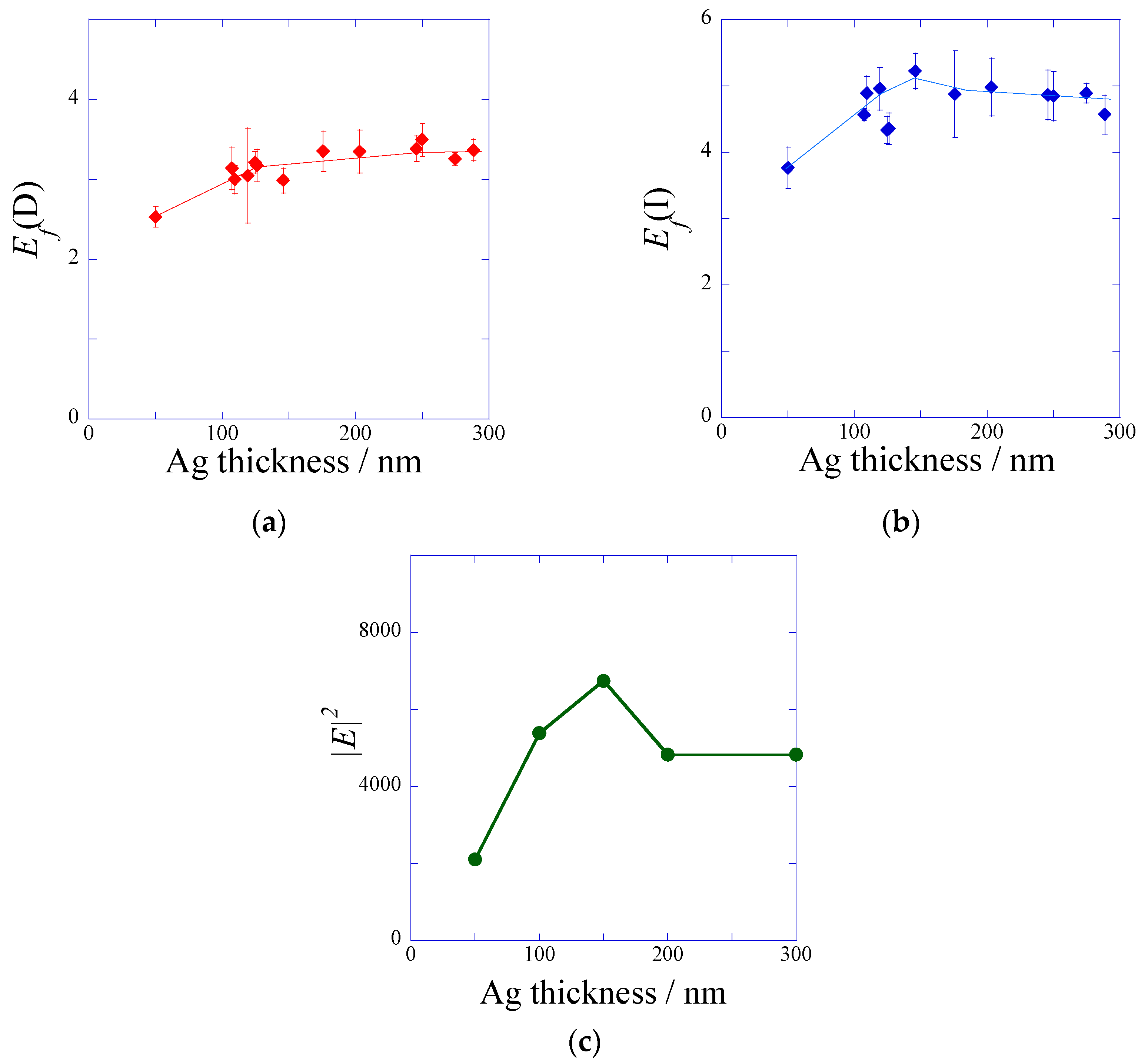
2.2.1. Silver-Film Thickness Dependence of the Fluorescence Intensity
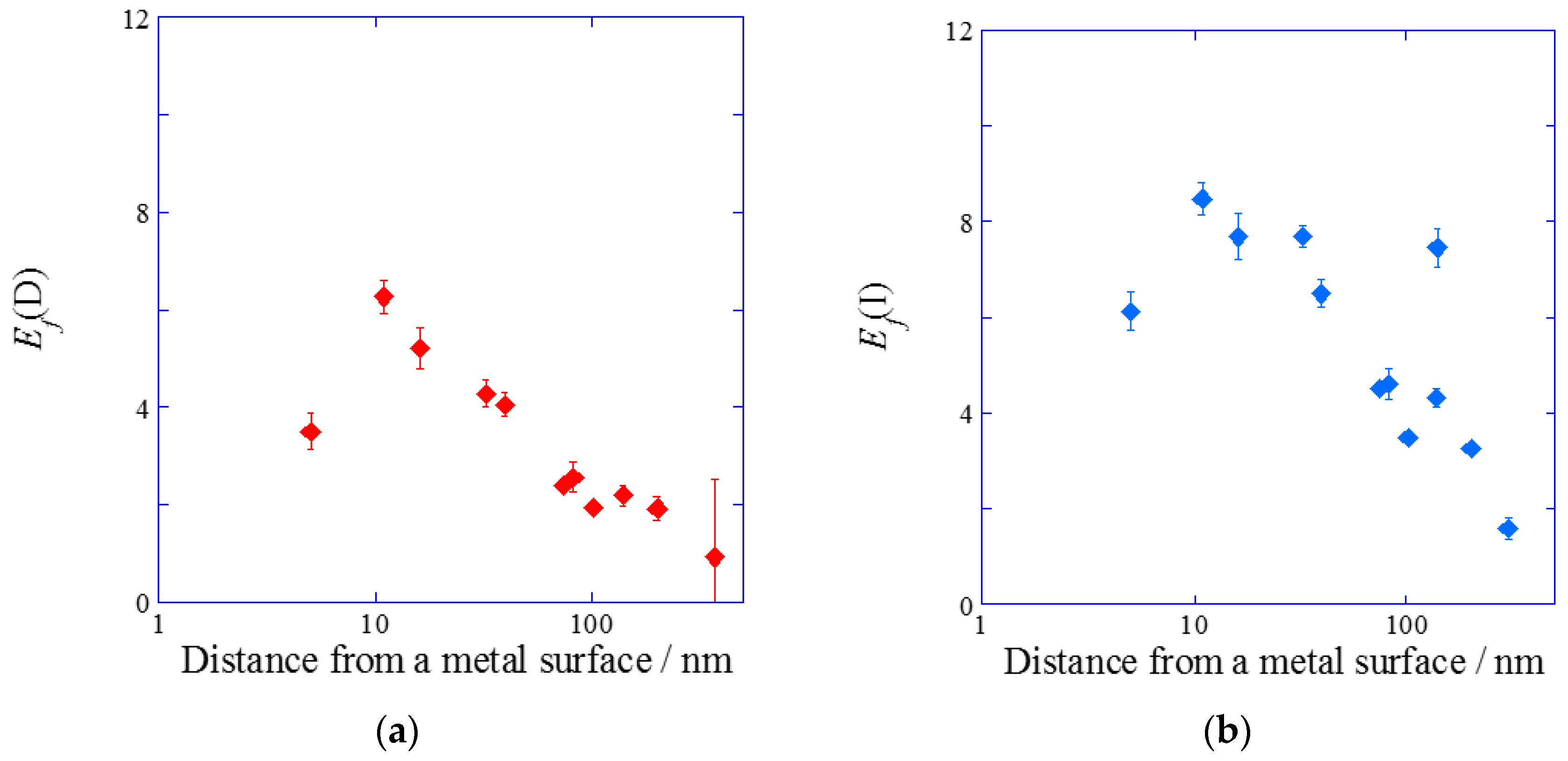
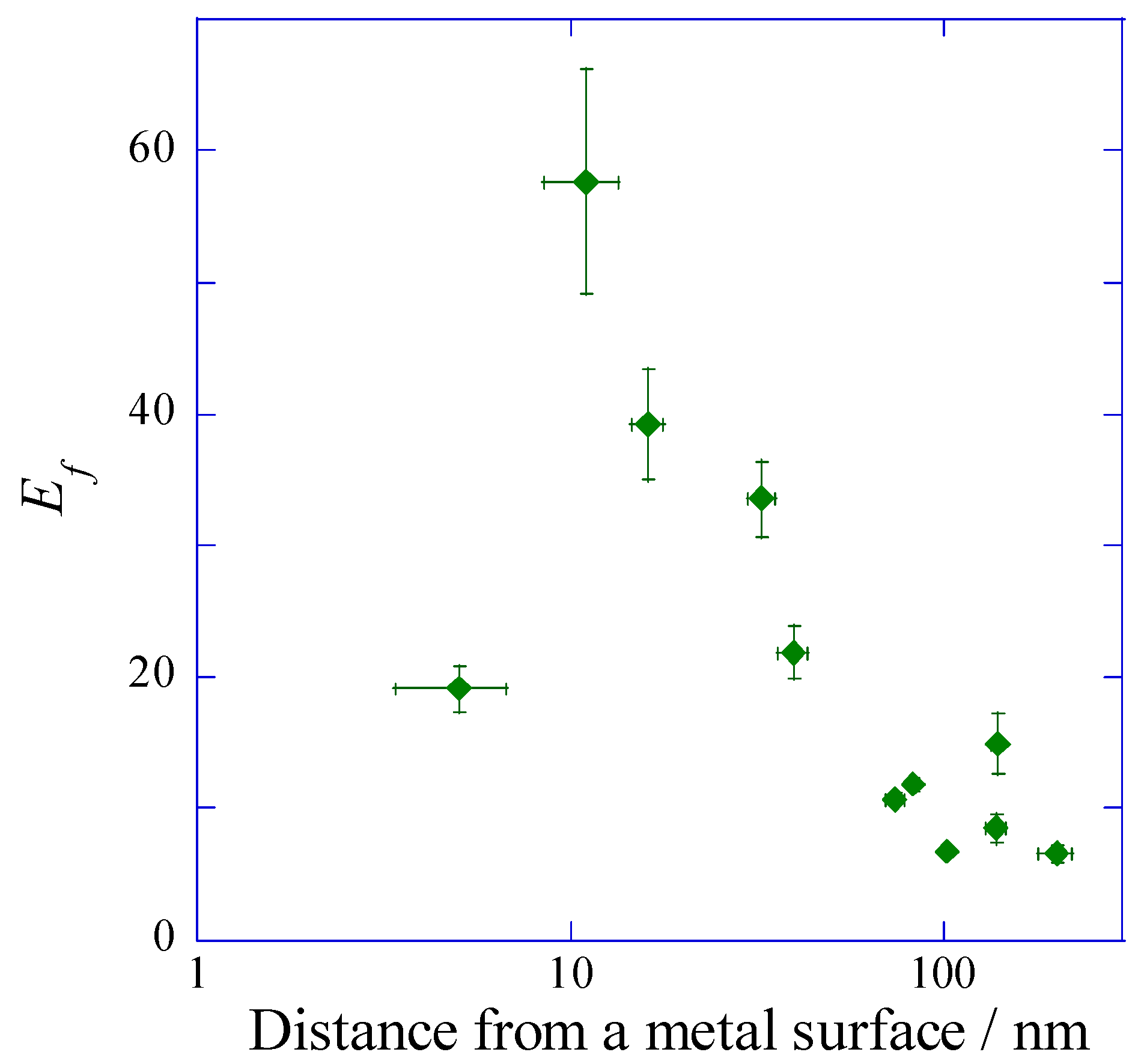
2.2.2. Dependence of the Fluorescence Intensity on the Distance from the Metal Surface
2.3. Limit of Detection for IAS and DAS
3. Discussion
4. Materials and Methods
4.1. Fabrication of a Plasmonic Chip
4.2. Surface Measurement
4.3. Samples
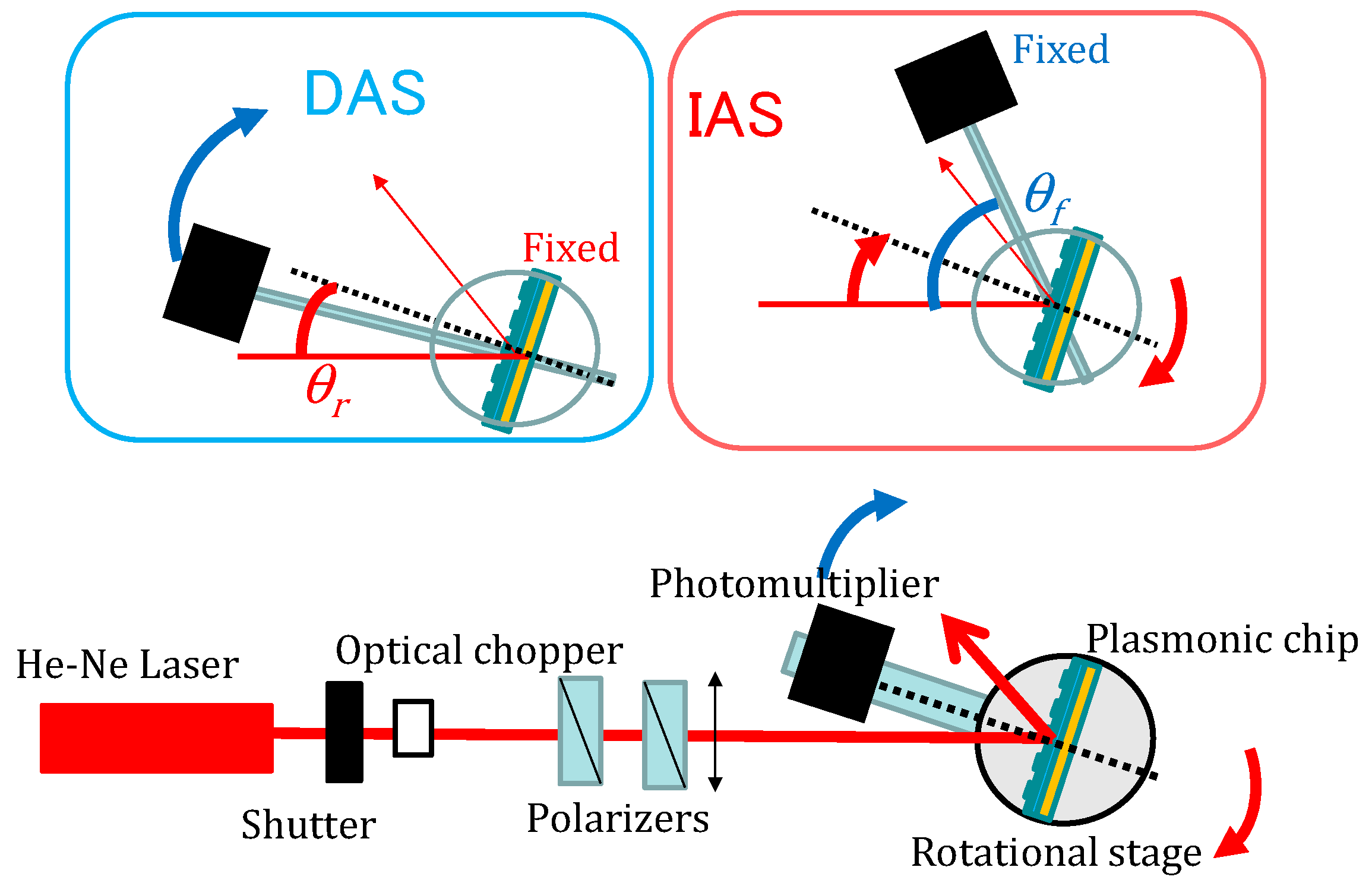
4.4. Fluorescence Measurement
4.5. Numerical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vareiro, M.L.M.; Liu, J.; Knoll, W.; Zak, K.; Williams, D.; Jenkins, A.T.A. Surface plasmon fluorescence measurements of human chorionic gonadotrophin: Role of antibody orientation in obtaining enhanced sensitivity and limit of detection. Anal. Chem. 2005, 77, 2426–2431. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.F.; Chen, R.C.; Lee, Y.J.; Chao, S.C.; Su, L.C.; Li, Y.C.; Chou, C. Localized surface plasmon coupled fluorescence fiber-optic biosensor for alpha-fetoprotein detection in human serum. Biosens. Bioelectron. 2009, 24, 1610–1614. [Google Scholar] [CrossRef] [PubMed]
- Willets, K.A.; Van Duyne, R.P. Localized Surface Plasmon Resonance Spectroscopy and Sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [PubMed]
- Hao, E.; Schatz, G.C. Electromagnetic fields around silver nanoparticles and dimers. J. Chem. Phys. 2004, 120, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.K.; Jeon, K.S.; Hwang, J.H.; Kim, H.; Kwon, S.; Suh, Y.D.; Nam, J.M. Highly uniform and reproducible surface-enhanced Raman scattering from DNA-tailorable nanoparticles with 1-nm interior gap. Nat. Nanotechnol. 2011, 6, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Ueno, K.; Misawa, H. Surface plasmon-enhanced photochemical reactions. J. Photochem. Photobiol. C-Photochem. Rev. 2013, 15, 31–52. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Beck, F.J.; Polman, A.; Catchpole, K.R. Tunable light trapping for solar cells using localized surface plasmons. Appl. Phys. 2009, 105, 114310. [Google Scholar] [CrossRef]
- Tagliabue, G.; Höller, C.; Eghlidi, H.; Poulikakos, D. Proximal gap-plasmon nanoresonators in the limit of vanishing inter-cavity separation. Nanoscale 2014, 6, 10274–10280. [Google Scholar] [CrossRef] [PubMed]
- Zayats, A.V.; Smolyaninov, I.I. Near-field photonics: Surface plasmon polaritons and localized surface plasmons. J. Opt. A Pure Appl. Opt. 2003, 5, S16. [Google Scholar] [CrossRef]
- Liebermann, T.; Knoll, W. Surface-plasmon field-enhanced fluorescence spectroscopy. Colloids Surf. A 2000, 177, 115–130. [Google Scholar] [CrossRef]
- Tawa, K.; Knoll, W. Mismatching base-pair dependence of the kinetics of DNA-DNA hybridization studied by surface plasmon fluorescence spectroscopy. Nucleic Acids Res. 2004, 32, 2372–2377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fu, Y.; Chowdhury, M.H.; Lakowicz, J.R. Metal-enhanced single-molecule fluorescence on silver particle monomer and dimer: Coupling effect between metal particles. Nano Lett. 2007, 7, 2101–2107. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Okamoto, T. Plasmonics: Visit the past to know the future. J. Phys. D Appl. Phys. 2012, 45, 433001. [Google Scholar] [CrossRef]
- Tawa, K.; Kondo, F.; Sasakawa, C.; Nagae, K.; Nakamura, Y.; Nozaki, A.; Kaya, T. Sensitive Detection of a Tumor Marker, α-Fetoprotein, with a Sandwich Assay on a Plasmonic Chip. Anal. Chem. 2015, 87, 3871–3876. [Google Scholar] [CrossRef]
- Matsuura, R.; Tawa, K.; Kitayama, Y.; Takeuchi, T. A Plasmonic Chip-Based Bio/Chemical Hybrid Sensing System for the Highly Sensitive Detection of C-Reactive Protein. Chem. Commun. 2016, 52, 3883–3886. [Google Scholar] [CrossRef] [PubMed]
- Tawa, K.; Umetsu, M.; Nakazawa, N.; Hattori, T.; Kumagai, I. Application of 300× Enhanced Fluorescence on a Plasmonic Chip Modified with a Bispecific Antibody to a Sensitive Immunosensor. ACS Appl. Mater. Interfaces 2013, 5, 8628–8632. [Google Scholar] [CrossRef] [PubMed]
- Tawa, K.; Hori, H.; Kintaka, K.; Kiyosue, K.; Tatsu, Y.; Nishii, J. Optical microscopic observation of fluorescence enhanced by grating-coupled surface plasmon resonance. Opt. Express 2008, 16, 9781–9790. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.Q.; Tawa, K.; Hori, H.; Nishii, J. Tailored Plasmonic Gratings for Enhanced Fluorescence Detection and Microscopic Imaging. Adv. Funct. Mater. 2010, 20, 546–553. [Google Scholar] [CrossRef]
- Tawa, K.; Yasui, C.; Hosokawa, C.; Aota, H.; Nishii, J. In Situ Sensitive Fluorescence Imaging of Neurons Cultured on a Plasmonic Dish Using Fluorescence Microscopy. ACS Appl. Mater. Interfaces 2014, 6, 20010–20015. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, S.; Sala, F.D. Active Role of Oxide Layers on the Polarization of Plasmonic Nanostructures. ACS Nano 2010, 4, 4117–4125. [Google Scholar] [CrossRef] [PubMed]
- Räther, H. Surface Plasmons on Smooth and Rough Surfaces and on Gratings; Springer: Heidelberg, Germany, 1988; pp. 1–133. [Google Scholar]
- Knoll, W. Interfaces and Thin Films as Seen by Bound Electromagnetic Waves. Annu. Rev. Phys. Chem. 1998, 49, 569–638. [Google Scholar] [CrossRef] [PubMed]
- Malicka, J.; Gryczynski, I.; Gryczynski, Z.; Lakowicz, J.R. DNA Hybridization Using Surface Plasmon-Coupled Emission. Anal. Chem. 2003, 75, 6629–6633. [Google Scholar] [CrossRef] [PubMed]
- Wintera, G.; Barnes, W.L. Emission of light through thin silver films via near-field coupling to surface plasmon polaritons. Appl. Phys. Lett. 2006, 88, 051109. [Google Scholar] [CrossRef] [Green Version]
- Tsuneyasu, M.; Sasakawa, C.; Naruishi, N.; Tanaka, Y.; Yoshida, Y.; Tawa, K. Sensitive detection of interleukin-6 (IL-6) on a plasmonic chip by grating-coupled surface-plasmon-field-enhanced fluorescence imaging. Jpn. J. Appl. Phys. 2014, 53, 06JL05. [Google Scholar] [CrossRef]
- Chance, R.R.; Prock, A.; Silbey, R. Molecular Fluorescence and Energy Transfer Near Interfaces. Adv. Chem. Phys. 1978, 37, 1–65. [Google Scholar] [CrossRef]
- Tawa, K.; Morigaki, K. Substrate-Supported Phospholipid Membranes Studied by Surface Plasmon Resonance and Surface Plasmon Fluorescence Spectroscopy. Biophys. J. 2005, 89, 2750–2758. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Jin, H.H.; Bae, G.; Lee, E.Y.; Im, S.H.; Kim, D.H.; Seo, T.S. Growth of Silver Nanowires from Controlled Silver Chloride Seeds and Their Application for Fluorescence Enhancement Based on Localized Surface Plasmon Resonance. Small 2017, 13, 1603392. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, L.; Wong, T.I.; Bauch, M.; Zhang, Q.; Zhang, J.; Liu, Z.; Zhou, X.; Bai, P.; Dostalek, J.; et al. Directional fluorescence emission co-enhanced by localized and propagating surface plasmons for biosensing. Nanoscale 2016, 8, 8008. [Google Scholar] [CrossRef] [PubMed]
- Akashi, N.; Tawa, K.; Tatsu, Y.; Kintaka, K.; Nishii, J. Grating Substrates Fabricated by Nanoimprint Lithography for Fluorescence Microscopy. Jpn. J. Appl. Phys. 2009, 48, 06FH17. [Google Scholar] [CrossRef]
- Hori, H.; Tawa, K.; Kintaka, K.; Nishii, J.; Tatsu, Y. Surface Profile Dependence on the Grating-Coupled Surface Plasmon Enhanced Fluorescence. J. Appl. Phys. 2010, 107, 114702. [Google Scholar] [CrossRef]


| Experiment | Silver/nm | SiO2/nm |
|---|---|---|
| Ag film thickness dependence | 50–288 | 20 ± 4 (fixed) |
| SiO2 film thickness dependence | 211 ± 8 (fixed) | 8–300 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tawa, K.; Nakayama, T.; Kintaka, K. Optimal Structure of a Plasmonic Chip for Sensitive Bio-Detection with the Grating-Coupled Surface Plasmon-Field Enhanced Fluorescence (GC-SPF). Materials 2017, 10, 1063. https://doi.org/10.3390/ma10091063
Tawa K, Nakayama T, Kintaka K. Optimal Structure of a Plasmonic Chip for Sensitive Bio-Detection with the Grating-Coupled Surface Plasmon-Field Enhanced Fluorescence (GC-SPF). Materials. 2017; 10(9):1063. https://doi.org/10.3390/ma10091063
Chicago/Turabian StyleTawa, Keiko, Takuya Nakayama, and Kenji Kintaka. 2017. "Optimal Structure of a Plasmonic Chip for Sensitive Bio-Detection with the Grating-Coupled Surface Plasmon-Field Enhanced Fluorescence (GC-SPF)" Materials 10, no. 9: 1063. https://doi.org/10.3390/ma10091063





