Review on the Antimicrobial Properties of Carbon Nanostructures
Abstract
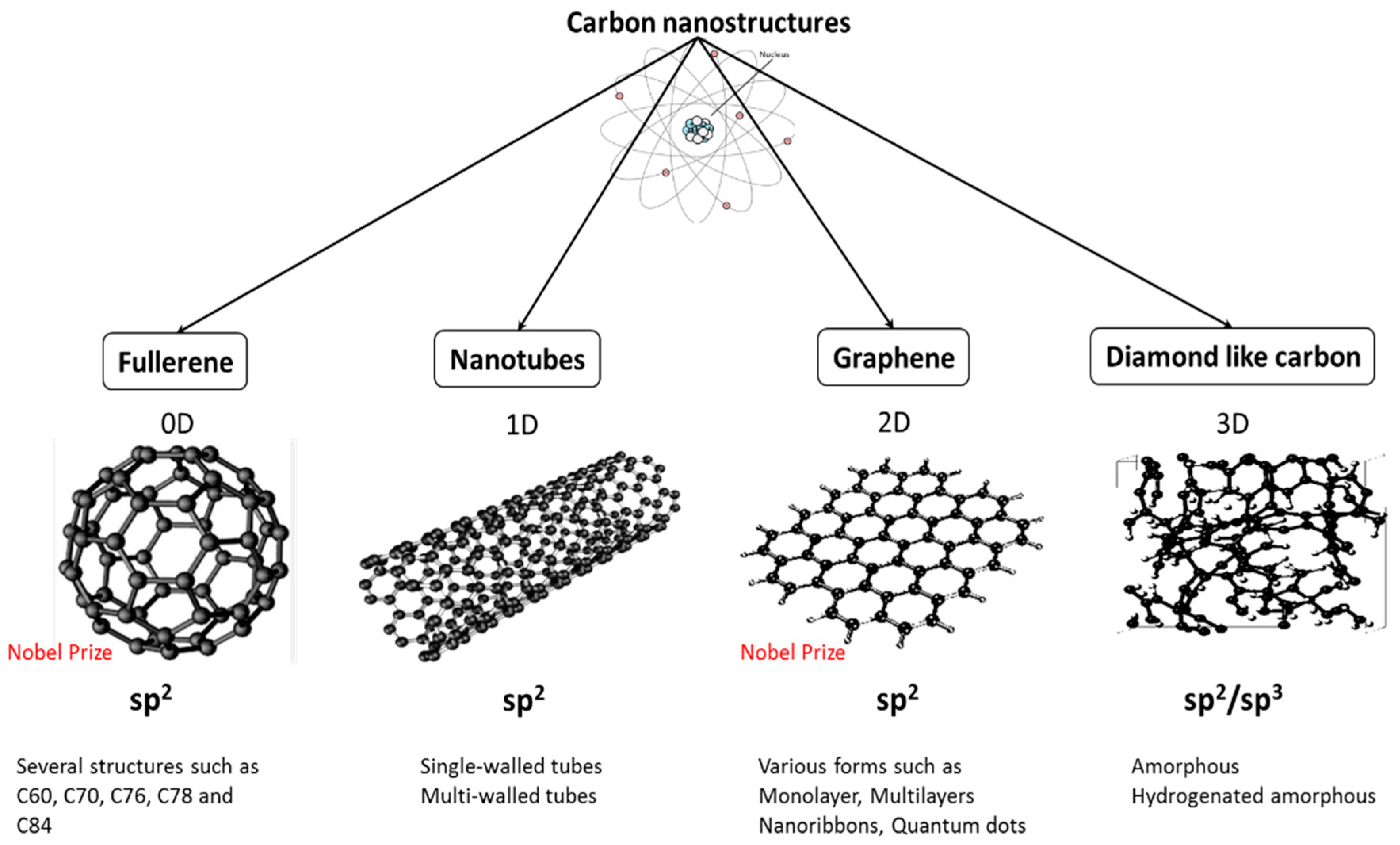
:1. Introduction
2. Antimicrobial Performance of Carbon Nanostructures
3. Fullerene
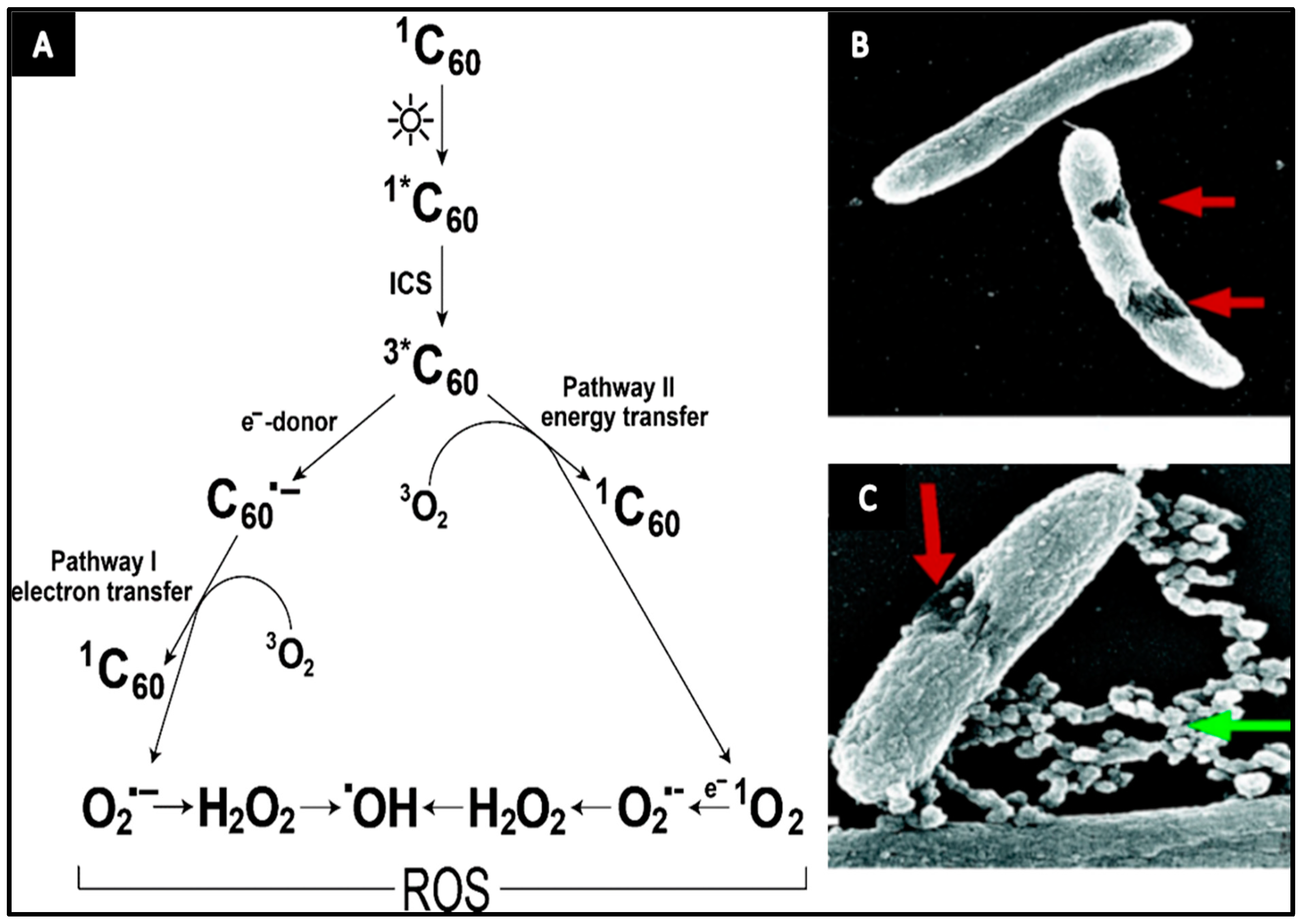
- Fullerene is capable of inducing cell membrane disruption and/or DNA cleavage in microorganisms.
- Fullerenes can inactivate microorganisms by impacting their cellular energy metabolism chain.
- Upon light illumination, fullerenes generally yield high rate of ROS that increase the antibacterial performance.
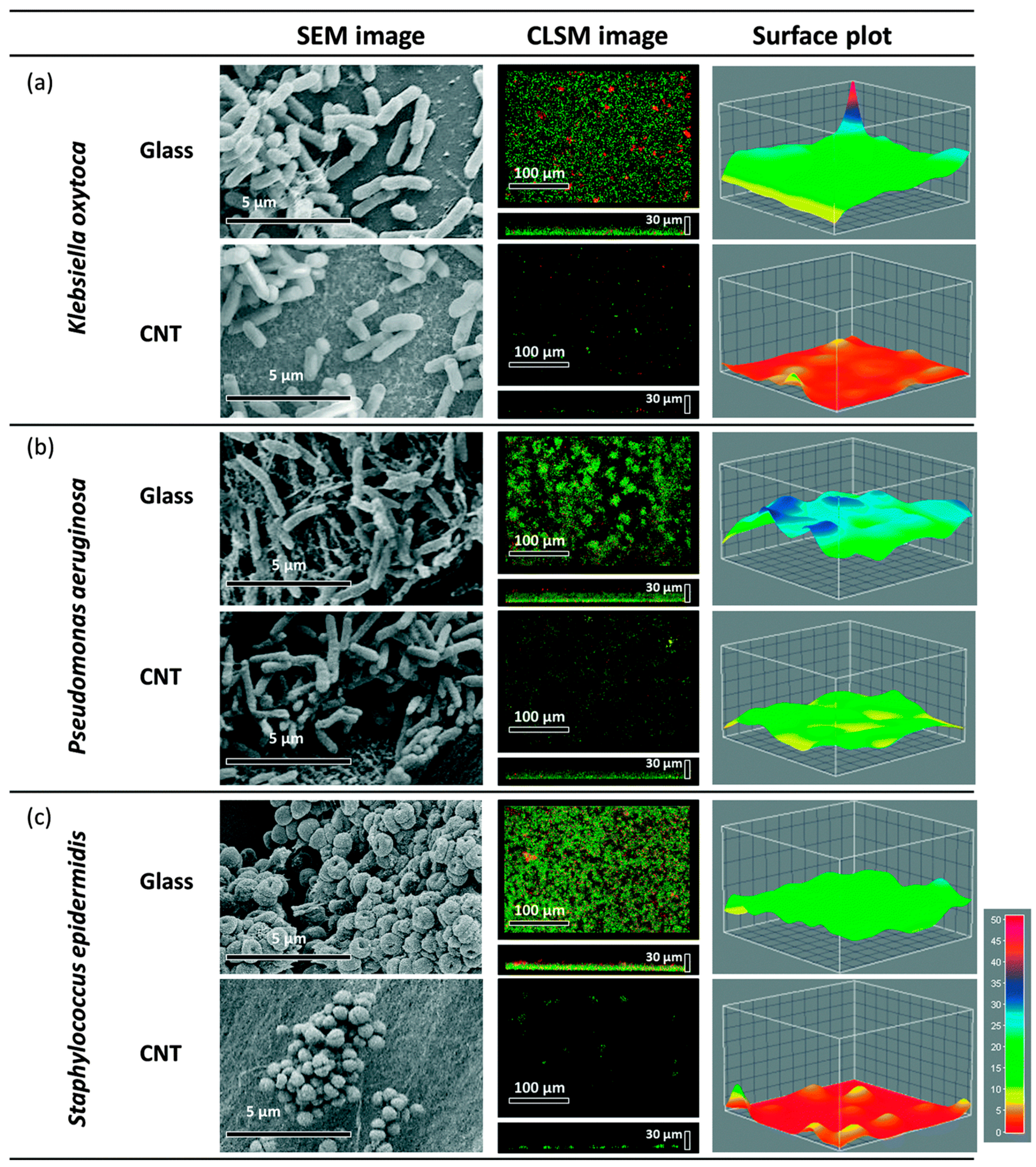
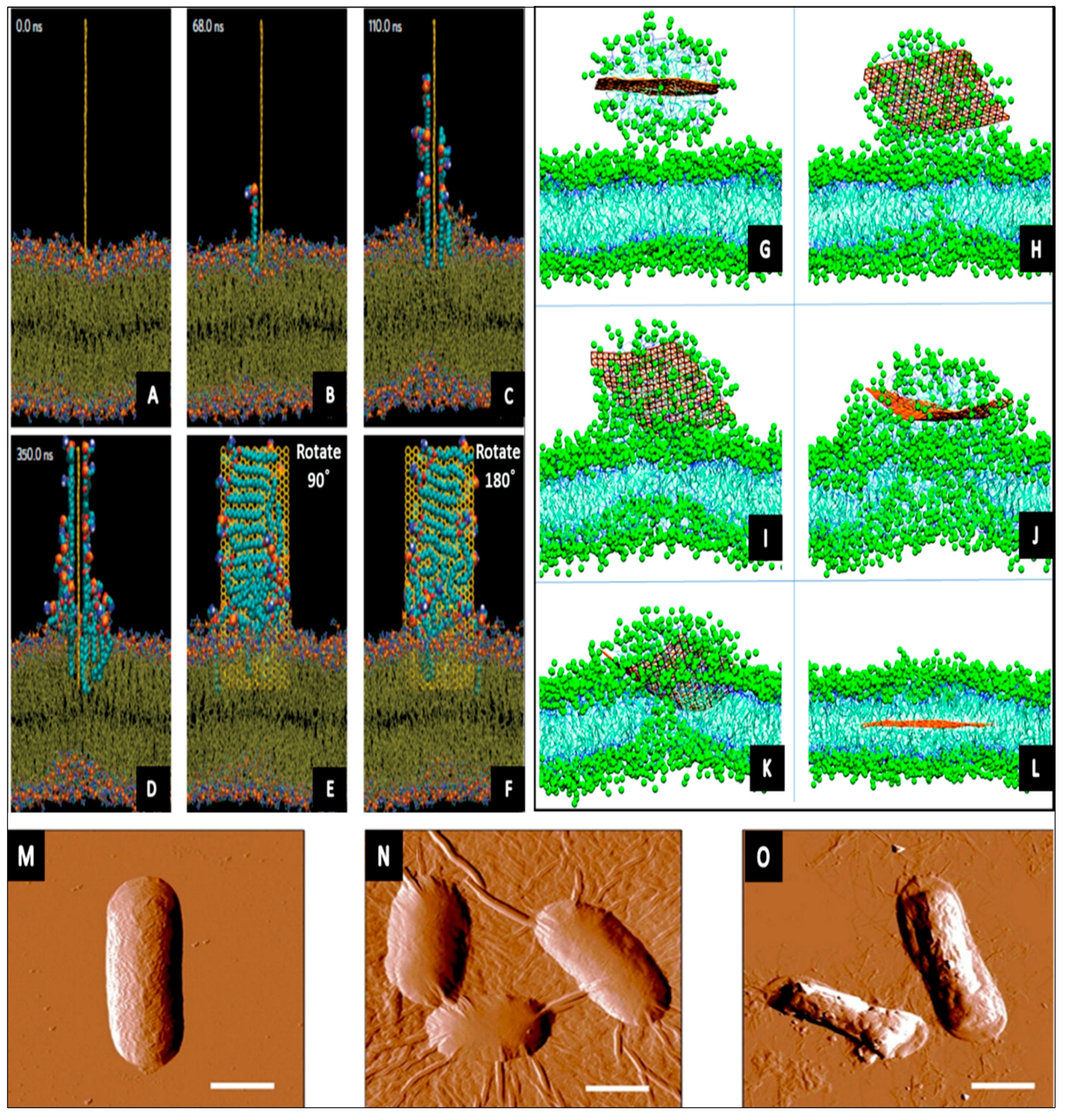
3.1. Carbon Nanotubes (CNTs)
- Disruption of membrane integrity by powerful electrostatic forces between microbial outer surface and CNTs, leading to oxidation of the membrane.
- Reactive oxygen species generation may directly harm biological molecules of bacteria and/or indirectly prompt DNA destruction.
- It is rationally possible to expect that some of antibacterial mechanisms associated with C60 can be applicable to SWCNT, in particular the bactericidal oxidative stress, since they are both are made of pure carbon and have similar diameters [116].
3.2. Graphene
- Serve cutting/damaging to the cell membrane.
- Destructive extraction of phospholipids from lipid membranes.
- Oxidative stress through ROS generation.
- Oxidative stress independent-ROS generation by charge transfer phenomena.
- Separating microorganisms from their microenvironment.
3.3. Diamond-Like Carbon (DLC)
- Strong hydrophobicity of DLC may cause variations of the bacterial cell membrane.
- DLC films reveal antibiofouling/antibacterial based on their surface profile.
- There is almost a specific property for each DLC film, depending on the fabrication conditions.
- Sp3/sp2 ratio play often important role in DLC biological activities.
4. Carbon Nanostructures Production Challenges
5. Conclusions
6. Future Outlook
Acknowledgments
Conflicts of Interest
References
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G. Graphene quantum dots derived from carbon fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Hahm, M.G.; Leela Mohana Reddy, A.; Cole, D.P.; Rivera, M.; Vento, J.A.; Nam, J.; Jung, H.Y.; Kim, Y.L.; Narayanan, N.T.; Hashim, D.P. Carbon nanotube—Nanocup hybrid structures for high power supercapacitor applications. Nano Lett. 2012, 12, 5616–5621. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Fal, V.; Colombo, L.; Gellert, P.; Schwab, M.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Shi, Y.; Shi, W.; Lu, G.; Huang, X.; Yan, Q.; Zhang, Q.; Zhang, H. Preparation of novel 3D graphene networks for supercapacitor applications. Small 2011, 7, 3163–3168. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, X.; Liu, Y.; Huang, H.; Lian, S.; Lee, S.-T.; Kang, Z. One-step ultrasonic synthesis of water-soluble carbon nanoparticles with excellent photoluminescent properties. Carbon 2011, 49, 605–609. [Google Scholar] [CrossRef]
- Cao, Q.; Rogers, J.A. Ultrathin Films of Single-Walled Carbon Nanotubes for Electronics and Sensors: A Review of Fundamental and Applied Aspects. Adv. Mater. 2009, 21, 29–53. [Google Scholar] [CrossRef]
- Choi, W.; Lahiri, I.; Seelaboyina, R.; Kang, Y.S. Synthesis of Graphene and Its Applications: A Review. Crit. Rev. Solid State Mater. Sci. 2010, 35, 52–71. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, X.; Zhang, M. Carbon nanotubes for thin film transistor: Fabrication, properties, and applications. J. Nanomater. 2013, 2013, 1–16. [Google Scholar] [CrossRef]
- Hecht, D.S.; Hu, L.; Irvin, G. Emerging Transparent Electrodes Based on Thin Films of Carbon Nanotubes, Graphene, and Metallic Nanostructures. Adv. Mater. 2011, 23, 1482–1513. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhi, L.; Müllen, K. Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 2008, 8, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.H.; Han, Z.J.; Kumar, S.; Ostrikov, K. Structure-Controlled, Vertical Graphene-Based, Binder-Free Electrodes from Plasma-Reformed Butter Enhance Supercapacitor Performance. Adv. Energy Mater. 2013, 3, 1316–1323. [Google Scholar] [CrossRef]
- Seo, D.H.; Pineda, S.; Yick, S.; Bell, J.; Han, Z.J.; Ostrikov, K.K. Plasma-enabled sustainable elemental lifecycles: Honeycomb-derived graphenes for next-generation biosensors and supercapacitors. Green Chem. 2015, 17, 2164–2171. [Google Scholar] [CrossRef]
- Stancu, E.C.; Stanciuc, A.-M.; Vizireanu, S.; Luculescu, C.; Moldovan, L.; Achour, A.; Dinescu, G. Plasma functionalization of carbon nanowalls and its effect on attachment of fibroblast-like cells. J. Phys. D Appl. Phys. 2014, 47, 1–10. [Google Scholar] [CrossRef]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.-T.; Liu, Z. Graphene in mice: Ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Star, A.; Vidal, S. Sweet carbon nanostructures: Carbohydrate conjugates with carbon nanotubes and graphene and their applications. Chem. Soc. Rev. 2013, 42, 4532–4542. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Mennati, A.; Jafari, S.; Khezri, K.; Adibkia, K. Antimicrobial activity of carbon-based nanoparticles. Adv. Pharm. Bull. 2015, 5, 19–23. [Google Scholar]
- Ji, H.; Sun, H.; Qu, X. Antibacterial applications of graphene-based nanomaterials: Recent achievements and challenges. Adv. Drug Deliv. Rev. 2016, 105, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Kholmanov, I.N.; Stoller, M.D.; Edgeworth, J.; Lee, W.H.; Li, H.; Lee, J.; Barnhart, C.; Potts, J.R.; Piner, R.; Akinwande, D. Nanostructured hybrid transparent conductive films with antibacterial properties. ACS Nano 2012, 6, 5157–5163. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Lekshmi, G.; Ostrikov, K.; Lussini, V.; Blinco, J.; Mohandas, M.; Vasilev, K.; Bottle, S.; Bazaka, K.; Ostrikov, K. Synergic bactericidal effects of reduced graphene oxide and silver nanoparticles against Gram-positive and Gram-negative bacteria. Sci. Rep. 2017. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5431540/ (accessed on 8 September 2017). [CrossRef] [PubMed]
- Li, J.; Wang, G.; Zhu, H.; Zhang, M.; Zheng, X.; Di, Z.; Liu, X.; Wang, X. Antibacterial activity of large-area monolayer graphene film manipulated by charge transfer. Sci. Rep. 2014, 4, 4359. Available online: http://www.nature.com/articles/srep04359 (accessed on 8 September 2017). [CrossRef] [PubMed]
- Bitounis, D.; Ali-Boucetta, H.; Hong, B.H.; Min, D.H.; Kostarelos, K. Prospects and challenges of graphene in biomedical applications. Adv. Mater. 2013, 25, 2258–2268. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, L.; Yan, M.; Yu, J. Carbon Nanostructures in Biology and Medicine. J. Mater. Chem. B 2017, 5, 6437–6450. [Google Scholar] [CrossRef]
- Goncalves, G.; Marques, P.; Vila, M. Graphene-Based Materials in Health and Environment; Springer: Berlin, Germany, 2017. [Google Scholar]
- Mocan, T.; Matea, C.T.; Pop, T.; Mosteanu, O.; Buzoianu, A.D.; Suciu, S.; Puia, C.; Zdrehus, C.; Iancu, C.; Mocan, L. Carbon nanotubes as anti-bacterial agents. Cell. Mol. Life Sci. 2017, 74, 3467–3479. [Google Scholar] [CrossRef] [PubMed]
- Botta, L.; Bizzarri, B.M.; Crucianelli, M.; Saladino, R. Advances in biotechnological synthetic applications of carbon nanostructured systems. J. Mater. Chem. B 2017, 5, 6490–9510. [Google Scholar] [CrossRef]
- V Kleandrova, V.; Luan, F.; Speck-Planche, A.; Cordeiro, M. Review of Structures Containing Fullerene-C60 for Delivery of Antibacterial Agents. Multitasking Model for Computational Assessment of Safety Profiles. Curr. Bioinform. 2015, 10, 565–578. [Google Scholar] [CrossRef]
- Szunerits, S.; Barras, A.; Boukherroub, R. Antibacterial applications of nanodiamonds. Int. J. Environ. Res. Public Health 2016, 13, 413. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ma, Z.; Liu, G.; Wei, H.; Xie, X. Antibacterial activity of cationic cyclen-functionalized fullerene derivatives: Membrane stress. Dig. J. Nanomater. Biostruct. (DJNB) 2016, 11, 753–761. [Google Scholar]
- Moor, K.J.; Osuji, C.O.; Kim, J.-H. Antimicrobial photodynamic therapy with fulleropyrrolidine: Photoinactivation mechanism of Staphylococcus aureus, in vitro and in vivo studies. Appl. Microbiol. Biotechnol. 2015, 99, 4031–4043. [Google Scholar]
- Moor, K.J.; Osuji, C.O.; Kim, J.-H. Dual-Functionality Fullerene and Silver Nanoparticle Antimicrobial Composites via Block Copolymer Templates. ACS Appl. Mater. Interfaces 2016, 8, 33583–33591. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial effects of carbon nanotubes: Size does matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef] [PubMed]
- Aslan, S.; Loebick, C.Z.; Kang, S.; Elimelech, M.; Pfefferle, L.D.; Van Tassel, P.R. Antimicrobial biomaterials based on carbon nanotubes dispersed in poly (lactic-co-glycolic acid). Nanoscale 2010, 2, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.R.; Yang, L. Inactivation of bacterial pathogens by carbon nanotubes in suspensions. Langmuir 2009, 25, 3003–3012. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Dayem, A.A.; Eppakayala, V.; Kim, J.-H. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed. 2012, 7, 5901–5914. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Lv, M.; Xiu, P.; Huynh, T.; Zhang, M.; Castelli, M.; Liu, Z.; Huang, Q.; Fan, C.; Fang, H. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nanotechnol. 2013, 8, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Deng, B.; Chen, G.; Lei, B.; Hua, H.; Peng, H.; Yan, Z. Large-area chemical vapor deposition-grown monolayer graphene-wrapped silver nanowires for broad-spectrum and robust antimicrobial coating. Nano Res. 2016, 9, 963–973. [Google Scholar] [CrossRef]
- Myllymaa, K.; Levon, J.; Tiainen, V.-M.; Myllymaa, S.; Soininen, A.; Korhonen, H.; Kaivosoja, E.; Lappalainen, R.; Konttinen, Y.T. Formation and retention of staphylococcal biofilms on DLC and its hybrids compared to metals used as biomaterials. Colloids Surf. B Biointerfaces 2013, 101, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.N.; Gibson, D.; MacKay, W.G.; Reid, S.; Williams, C.; Birney, R. Investigation of the antimicrobial properties of modified multilayer diamond-like carbon coatings on 316 stainless steel. Surf. Coat. Technol. 2017, 314, 72–78. [Google Scholar] [CrossRef]
- Su, X.; Zhao, Q.; Wang, S.; Bendavid, A. Modification of diamond-like carbon coatings with fluorine to reduce biofouling adhesion. Surf. Coat. Technol. 2010, 204, 2454–2458. [Google Scholar] [CrossRef]
- Honglertkongsakul, K.; May, P.W.; Paosawatyanyong, B. Electrical and optical properties of diamond-like carbon films deposited by pulsed laser ablation. Diam. Relat. Mater. 2010, 19, 999–1002. [Google Scholar] [CrossRef]
- Skariyachan, S.; Parveen, A.; Garka, S. Nanoparticle Fullerene (C60) demonstrated stable binding with antibacterial potential towards probable targets of drug resistant Salmonella typhi—A computational perspective and in vitro investigation. J. Biomol. Struct. Dyn. 2016, 34, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Dostalova, S.; Moulick, A.; Milosavljevic, V.; Guran, R.; Kominkova, M.; Cihalova, K.; Heger, Z.; Blazkova, L.; Kopel, P.; Hynek, D.; et al. Antiviral activity of fullerene C60 nanocrystals modified with derivatives of anionic antimicrobial peptide maximin H5. Mon. Chem. Chem. Mon. 2016, 147, 905–918. [Google Scholar] [CrossRef]
- Sharma, S.K.; Chiang, L.Y.; Hamblin, M.R. Photodynamic therapy with fullerenes in vivo: Reality or a dream? Nanomedicine 2011, 6, 1813–1825. [Google Scholar] [CrossRef] [PubMed]
- Rondags, A.; Yuen, W.Y.; Jonkman, M.F.; Horváth, B. Fullerene C60 with cytoprotective and cytotoxic potential: Prospects as a novel treatment agent in Dermatology? Exp. Dermatol. 2017, 26, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Naddeo, J.; Ratti, M.; O'Malley, S.M.; Griepenburg, J.C.; Bubb, D.M.; Klein, E.A. Antibacterial Properties of Nanoparticles: A Comparative Review of Chemically Synthesized and Laser-Generated Particles. Adv. Sci. Eng. Med. 2015, 7, 1044–1057. [Google Scholar] [CrossRef]
- Boonstra, J.; Post, J.A. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene 2004, 337, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, M.; Bazaka, K.; Ostrikov, K. Pro-apoptotic NOXA is implicated in atmospheric-pressure plasma-induced melanoma cell death. J. Phys. D Appl. Phys. 2015, 48, 464002. [Google Scholar] [CrossRef]
- Ishaq, M.; Bazaka, K.; Ostrikov, K. Intracellular effects of atmospheric-pressure plasmas on melanoma cancer cells. Phys. Plasmas 2015, 22, 122003. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Zhou, R.; Zhang, X.; Zhuang, J.; Yang, S.; Bazaka, K.; Ostrikov, K. Effects of atmospheric-pressure N2, He, Air, and O2 microplasmas on mung bean seed germination and seedling growth. Sci. Rep. 2016, 6, 32603. Available online: https://www.nature.com/articles/srep32603 (accessed on 8 September 2017). [CrossRef] [PubMed]
- Zhou, R.; Zhou, R.; Zhang, X.; Li, J.; Wang, X.; Chen, Q.; Yang, S.; Chen, Z.; Bazaka, K.; Ostrikov, K. Synergistic effect of atmospheric pressure plasma and TiO2 photocatalysis on inactivation of Escherichia coli cells in aqueous media. Sci. Rep. 2016, 6, 39552. Available online: http://www.nature.com/articles/srep39552?WT.feed_name=subjects_physical-sciences (accessed on 8 September 2017). [CrossRef] [PubMed]
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.-J.; Quigg, A.; Santschi, P.H.; Sigg, L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Markovic, Z.; Trajkovic, V. Biomedical potential of the reactive oxygen species generation and quenching by fullerenes (C60). Biomaterials 2008, 29, 3561–3573. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Lyon, D.Y.; Wiesner, M.R.; Dong, J. Alvarez. Effect of a Fullerene Water Suspension on Bacterial Phospholipids and Membrane Phase Behavior. Environ. Sci. Technol. 2007, 41, 2636–2642. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.J.; Ashcroft, J.M.; Chen, D.; Min, G.; Kim, C.-H.; Murkhejee, B.; Larabell, C.; Keasling, J.D.; Chen, F.F. Charge-Associated Effects of Fullerene Derivatives on Microbial Structural Integrity and Central Metabolism. Nano Lett. 2007, 7, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Mashino, T.; Nishikawa, D.; Takahashi, K.; Usui, N.; Yamori, T.; Seki, M.; Endo, T.; Mochizuki, M. Antibacterial and antiproliferative activity of cationic fullerene derivatives. Bioorg. Med. Chem. Lett. 2003, 13, 4395–4397. [Google Scholar] [CrossRef] [PubMed]
- Mashino, T.; Usui, N.; Okuda, K.; Hirota, T.; Mochizuki, M. Respiratory chain inhibition by fullerene derivatives: Hydrogen peroxide production caused by fullerene derivatives and a respiratory chain system. Bioorg. Med. Chem. 2003, 11, 1433–1438. [Google Scholar] [CrossRef]
- Hsu, P.-C.; Jefferies, D.; Khalid, S. Molecular Dynamics Simulations Predict the Pathways via Which Pristine Fullerenes Penetrate Bacterial Membranes. J. Phys. Chem. B 2016, 120, 11170–11179. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Veerapandian, M.; Zhang, L.-H.; Yun, K.; Kim, S.J. Antibacterial efficiency of graphene nanosheets against pathogenic bacteria via lipid peroxidation. J. Phys. Chem. C 2012, 116, 17280–17287. [Google Scholar] [CrossRef]
- Yang, X.; Ebrahimi, A.; Li, J.; Cui, Q. Fullerene-biomolecule conjugates and their biomedicinal applications. Int. J. Nanomed. 2014, 9, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, M.; Dai, T.; Sperandio, F.F.; Huang, Y.-Y.; Xuan, Y.; Chiang, L.Y.; Hamblin, M.R. Antimicrobial photodynamic therapy with decacationic monoadducts and bisadducts of [70] fullerene: In vitro and in vivo studies. Nanomedicine 2014, 9, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dai, T.; Wang, M.; Vecchio, D.; Chiang, L.Y.; Hamblin, M.R. Potentiation of antimicrobial photodynamic inactivation mediated by a cationic fullerene by added iodide: In vitro and in vivo studies. Nanomedicine 2015, 10, 603–614. [Google Scholar] [CrossRef] [PubMed]
- McCluskey, D.M.; Smith, T.N.; Madasu, P.K.; Coumbe, C.E.; Mackey, M.A.; Fulmer, P.A.; Wynne, J.H.; Stevenson, S.; Phillips, J.P. Evidence for Singlet-Oxygen Generation and Biocidal Activity in Photoresponsive Metallic Nitride Fullerene—Polymer Adhesive Films. ACS Appl. Mater.Interfaces 2009, 1, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A. Advances in Polymer/Fullerene Nanocomposite: A Review on Essential Features and Applications. Polym. Plast. Technol. Eng. 2017, 56, 594–605. [Google Scholar] [CrossRef]
- Zhou, G.; Li, Y.; Xiao, W.; Zhang, L.; Zuo, Y.; Xue, J.; Jansen, J.A. Synthesis, characterization, and antibacterial activities of a novel nanohydroxyapatite/zinc oxide complex. J. Biomed. Mater. Res. Part A 2008, 85, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Lyon, D.Y.; Alvarez, P.J. Fullerene water suspension (nC60) exerts antibacterial effects via ROS-independent protein oxidation. Environ. Sci. Technol. 2008, 42, 8127–8132. [Google Scholar] [CrossRef] [PubMed]
- Lyon, D.Y.; Brunet, L.; Hinkal, G.W.; Wiesner, M.R.; Alvarez, P.J. Antibacterial activity of fullerene water suspensions (nC60) is not due to ROS-mediated damage. Nano Lett. 2008, 8, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Kai, Y.; Komazawa, Y.; Miyajima, A.; Miyata, N.; Yamakoshi, Y. [60] Fullerene as a novel photoinduced antibiotic. Fuller. Nanotub. Carbon Nanostruct. 2003, 11, 79–87. [Google Scholar] [CrossRef]
- Moor, K.J.; Snow, S.D.; Kim, J.-H. Differential photoactivity of aqueous [C60] and [C70] fullerene aggregates. Environ. Sci. Technol. 2015, 49, 5990–5998. [Google Scholar] [CrossRef] [PubMed]
- Tegos, G.P.; Demidova, T.N.; Arcila-Lopez, D.; Lee, H.; Wharton, T.; Gali, H.; Hamblin, M.R. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem. Biol. 2005, 12, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Badireddy, A.R.; Hotze, E.M.; Chellam, S.; Alvarez, P.; Wiesner, M.R. Inactivation of Bacteriophages via Photosensitization of Fullerol Nanoparticles. Environ. Sci. Technol. 2007, 41, 6627–6632. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Agrawal, T.; Khan, U.; Gupta, G.K.; Rai, V.; Huang, Y.-Y.; Hamblin, M.R. Antimicrobial photodynamic inactivation in nanomedicine: Small light strides against bad bugs. Nanomedicine 2015, 10, 2379–2404. [Google Scholar] [CrossRef] [PubMed]
- Lyon, D.Y.; Adams, L.K.; Falkner, J.C.; Alvarez, P.J. Antibacterial activity of fullerene water suspensions: Effects of preparation method and particle size. Environ. Sci. Technol. 2006, 40, 4360–4366. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Son, Y.; Yoon, T.K.; Kim, S.; Kim, W. The effect of multi-walled carbon nanotubes on soil microbial activity. Ecotoxicol. Environ. Saf. 2011, 74, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Son, Y.; Yoon, T.K.; Kang, Y.J.; Kim, W.; Chung, H. High concentrations of single-walled carbon nanotubes lower soil enzyme activity and microbial biomass. Ecotoxicol. Environ. Saf. 2013, 88, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, J.; Akinoglu, E.M.; Wirtz, D.C.; Hoerauf, A.; Bekeredjian-Ding, I.; Jepsen, S.; Haddouti, E.-M.; Limmer, A.; Giersig, M. Long-term release of antibiotics by carbon nanotube-coated titanium alloy surfaces diminish biofilm formation by staphylococcus epidermidis. Nanomedicine 2017, 13, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Oyelami, A.O.; Semple, K.T. Impact of carbon nanomaterials on microbial activity in soil. Soil Biol. Biochem. 2015, 86, 172–180. [Google Scholar] [CrossRef]
- Jackson, P.; Jacobsen, N.R.; Baun, A.; Birkedal, R.; Kühnel, D.; Jensen, K.A.; Vogel, U.; Wallin, H. Bioaccumulation and ecotoxicity of carbon nanotubes. Chem. Cent. J. 2013, 7, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Wang, B.; Gao, D.; Guan, M.; Zheng, L.; Ouyang, H.; Chai, Z.; Zhao, Y.; Feng, W. Broad-Spectrum Antibacterial Activity of Carbon Nanotubes to Human Gut Bacteria. Small 2013, 9, 2735–2746. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Mamouni, J.; Tang, Y.; Yang, L. Antimicrobial activity of single-walled carbon nanotubes: Length effect. Langmuir 2010, 26, 16013–16019. [Google Scholar] [CrossRef] [PubMed]
- Johnston, H.J.; Hutchison, G.R.; Christensen, F.M.; Peters, S.; Hankin, S.; Aschberger, K.; Stone, V. A critical review of the biological mechanisms underlying the in vivo and in vitro toxicity of carbon nanotubes: The contribution of physico-chemical characteristics. Nanotoxicology 2010, 4, 207–246. [Google Scholar] [CrossRef] [PubMed]
- Saifuddin, N.; Raziah, A.; Junizah, A. Carbon nanotubes: A review on structure and their interaction with proteins. J. Chem. 2012, 2013. Available online: https://www.hindawi.com/journals/jchem/2013/676815/ (accessed on 8 September 2017). [CrossRef]
- Smith, S.C.; Rodrigues, D.F. Carbon-based nanomaterials for removal of chemical and biological contaminants from water: A review of mechanisms and applications. Carbon 2015, 91, 122–143. [Google Scholar] [CrossRef]
- Rhiem, S. End of Life Cycle Assessment for Carbon Nanotube (CNT) Containing Composites: Release of CNT and Ecotoxicological Consequences. Hochschulbibliothek der Rheinisch-Westfälischen Technischen Hochschule Aachen. 2014. Available online: http://publications.rwth-aachen.de/record/444979/files/5137.pdf (accessed on 8 September 2017).
- Xu, H.; Zhang, S.; Anlage, S.M.; Hu, L.; Grüner, G. Frequency-and electric-field-dependent conductivity of single-walled carbon nanotube networks of varying density. Phys. Rev. B 2008, 77, 075418. Available online: http://www.physics.ucla.edu/research/Gruner_Group/pubs/pdf/pr_freq_depend.pdf (accessed on 8 September 2017). [CrossRef]
- Skákalová, V.; Vretenár, V.; Kopera, Ľ.; Kotrusz, P.; Mangler, C.; Meško, M.; Meyer, J.C.; Hulman, M. Electronic transport in composites of graphite oxide with carbon nanotubes. Carbon 2014, 72, 224–232. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Zodrow, K.R.; Kang, S.; Elimelech, M. Electronic-structure-dependent bacterial cytotoxicity of single-walled carbon nanotubes. ACS Nano 2010, 4, 5471–5479. [Google Scholar] [CrossRef] [PubMed]
- Smotlacha, J.; Pincak, R. Electronic Properties of Carbon Nanostructures. arXiv 2016, arXiv:1612.01433. [Google Scholar]
- Yang, S.; Huang, Y.C.; Luo, C.H.; Lin, Y.C.; Huang, J.W.; Chuang, C.P.J.; Chen, C.J.; Fang, W.; Chuang, C.Y. Inactivation Efficiency of Bioaerosols Using Carbon Nanotube Plasma. CLEAN Soil Air Water 2011, 39, 201–205. [Google Scholar] [CrossRef]
- Lolla, D.; Lolla, M.; Abutaleb, A.; Shin, H.U.; Reneker, D.H.; Chase, G.G. Fabrication, Polarization of Electrospun Polyvinylidene Fluoride Electret Fibers and Effect on Capturing Nanoscale Solid Aerosols. Materials 2016, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Kranz, M.; Kim, S.; Allen, M. Micropatternable elastic electrets based on a PDMS/carbon nanotube composite. J. Micromech. Microeng. 2010, 20, 104003-1–104003-7. [Google Scholar] [CrossRef]
- Bazaka, K.; Crawford, R.J.; Nazarenko, E.L.; Ivanova, E.P. Bacterial extracellular polysaccharides. In Bacterial Adhesion; Springer: Dordrecht, The Netherlands; Houten, The Netherlands, 2011; pp. 213–226. [Google Scholar]
- Bazaka, K.; Jacob, M.V.; Crawford, R.J.; Ivanova, E.P. Efficient surface modification of biomaterial to prevent biofilm formation and the attachment of microorganisms. Appl. Microbiol. Biotechnol. 2012, 95, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Bazaka, O.; Bazaka, K. Surface modification of biomaterials for biofilm control. Biomater. Med. Device Assoc. Infect. 2014, 6, 103–132. [Google Scholar] [CrossRef]
- Ahmed, F.; Santos, C.M.; Vergara, R.A.M.V.; Tria, M.C.R.; Advincula, R.; Rodrigues, D.F. Antimicrobial applications of electroactive PVK-SWNT nanocomposites. Environ. Sci. Technol. 2012, 46, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Yang, L. Inhibitory effects of single-walled carbon nanotubes on biofilm formation from Bacillus anthracis spores. Biofouling 2014, 30, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.F.; Elimelech, M. Toxic effects of single-walled carbon nanotubes in the development of E. coli biofilm. Environ. Sci. Technol. 2010, 44, 4583–4589. [Google Scholar] [CrossRef] [PubMed]
- Malek, I.; Schaber, C.; Heinlein, T.; Schneider, J.; Gorb, S.; Schmitz, R. Vertically aligned multi walled carbon nanotubes prevent biofilm formation of medically relevant bacteria. J. Mater. Chem. B 2016, 4, 5228–5235. [Google Scholar] [CrossRef]
- Yick, S.; Mai-Prochnow, A.; Levchenko, I.; Fang, J.; Bull, M.K.; Bradbury, M.; Murphy, A.B.; Ostrikov, K.K. The effects of plasma treatment on bacterial biofilm formation on vertically-aligned carbon nanotube arrays. RSC Adv. 2015, 5, 5142–5148. [Google Scholar] [CrossRef]
- Chae, S.-R.; Watanabe, Y.; Wiesner, M.R. Comparative photochemical reactivity of spherical and tubular fullerene nanoparticles in water under ultraviolet (UV) irradiation. Water Res. 2011, 45, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Abdolahad, M.; Abdi, Y.; Mohajerzadeh, S. Synthesis of titania/carbon nanotube heterojunction arrays for photoinactivation of E. coli in visible light irradiation. Carbon 2009, 47, 3280–3287. [Google Scholar] [CrossRef]
- Darbari, S.; Abdi, Y.; Haghighi, F.; Mohajerzadeh, S.; Haghighi, N. Investigating the antifungal activity of TiO2 nanoparticles deposited on branched carbon nanotube arrays. J. Phys. D Appl. Phys. 2011, 44, 245401. [Google Scholar] [CrossRef]
- Akhavan, O.; Abdolahad, M.; Abdi, Y.; Mohajerzadeh, S. Silver nanoparticles within vertically aligned multi-wall carbon nanotubes with open tips for antibacterial purposes. J. Mater. Chem. 2011, 21, 387–393. [Google Scholar] [CrossRef]
- Egerton, R.; Li, P.; Malac, M. Radiation damage in the TEM and SEM. Micron 2004, 35, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Crespi, V.H.; Chopra, N.G.; Cohen, M.L.; Zettl, A.; Louie, S.G. Anisotropic electron-beam damage and the collapse of carbon nanotubes. Phys. Rev. B 1996, 54, 5927–5931. [Google Scholar] [CrossRef]
- Lolla, D.; Gorse, J.; Kisielowski, C.; Miao, J.; Taylor, P.L.; Chase, G.G.; Reneker, D.H. Polyvinylidene fluoride molecules in nanofibers, imaged at atomic scale by aberration corrected electron microscopy. Nanoscale 2016, 8, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Fu, C.; Lu, Q. Advanced technology for functionalization of carbon nanotubes. Prog. Nat. Sci. 2009, 19, 801–810. [Google Scholar] [CrossRef]
- Gu, L.; Luo, P.G.; Wang, H.; Meziani, M.J.; Lin, Y.; Veca, L.M.; Cao, L.; Lu, F.; Wang, X.; Quinn, R.A. Single-walled carbon nanotube as a unique scaffold for the multivalent display of sugars. Biomacromolecules 2008, 9, 2408–2418. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Azimirad, R.; Safa, S. Functionalized carbon nanotubes in ZnO thin films for photoinactivation of bacteria. Mater. Chem. Phys. 2011, 130, 598–602. [Google Scholar] [CrossRef]
- Zardini, H.Z.; Davarpanah, M.; Shanbedi, M.; Amiri, A.; Maghrebi, M.; Ebrahimi, L. Microbial toxicity of ethanolamines—Multiwalled carbon nanotubes. J. Biomed. Mater. Res. Part A 2014, 102, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Varshney, K. Carbon nanotubes: A review on synthesis, properties and applications. Int. J. Eng.Res. 2014, 2, 660–677. [Google Scholar]
- Upadhyayula, V.K.K.; Gadhamshetty, V. Appreciating the role of carbon nanotube composites in preventing biofouling and promoting biofilms on material surfaces in environmental engineering: A review. Biotechnol. Adv. 2010, 28, 802–816. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.R.; Waiser, M.J.; Swerhone, G.D.W.; Roy, J.; Tumber, V.; Paule, A.; Hitchcock, A.P.; Dynes, J.J.; Korber, D.R. Effects of fullerene (C60), multi-wall carbon nanotubes (MWCNT), single wall carbon nanotubes (SWCNT) and hydroxyl and carboxyl modified single wall carbon nanotubes on riverine microbial communities. Environ. Sci. Pollut. Res. 2016, 23, 10090–10102. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wei, L.; Hao, L.; Fang, N.; Chang, M.W.; Xu, R.; Yang, Y.; Chen, Y. Sharper and faster “nano darts” kill more bacteria: A study of antibacterial activity of individually dispersed pristine single-walled carbon nanotube. ACS Nano 2009, 3, 3891–3902. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, J.; Hou, J.; Zhang, Y.; Liu, J.; Van der Bruggen, B. Graphene-based antimicrobial polymeric membranes: A review. J. Mater. Chem. A 2017, 5, 6776–6793. [Google Scholar] [CrossRef]
- Zhou, R.; Gao, H. Cytotoxicity of graphene: Recent advances and future perspective. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 452–474. [Google Scholar] [CrossRef] [PubMed]
- Hegab, H.M.; ElMekawy, A.; Zou, L.; Mulcahy, D.; Saint, C.P.; Ginic-Markovic, M. The controversial antibacterial activity of graphene-based materials. Carbon 2016, 105, 362–376. [Google Scholar] [CrossRef]
- Titov, A.V.; Král, P.; Pearson, R. Sandwiched graphene—Membrane superstructures. ACS Nano 2009, 4, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Dallavalle, M.; Calvaresi, M.; Bottoni, A.; Melle-Franco, M.; Zerbetto, F. Graphene can wreak havoc with cell membranes. ACS Appl. Mater. Interfaces 2015, 7, 4406–4414. [Google Scholar] [CrossRef] [PubMed]
- De Leon, A.C.C. On the antibacterial mechanism of graphene oxide (GO) Langmuir—Blodgett films. Chem. Commun. 2015, 51, 2886–2889. [Google Scholar]
- Hui, L.; Piao, J.-G.; Auletta, J.; Hu, K.; Zhu, Y.; Meyer, T.; Liu, H.; Yang, L. Availability of the basal planes of graphene oxide determines whether it is antibacterial. ACS Appl. Mater. Interfaces 2014, 6, 13183–13190. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E.; Esfandiar, A. Wrapping bacteria by graphene nanosheets for isolation from environment, reactivation by sonication, and inactivation by near-infrared irradiation. J. Phys. Chem. B 2011, 115, 6279–6288. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O. Photocatalytic reduction of graphene oxides hybridized by ZnO nanoparticles in ethanol. Carbon 2011, 49, 11–18. [Google Scholar] [CrossRef]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological interactions of graphene-family nanomaterials: An interdisciplinary review. Chem. Res. Toxicol. 2011, 25, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Perreault, F.; De Faria, A.F.; Nejati, S.; Elimelech, M. Antimicrobial properties of graphene oxide nanosheets: Why size matters. ACS Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef] [PubMed]
- Romero-Vargas Castrillón, S.; Perreault, F.o.; De Faria, A.F.; Elimelech, M. Interaction of graphene oxide with bacterial cell membranes: Insights from force spectroscopy. Environ. Sci. Technol. Lett. 2015, 2, 112–117. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, M.; Zeng, T.H.; Wu, R.; Jiang, R.; Wei, J.; Wang, L.; Kong, J.; Chen, Y. Lateral dimension-dependent antibacterial activity of graphene oxide sheets. Langmuir 2012, 28, 12364–12372. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Mashayekhi, H.; Xing, B. Bacterial toxicity comparison between nano-and micro-scaled oxide particles. Environ. Pollut. 2009, 157, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, J.; Xiong, Z.; Yong, Y.; Zhao, X. Preparation, characterization and antibacterial properties of silver-modified graphene oxide. J. Mater. Chem. 2011, 21, 3350–3352. [Google Scholar] [CrossRef]
- Hussain, N.; Gogoi, A.; Sarma, R.K.; Sharma, P.; Barras, A.; Boukherroub, R.; Saikia, R.; Sengupta, P.; Das, M.R. Reduced graphene oxide nanosheets decorated with Au nanoparticles as an effective bactericide: Investigation of biocompatibility and leakage of sugars and proteins. ChemPlusChem 2014, 79, 1774–1784. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Zhang, B.; Liu, J. Enhanced antibacterial activity of silver nanoparticles/halloysite nanotubes/graphene nanocomposites with sandwich-like structure. Sci. Rep. 2014, 4, 4551. Available online: https://www.nature.com/articles/srep04551 (accessed on 8 September 2017). [CrossRef] [PubMed]
- Kellici, S.; Acord, J.; Vaughn, A.; Power, N.P.; Morgan, D.J.; Heil, T.; Facq, S.P.; Lampronti, G.I. Calixarene Assisted Rapid Synthesis of Silver-Graphene Nanocomposites with Enhanced Antibacterial Activity. ACS Appl. Mater. Interfaces 2016, 8, 19038–19046. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Guo, J.; Dong, J.; Cui, Z.; Lu, T.; Zhu, C.; Zhang, D.; Ma, J. Sonochemical fabrication of Fe3O4 nanoparticles on reduced graphene oxide for biosensors. Ultrason. Sonochem. 2013, 20, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Mansukhani, N.D.; Guiney, L.M.; Ji, Z.; Zhao, Y.; Chang, C.H.; French, C.T.; Miller, J.F.; Hersam, M.C.; Nel, A.E. Identification and Optimization of Carbon Radicals on Hydrated Graphene Oxide for Ubiquitous Antibacterial Coatings. ACS Nano 2016, 10, 10966–10980. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhang, Y.; Zhang, B.; Liu, J.; Zhang, H.; Song, C. Preparation and characterization of HPEI-GO/PES ultrafiltration membrane with antifouling and antibacterial properties. J. Membr. Sci. 2013, 447, 452–462. [Google Scholar] [CrossRef]
- Carpio, I.E.M.; Santos, C.M.; Wei, X.; Rodrigues, D.F. Toxicity of a polymer—Graphene oxide composite against bacterial planktonic cells, biofilms, and mammalian cells. Nanoscale 2012, 4, 4746–4756. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Marsh, K.L.; McVerry, B.T.; Hoek, E.M.; Kaner, R.B. Low-Fouling Antibacterial Reverse Osmosis Membranes via Surface Grafting of Graphene Oxide. ACS Appl. Mater. Interfaces 2016, 8, 14334–14338. [Google Scholar] [CrossRef] [PubMed]
- Pandey, H.; Parashar, V.; Parashar, R.; Prakash, R.; Ramteke, P.W.; Pandey, A.C. Controlled drug release characteristics and enhanced antibacterial effect of graphene nanosheets containing gentamicin sulfate. Nanoscale 2011, 3, 4104–4108. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Qian, F.; Saltikov, C.W.; Jiao, Y.; Li, Y. Microbial reduction of graphene oxide by Shewanella. Nano Res. 2011, 4, 563–570. [Google Scholar] [CrossRef]
- Ruiz, O.N.; Fernando, K.S.; Wang, B.; Brown, N.A.; Luo, P.G.; McNamara, N.D.; Vangsness, M.; Sun, Y.-P.; Bunker, C.E. Graphene oxide: A nonspecific enhancer of cellular growth. ACS Nano 2011, 5, 8100–8107. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, Q.; Liu, Y.; Wang, S.; Abel, E.W. Reduction of bacterial adhesion on modified DLC coatings. Colloids Surf. B Biointerfaces 2008, 61, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, N.; Pan, C.; Kwok, S.; Yang, P.; Leng, Y.; Chen, J.; Sun, H.; Wan, G.; Liu, Z. Bacterial repellence from polyethylene terephthalate surface modified by acetylene plasma immersion ion implantation–deposition. Surf. Coat. Technol. 2004, 186, 299–304. [Google Scholar] [CrossRef]
- Kinnari, T.J.; Soininen, A.; Esteban, J.; Zamora, N.; Alakoski, E.; Kouri, V.P.; Lappalainen, R.; Konttinen, Y.T.; Gomez-Barrena, E.; Tiainen, V.M. Adhesion of staphylococcal and Caco-2 cells on diamond-like carbon polymer hybrid coating. J. Biomed. Mater. Res. Part A 2008, 86, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Marciano, F.; Bonetti, L.; Santos, L.; Da-Silva, N.; Corat, E.; Trava-Airoldi, V. Antibacterial activity of DLC and Ag–DLC films produced by PECVD technique. Diam. Relat. Mater. 2009, 18, 1010–1014. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, L.; Ogino, A.; Nagatsu, M. Investigation into the antibacterial property of carbon films. Diam. Relat. Mater. 2008, 17, 1416–1419. [Google Scholar] [CrossRef]
- Gorzelanny, C.; Kmeth, R.; Obermeier, A.; Bauer, A.T.; Halter, N.; Kümpel, K.; Schneider, M.F.; Wixforth, A.; Gollwitzer, H.; Burgkart, R.; et al. Silver nanoparticle-enriched diamond-like carbon implant modification as a mammalian cell compatible surface with antimicrobial properties. Sci. Rep. 2016, 6, 22849. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4783782/ (accessed on 8 September 2017). [CrossRef] [PubMed]
- Endrino, J.; Anders, A.; Albella, J.; Horton, J.; Horton, T.; Ayyalasomayajula, P.; Allen, M. Antibacterial efficacy of advanced silver-amorphous carbon coatings deposited using the pulsed dual cathodic arc technique. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2010. [Google Scholar]
- Almaguer-Flores, A.; Olivares-Navarrete, R.; Lechuga-Bernal, A.; Ximénez-Fyvie, L.A.; Rodil, S.E. Oral bacterial adhesion on amorphous carbon films. Diam. Relat. Mater. 2009, 18, 1179–1185. [Google Scholar] [CrossRef]
- Jelinek, M.; Voss, A.; Kocourek, T.; Mozafari, M.; Vymětalová, V.; Zezulová, M.; Písařík, P.; Kotzianová, A.; Popov, C.; Mikšovský, J. Comparison of the surface properties of DLC and ultrananocrystalline diamond films with respect to their bio-applications. Phys. Status Solidi (a) 2013, 210, 2106–2110. [Google Scholar] [CrossRef]
- Maas, M. Carbon Nanomaterials as Antibacterial Colloids. Materials 2016, 9, E617. [Google Scholar] [CrossRef] [PubMed]
- Bendavid, A.; Martin, P.; Randeniya, L.; Amin, M. The properties of fluorine containing diamond-like carbon films prepared by plasma-enhanced chemical vapour deposition. Diam. Relat. Mater. 2009, 18, 66–71. [Google Scholar] [CrossRef]
- Nobili, L.; Guglielmini, A. Thermal stability and mechanical properties of fluorinated diamond-like carbon coatings. Surf. Coat. Technol. 2013, 219, 144–150. [Google Scholar] [CrossRef]
- Chan, Y.-H.; Huang, C.-F.; Ou, K.-L.; Peng, P.-W. Mechanical properties and antibacterial activity of copper doped diamond-like carbon films. Surf. Coat. Technol. 2011, 206, 1037–1040. [Google Scholar] [CrossRef]
- Ji, L.; Li, H.; Zhao, F.; Chen, J.; Zhou, H. Microstructure and mechanical properties of Mo/DLC nanocomposite films. Diam. Relat. Mater. 2008, 17, 1949–1954. [Google Scholar] [CrossRef]
- Bociąga, D.; Jakubowski, W.; Komorowski, P.; Sobczyk-Guzenda, A.; Jędrzejczak, A.; Batory, D.; Olejnik, A. Surface characterization and biological evaluation of silver-incorporated DLC coatings fabricated by hybrid RF PACVD/MS method. Mater. Sci. Eng. C 2016, 63, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, P.; He, X.; Li, L.; Wang, A.; Li, H. Developing transparent copper-doped diamond-like carbon films for marine antifouling applications. Diam. Relat. Mater. 2016, 69, 144–151. [Google Scholar] [CrossRef]
- Love, C.A.; Cook, R.B.; Harvey, T.J.; Dearnley, P.A.; Wood, R.J.K. Diamond like carbon coatings for potential application in biological implants—A review. Tribol. Int. 2013, 63, 141–150. [Google Scholar] [CrossRef]
- Lan, W.-C.; Ou, S.-F.; Lin, M.-H.; Ou, K.-L.; Tsai, M.-Y. Development of silver-containing diamond-like carbon for biomedical applications. Part I: Microstructure characteristics, mechanical properties and antibacterial mechanisms. Ceram. Int. 2013, 39, 4099–4104. [Google Scholar] [CrossRef]
- Cloutier, M.; Turgeon, S.; Busby, Y.; Tatoulian, M.; Pireaux, J.J.; Mantovani, D. Controlled Distribution and Clustering of Silver in Ag-DLC Nanocomposite Coatings Using a Hybrid Plasma Approach. ACS Appl. Mater. Interfaces 2016, 8, 21020–21027. [Google Scholar] [CrossRef] [PubMed]
- Dearnaley, G.; Arps, J.H. Biomedical applications of diamond-like carbon (DLC) coatings: A review. Surf. Coat. Technol. 2005, 200, 2518–2524. [Google Scholar] [CrossRef]
- Juknius, T.; Ružauskas, M.; Tamulevičius, T.; Šiugždinienė, R.; Juknienė, I.; Vasiliauskas, A.; Jurkevičiūtė, A.; Tamulevičius, S. Antimicrobial Properties of Diamond-Like Carbon/Silver Nanocomposite Thin Films Deposited on Textiles: Towards Smart Bandages. Materials 2016, 9, 371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhao, J.; Yang, Y. A novel diamond-like carbon film. Surf. Interfaces 2017, 7, 1–5. [Google Scholar] [CrossRef]
- Swiatek, L.; Olejnik, A.; Grabarczyk, J.; Jedrzejczak, A.; Sobczyk-Guzenda, A.; Kaminska, M.; Jakubowski, W.; Szymanski, W.; Bociaga, D. Multi-doped diamond like-carbon coatings (DLC-Si/Ag) for biomedical applications fabricated using the modified chemical vapour deposition method. Diam. Relat. Mater. 2016, 67, 54–62. [Google Scholar] [CrossRef]
- Zhang, L.L.; Yang, Q.; Tang, Y.; Yang, L.; Zhang, C.; Hu, Y.; Cui, X. Synthesis and characterization of boron incorporated diamond-like carbon thin films. Thin Solid Films 2015, 589, 457–464. [Google Scholar] [CrossRef]
- Luo, J.; Fu, Y.Q.; Le, H.; Williams, J.A.; Spearing, S.; Milne, W. Diamond and diamond-like carbon MEMS. J. Micromech. Microeng. 2007, 17, S147–S163. [Google Scholar] [CrossRef]
- Dwivedi, N.; Kumar, S.; Malik, H.K. Strange hardness characteristic of hydrogenated diamond-like carbon thin film by plasma enhanced chemical vapor deposition process. Appl. Phys. Lett. 2013, 102, 011917. [Google Scholar] [CrossRef]
- Santos, T.B.; Vieira, A.A.; Paula, L.O.; Santos, E.D.; Radi, P.A.; Khouri, S.; Maciel, H.S.; Pessoa, R.S.; Vieira, L. Flexible camphor diamond-like carbon coating on polyurethane to prevent Candida albicans biofilm growth. J. Mech. Behav. Biomed. Mater. 2017, 68, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.I.; Javaherian, Z.; Minai-Tehrani, D.; Ghasemi, R.; Ghaempanah, Z.; Firouzjah, M.A.; Shokri, B. Antibacterial properties of fluorinated diamond-like carbon films deposited by direct and remote plasma. Mater. Lett. 2017, 188, 84–87. [Google Scholar] [CrossRef]
- Fadel, T. Realizing the Promise of Carbon Nanotubes: Challenges, Opportunities, and the Pathway to Commercialization. 2015. Available online: https://www.nano.gov/sites/default/files/pub_resource/2014_nni_cnt_tech_meeting_report.pdf (accessed on 8 September 2017).
- Socio-Environmental Systems (SES) Research. Fullerene; SES Research: Houston, TX, USA, 2017; Available online: https://www.sesres.com/fullerene/ (accessed on 8 September 2017).
- Norinaga, K.; Deutschmann, O. Detailed kinetic modeling of gas-phase reactions in the chemical vapor deposition of carbon from light hydrocarbons. Ind. Eng. Chem. Res. 2007, 46, 3547–3557. [Google Scholar] [CrossRef]
- Sun, L.; Tian, C.; Li, M.; Meng, X.; Wang, L.; Wang, R.; Yin, J.; Fu, H. From coconut shell to porous graphene-like nanosheets for high-power supercapacitors. J. Mater. Chem. A 2013, 1, 6462–6470. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Darder, M.; Fernandes, F.M.; Zatile, E.; Palomares, F.J.; Aranda, P. Supported graphene from natural resources: Easy preparation and applications. Adv. Mater. 2011, 23, 5250–5255. [Google Scholar] [CrossRef] [PubMed]
- Kawale, S.S.; Bhardwaj, S.; Kshirsagar, D.; Bhosale, C.; Sharon, M.; Sharon, M. Thin Films of Carbon Nanomaterial from Natural Precursor by Hot Wire CVD. Fuller. Nanotub. Carbon Nanostruct. 2011, 19, 540–549. [Google Scholar] [CrossRef]
- Jacob, M.V.; Rawat, R.S.; Ouyang, B.; Bazaka, K.; Kumar, D.S.; Taguchi, D.; Iwamoto, M.; Neupane, R.; Varghese, O.K. Catalyst-Free Plasma Enhanced Growth of Graphene from Sustainable Sources. Nano Lett. 2015, 15, 5702–5708. [Google Scholar] [CrossRef] [PubMed]
| CNSs | Type | Synthesis Method | Modification/Catalyst | Dimensions | Concentration | Species of Bacteria | Antibacterial Efficacy | Antibacterial Mechanism | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Fullerene | C60 | Four-step reaction | Cyclen-functionalized fullerene derivative | 150 to 320 nm | 7.5 μg/mL | E. coli, S. aureus | 86.1% 40.7% | Electrostatic attraction plays a major role | [29] |
| C60 | - | Light (160 J/cm2 of 385–780 nm) | - | 100 μM | S. aureus, P. aeruginosa, E. coli, C. albicans | 6 log10 1 log10 3 log10 3 log10 | Increase in membrane permeability | [30] | |
| C70 | SES Research production | Ag-NP and polystyrene-(PS-P4VP) | - | 2 wt % of PS-P4VP | E. coli | ~5 log | C70 and Ag-NPs, synergistically target bacterial cells that increase photo-generated ROS | [31] | |
| CNT | SWCNT | CO disprop-ortionation | - | d = ~1 nm l = 1–3 µm | 5 μg/mL | E. coli | 86.8% | Irrecoverable damages to the outer membrane, releasing the intracellular content | [32] |
| SWCNT | CO incorporated MCM-41 | - | d = 0.9 nm l = 2 µm | 5 µg/mL | E. coli | 80.1% | Cells lost their cellular integrity | [33] | |
| MWCNT | CVD method | - | d = 30 nm l = 70 µm | 5 µg/mL | E. coli | 24.4% | The majority of cells still intact and maintain their outer membrane | [33] | |
| SWCNT | CO decomposition | Poly(lactic-co-glycolic acid) | d = ~1 nm l = 300 nm | 1/70 CNT/polymer | S. epidermidis | 98% | Cells loss their viability and deactivated | [34] | |
| SWCNT | NanoLab productions | Functional groups (−OH, −COOH, and −NH2) | d = ~1.5 nm l = 10 µm | 200–250 μg/mL | S.typhimuriu, B. subtilis, S. aureus | ~7 log | Form aggregates that act like needles surrounding the cells | [35] | |
| MWCNT | NanoLab productions | Functional groups (−OH, −COOH, and −NH2) | d = 15–30 nm l = 1–5 μm | 500 μg/mL | S.typhimuriu, B. subtilis, S. aureus | Minor | - | [35] | |
| Graphene | Graphene oxide | Hummers and Offeman | - | 0.525 μm | P.aeruginosa | 92% | Oxidative stress, ROS generation, and laddering of DNA | [36] | |
| Reduce graphene oxide | Synthesized from GO | - | 3.40 μm | 0.1 mg/mL | P.aeruginosa | 90% | Oxidative stress and ROS generation | [36] | |
| Graphene oxide | Modified Hummers’ procedure | - | 205 nm | 100 mg/mL | E. coli | - | Extraction of phospholipids from the cell membrane | [37] | |
| Graphene | low-pressure CVD | AgNW/water electrolysis | - | - | C. albicans | 100% | Graphene layer reduces the attachment of microbes | [38] | |
| DLC * | - | DC sputtering | Polytetrafluoroethylene hybrid | t = 200 nm | - | S. epidermidis, S. aureus | 56% 51% | Reduce biofilm formation and cell attachments | [39] |
| Multilayer films | Pulsed-DC-PECVD | Germanium | t = 1–2 μm | 28.9% germanium | P. aeruginosa | 62.6% | Disruption to the outer cell wall and leakage of cellular components. | [40] | |
| Multilayer films | Pulsed-DC-PECVD | Germanium | t = 1–2 μm | 28.9% germanium | S. aureus | - | Minor reduction in biofilm | [40] | |
| Two layers (a-SiC:H/F-DLC) | RF-PECVD | Fluorine | t = 1 µm | 6.5–39.2 at % F | P. fluorescens | 48.8% | Reduce bacterial attachment and proliferation. | [41] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M.V. Review on the Antimicrobial Properties of Carbon Nanostructures. Materials 2017, 10, 1066. https://doi.org/10.3390/ma10091066
Al-Jumaili A, Alancherry S, Bazaka K, Jacob MV. Review on the Antimicrobial Properties of Carbon Nanostructures. Materials. 2017; 10(9):1066. https://doi.org/10.3390/ma10091066
Chicago/Turabian StyleAl-Jumaili, Ahmed, Surjith Alancherry, Kateryna Bazaka, and Mohan V. Jacob. 2017. "Review on the Antimicrobial Properties of Carbon Nanostructures" Materials 10, no. 9: 1066. https://doi.org/10.3390/ma10091066





