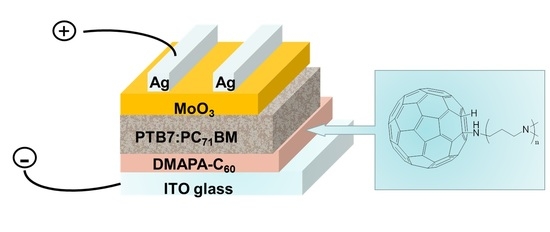
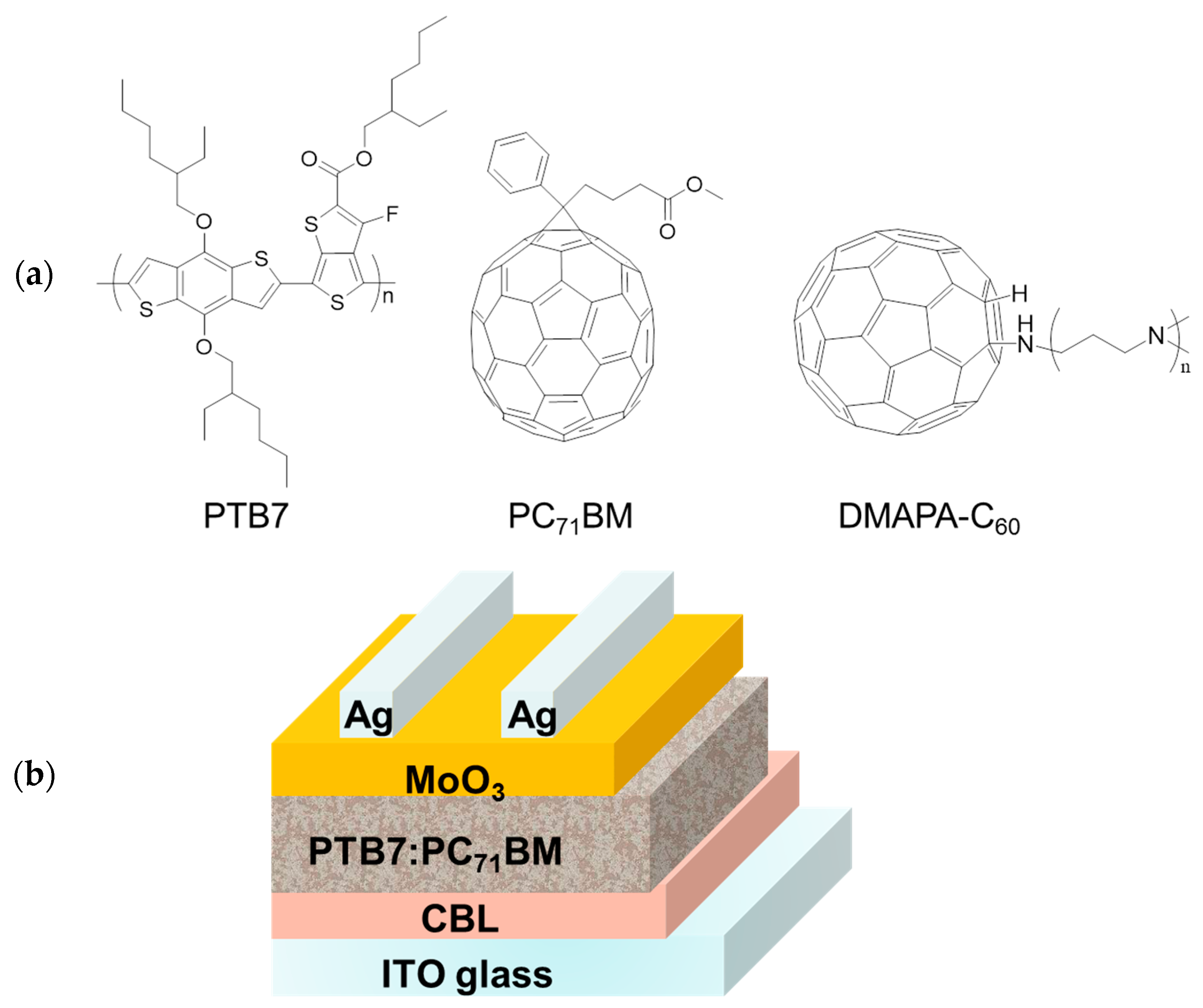
Efficient Inverted Organic Solar Cells Based on a Fullerene Derivative-Modified Transparent Cathode
Abstract
:1. Introduction
2. Results and Discussion
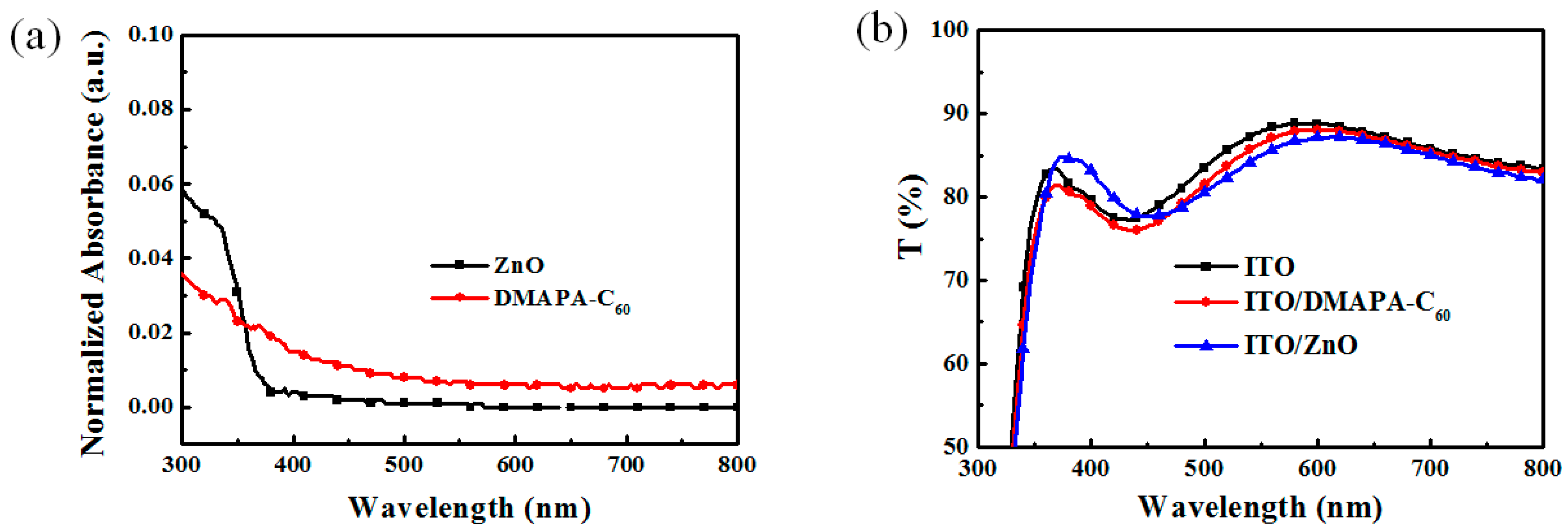
2.1. Absorption and Transmittance
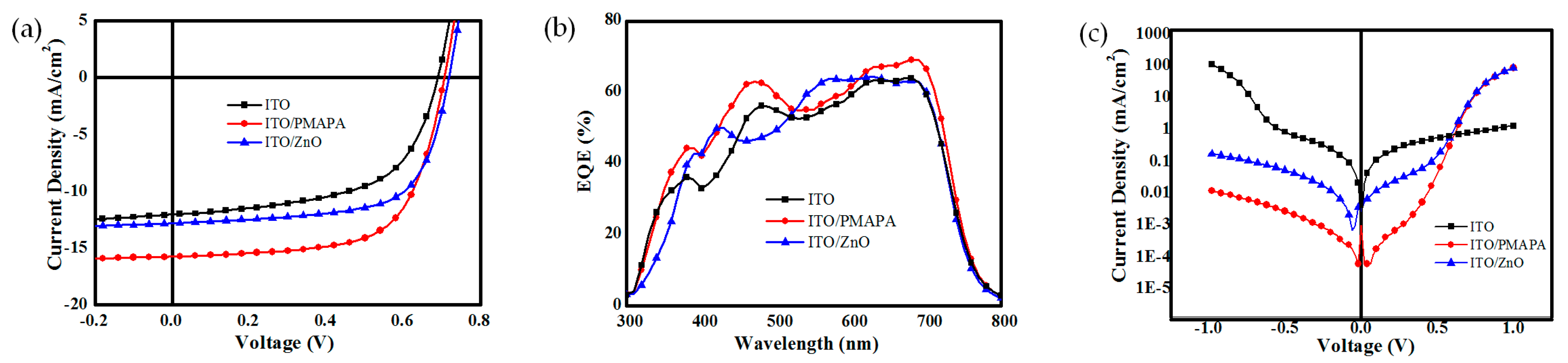
2.2. Photovoltaic Performances
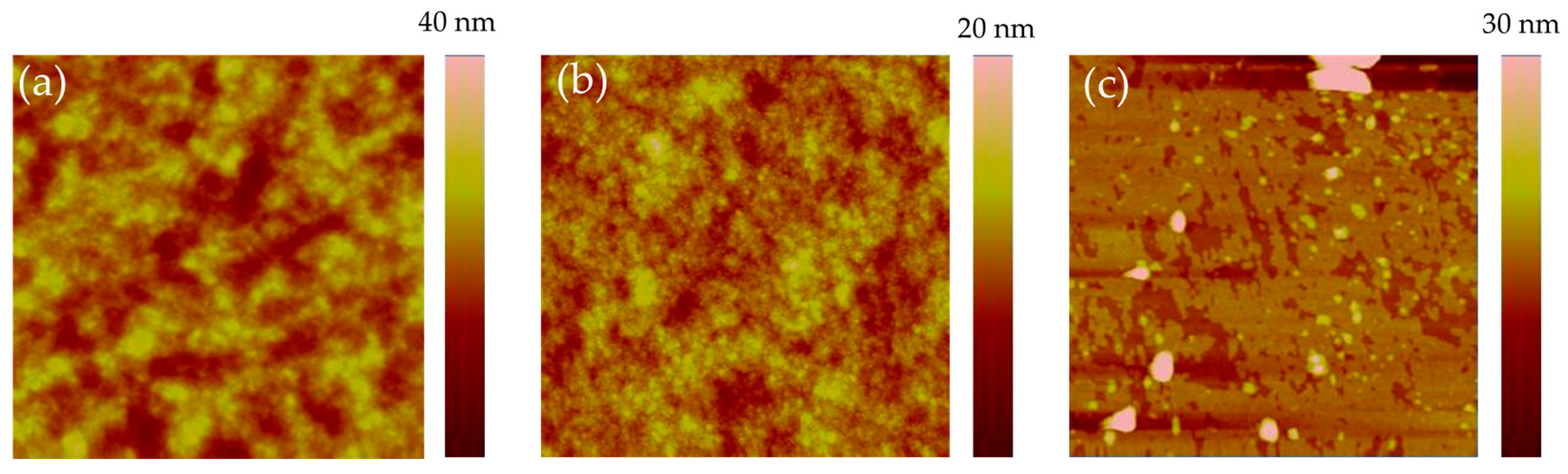
2.3. Morphology
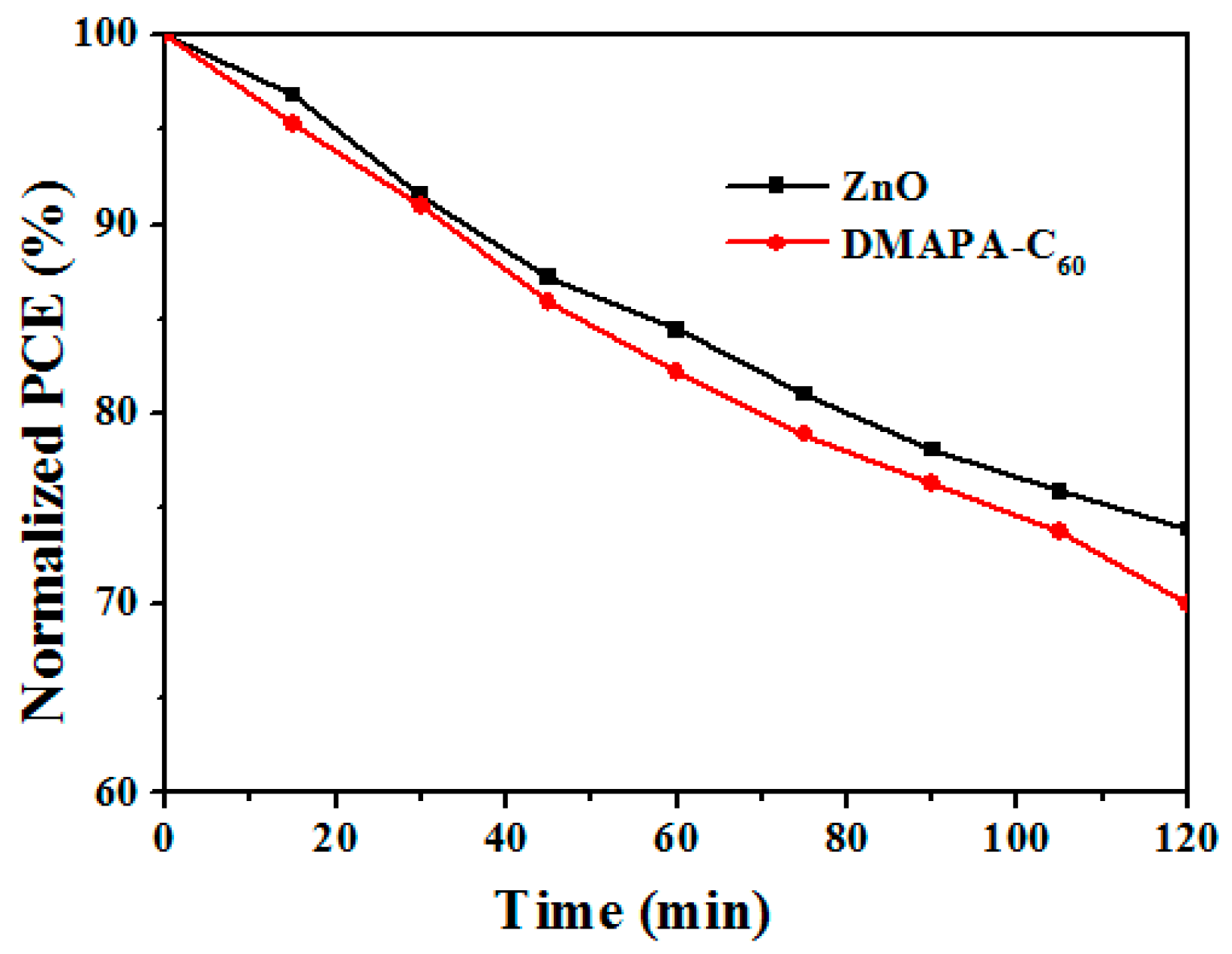
2.4. Stability
3. Materials and Methods
3.1. Materials
3.2. Preparation of ZnO
3.3. Fabrication of the OSCs
3.4. Characterizations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, Y.F. Molecular Design of Photovoltaic Materials for Polymer Solar Cells: Toward Suitable Electronic Energy Levels and Broad Absorption. Acc. Chem. Res. 2012, 45, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Heeger, A.J. 25th anniversary article: Bulk heterojunction solar cells: Understanding the mechanism of operation. Adv. Mater. 2014, 26, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Zhan, X.W. Stability of organic solar cells: Challenges and strategies. Chem. Soc. Rev. 2016, 45, 2544–2582. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.; Han, D.; Sun, B.; Zhou, D.; Wang, C.; Liu, P.; Feng, L. Facile Approach to Preparing a Vanadium Oxide Hydrate Layer as a Hole-Transport Layer for High-Performance Polymer Solar Cells. ACS Appl. Mater. Int. 2017, 9, 18087–18094. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chang, W.H.; Yoshimura, K.; Ohya, K.; You, J.; Gao, J.; Hong, Z.; Yang, Y. An efficient triple-junction polymer solar cell having a power conversion efficiency exceeding 11%. Adv. Mater. 2014, 26, 5670–5677. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Dai, S.; Wu, Y.; Zhang, Q.; Wang, J.; Jiang, L.; Ling, Q.; Wei, Z.; Ma, W.; You, W.; et al. Single-Junction Binary-Blend Nonfullerene Polymer Solar Cells with 12.1% Efficiency. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.Z.; Zhao, F.W.; Wu, Y.; Chen, K.; Xia, Y.X.; Li, G.W.; Prasad, S.K.K.; Zhu, J.S.; Huo, L.J.; Bin, H.J.; et al. Mapping Polymer Donors toward High-Efficiency Fullerene Free Organic Solar Cells. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Zhao, F.; Zhang, Q.; Lau, T.-K.; Li, T.; Liu, K.; Ling, Q.; Wang, C.; Lu, X.; You, W.; et al. Fused Nonacyclic Electron Acceptors for Efficient Polymer Solar Cells. J. Am. Chem. Soc. 2017, 139, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Y.; Zhang, L.; Chow, P.C.Y.; Wang, Z.; Zhang, G.; Ma, W.; Yan, H. A Wide-Bandgap Donor Polymer for Highly Efficient Non-fullerene Organic Solar Cells with a Small Voltage Loss. J. Am. Chem. Soc. 2017, 139, 6298–6301. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhong, C.; Su, S.; Xu, M.; Wu, H.; Cao, Y. Enhanced power-conversion efficiency in polymer solar cells using an inverted device structure. Nat. Photonics 2012, 6, 591–595. [Google Scholar] [CrossRef]
- Wang, K.; Liu, C.; Meng, T.Y.; Yi, C.; Gong, X. Inverted organic photovoltaic cells. Chem. Soc. Rev. 2016, 45, 2937–2975. [Google Scholar] [CrossRef] [PubMed]
- Etxebarria, I.; Ajuria, J.; Pacios, R. Solution-processable polymeric solar cells: A review on materials, strategies and cell architectures to overcome 10%. Org. Electron. 2015, 19, 34–60. [Google Scholar] [CrossRef]
- Cahen, D.; Kahn, A. Electron Energetics at Surfaces and Interfaces: Concepts and Experiments. Adv. Mater. 2003, 15, 271–277. [Google Scholar] [CrossRef]
- Woo, S.; Kim, W.H.; Kim, H.; Yi, Y.; Lyu, H.K.; Kim, Y. 8.9% Single-Stack Inverted Polymer Solar Cells with Electron-Rich Polymer Nanolayer-Modified Inorganic Electron-Collecting Buffer Layers. Adv. Energy Mater. 2014, 4, 1301692. [Google Scholar] [CrossRef]
- Ma, D.; Lv, M.L.; Lei, M.; Zhu, J.; Wang, H.Q.; Chen, X.W. Self-Organization of Amine-Based Cathode Interfacial Materials in Inverted Polymer Solar Cells. ACS Nano 2014, 8, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xu, H.; Tian, H.; Yin, S.; Zhang, F. Effect of cathode buffer layer on the stability of polymer bulk heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2010, 94, 1831–1834. [Google Scholar] [CrossRef]
- Lee, Y.I.; Youn, J.H.; Ryu, M.S.; Kim, J.; Moon, H.T.; Jang, J. Highly efficient inverted poly(3-hexylthiophene): Methano-fullerene 6,6-phenyl C71-butyric acid methyl ester bulk heterojunction solar cell with Cs2CO3 and MoO3. Org. Electron. 2011, 12, 353–357. [Google Scholar] [CrossRef]
- Kuwabara, T.; Nakayama, T.; Uozumi, K.; Yamaguchi, T.; Takahashi, K. Highly durable inverted-type organic solar cell using amorphous titanium oxide as electron collection electrode inserted between ITO and organic layer. Sol. Energy Mater. Sol. Cells 2008, 92, 1476–1482. [Google Scholar] [CrossRef]
- Guerrero, A.; Chambon, S.; Hirsch, L.; Garcia-Belmonte, G. Light-modulated TiOx Interlayer Dipole and Contact Activation in Organic Solar Cell Cathodes. Adv. Funct. Mater. 2014, 24, 6234–6240. [Google Scholar] [CrossRef]
- Liang, Z.Q.; Zhang, Q.F.; Jiang, L.; Cao, G.Z. ZnO cathode buffer layers for inverted polymer solar cells. Energy Environ. Sci. 2015, 8, 3442–3476. [Google Scholar] [CrossRef]
- Bai, S.; Jin, Y.Z.; Liang, X.Y.; Ye, Z.Z.; Wu, Z.W.; Sun, B.Q.; Ma, Z.F.; Tang, Z.; Wang, J.P.; Wurfel, U.; et al. Ethanedithiol Treatment of Solution-Processed ZnO Thin Films: Controlling the Intragap States of Electron Transporting Interlayers for Efficient and Stable Inverted Organic Photovoltaics. Adv. Energy Mater. 2015, 5, 1401606. [Google Scholar] [CrossRef]
- MacLeod, B.A.; de Villers, B.J.T.; Schulz, P.; Ndione, P.F.; Kim, H.; Giordano, A.J.; Zhu, K.; Marder, S.R.; Graham, S.; Berry, J.J.; et al. Stability of inverted organic solar cells with ZnO contact layers deposited from precursor solutions. Energy Environ. Sci. 2015, 8, 592–601. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Qi, B.Y.; Jin, Z.W.; Chi, D.; Qi, Z.; Li, Y.F.; Wang, J.Z. Perylene diimides: A thickness-insensitive cathode interlayer for high performance polymer solar cells. Energy Environ. Sci. 2014, 7, 1966–1973. [Google Scholar] [CrossRef]
- Page, Z.A.; Liu, Y.; Duzhko, V.V.; Russell, T.P.; Emrick, T. Fulleropyrrolidine interlayers: Tailoring electrodes to raise organic solar cell efficiency. Science 2014, 346, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Kan, B.; Liu, F.; Long, G.K.; Wan, X.J.; Chen, X.Q.; Zuo, Y.; Ni, W.; Zhang, H.J.; Li, M.M.; et al. Small-molecule solar cells with efficiency over 9%. Nat. Photonics 2015, 9, 35–41. [Google Scholar] [CrossRef]
- Hu, Z.C.; Zhang, K.; Huang, F.; Cao, Y. Water/alcohol soluble conjugated polymers for the interface engineering of highly efficient polymer light-emitting diodes and polymer solar cells. Chem. Commun. 2015, 51, 5572–5585. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fuentes-Hernandez, C.; Shim, J.; Meyer, J.; Giordano, A.J.; Li, H.; Winget, P.; Papadopoulos, T.; Cheun, H.; Kim, J.; et al. A Universal Method to Produce Low–Work Function Electrodes for Organic Electronics. Science 2012, 336, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.A.; Zhang, W.; Zhang, Z.; Qian, D.; Huang, Y.; Hou, J.; Li, Y. High-Performance Inverted Polymer Solar Cells with Solution-Processed Titanium Chelate as Electron-Collecting Layer on ITO Electrode. Adv. Mater. 2012, 24, 1476–1481. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-H.; Cheng, Y.-J.; Li, P.-J.; Chen, C.-H.; Dubosc, M.; Liang, R.-M.; Hsu, C.-S. Highly Efficient and Stable Inverted Polymer Solar Cells Integrated with a Cross-Linked Fullerene Material as an Interlayer. J. Am. Chem. Soc. 2010, 132, 4887–4893. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-J.; Hsieh, C.-H.; He, Y.; Hsu, C.-S.; Li, Y. Combination of Indene-C60 Bis-Adduct and Cross-Linked Fullerene Interlayer Leading to Highly Efficient Inverted Polymer Solar Cells. J. Am. Chem. Soc. 2010, 132, 17381–17383. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.H.; Zhong, C.M.; Liu, C.C.; Huang, F.; Cao, Y. Highly Efficient Inverted Polymer Solar Cells Based on an Alcohol Soluble Fullerene Derivative Interfacial Modification Material. Chem. Mater. 2012, 24, 1682–1689. [Google Scholar] [CrossRef]
- Jung, J.W.; Jo, J.W.; Jo, W.H. Enhanced Performance and Air Stability of Polymer Solar Cells by Formation of a Self-Assembled Buffer Layer from Fullerene-End-Capped Poly(ethylene glycol). Adv. Mater. 2011, 23, 1782–1787. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Z.; Chang, C.Y.; Zang, Y.; Ju, H.X.; Chueh, C.C.; Liang, P.W.; Cho, N.; Ginger, D.S.; Jen, A.K. Suppressed charge recombination in inverted organic photovoltaics via enhanced charge extraction by using a conductive fullerene electron transport layer. Adv. Mater. 2014, 26, 6262–6267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-G.; Li, H.; Qi, B.; Chi, D.; Jin, Z.; Qi, Z.; Hou, J.; Li, Y.; Wang, J. Amine group functionalized fullerene derivatives as cathode buffer layers for high performance polymer solar cells. J. Mater. Chem. A 2013, 1, 9624–9629. [Google Scholar] [CrossRef]
| DMAPA-C60 (mg/mL) | VOC (V) | JSC (mA·cm−2) | FF (%) | PCE (%) | |
|---|---|---|---|---|---|
| Best | Average a | ||||
| 1 | 0.69 | 14.2 | 57.3 | 5.61 | 5.48 |
| 1.5 | 0.69 | 14.3 | 63.4 | 6.26 | 6.1 |
| 2 | 0.70 | 15.7 | 65.7 | 7.43 | 7.27 |
| 2.5 | 0.70 | 15.4 | 61.4 | 6.62 | 6.45 |
| 3 | 0.69 | 14.8 | 62.9 | 6.42 | 6.29 |
| Structure | VOC (V) | JSC (mA·cm−2) | FF (%) | PCE (%) | RS (Ω·cm−2) | RSH (Ω·cm−2) | |
|---|---|---|---|---|---|---|---|
| Best | Average a | ||||||
| ITO | 0.69 | 12.0 | 58.5 | 4.93 | 4.59 | 7.03 | 427.3 |
| ITO/DMAPA-C60 | 0.70 | 15.7 | 65.7 | 7.43 | 7.27 | 3.50 | 580.2 |
| ITO/ZnO | 0.71 | 12.8 | 66.9 | 6.24 | 6.01 | 6.22 | 685.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Cong, H.; Yu, B.; Zhang, Z.; Zhan, X. Efficient Inverted Organic Solar Cells Based on a Fullerene Derivative-Modified Transparent Cathode. Materials 2017, 10, 1064. https://doi.org/10.3390/ma10091064
Wang Y, Cong H, Yu B, Zhang Z, Zhan X. Efficient Inverted Organic Solar Cells Based on a Fullerene Derivative-Modified Transparent Cathode. Materials. 2017; 10(9):1064. https://doi.org/10.3390/ma10091064
Chicago/Turabian StyleWang, Yifan, Hailin Cong, Bing Yu, Zhiguo Zhang, and Xiaowei Zhan. 2017. "Efficient Inverted Organic Solar Cells Based on a Fullerene Derivative-Modified Transparent Cathode" Materials 10, no. 9: 1064. https://doi.org/10.3390/ma10091064