The Role of Controlled Surface Topography and Chemistry on Mouse Embryonic Stem Cell Attachment, Growth and Self-Renewal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates
2.2. Plasma Polymer Coating
2.3. Nanotopography Substrate Modification
2.4. Cell Culture
2.5. Mouse ES Cells Culture on Substrates
2.6. Immunostaining
2.7. Cell Adhesion, Morphology and Pluripotency
2.8. Statistical Analysis
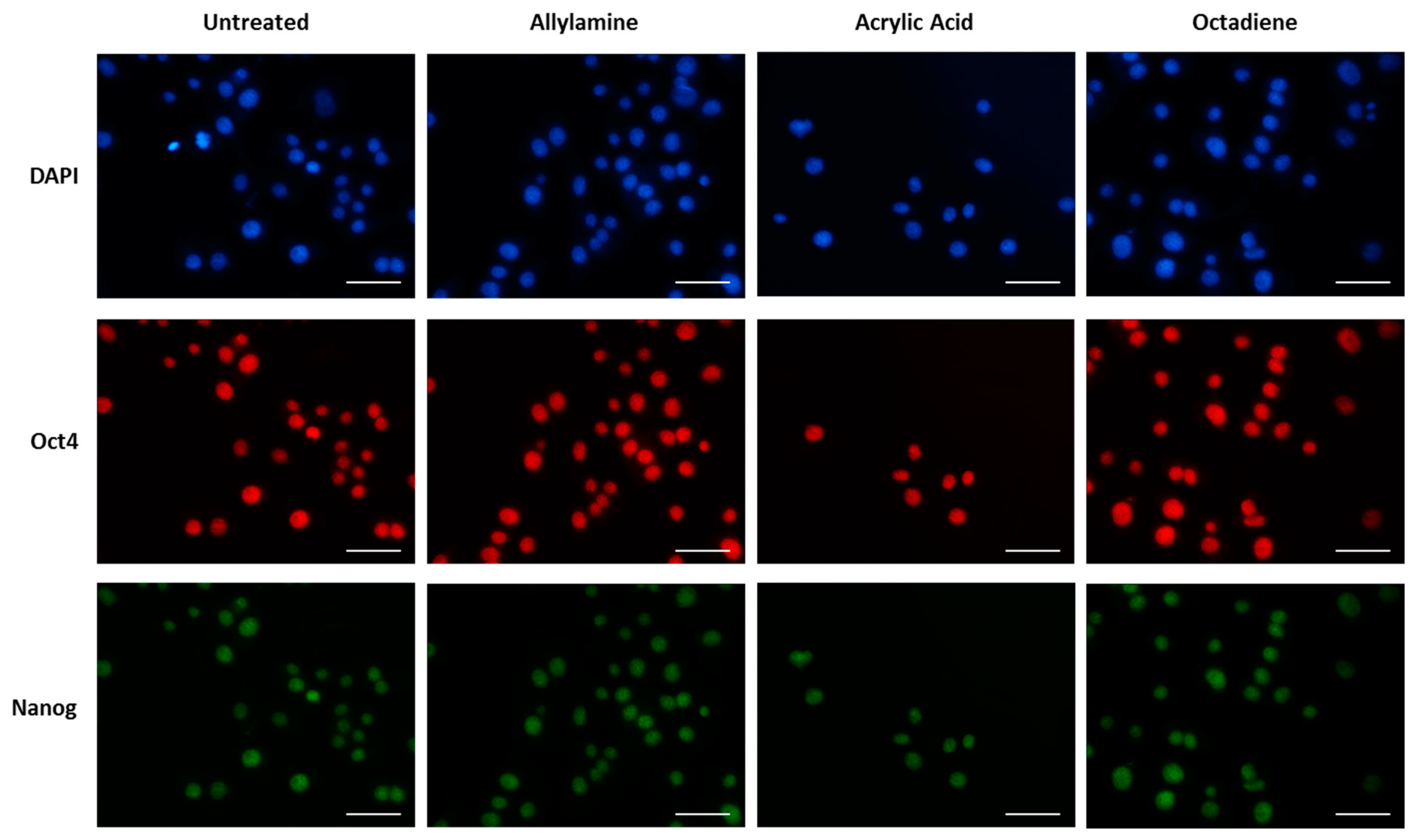
3. Results
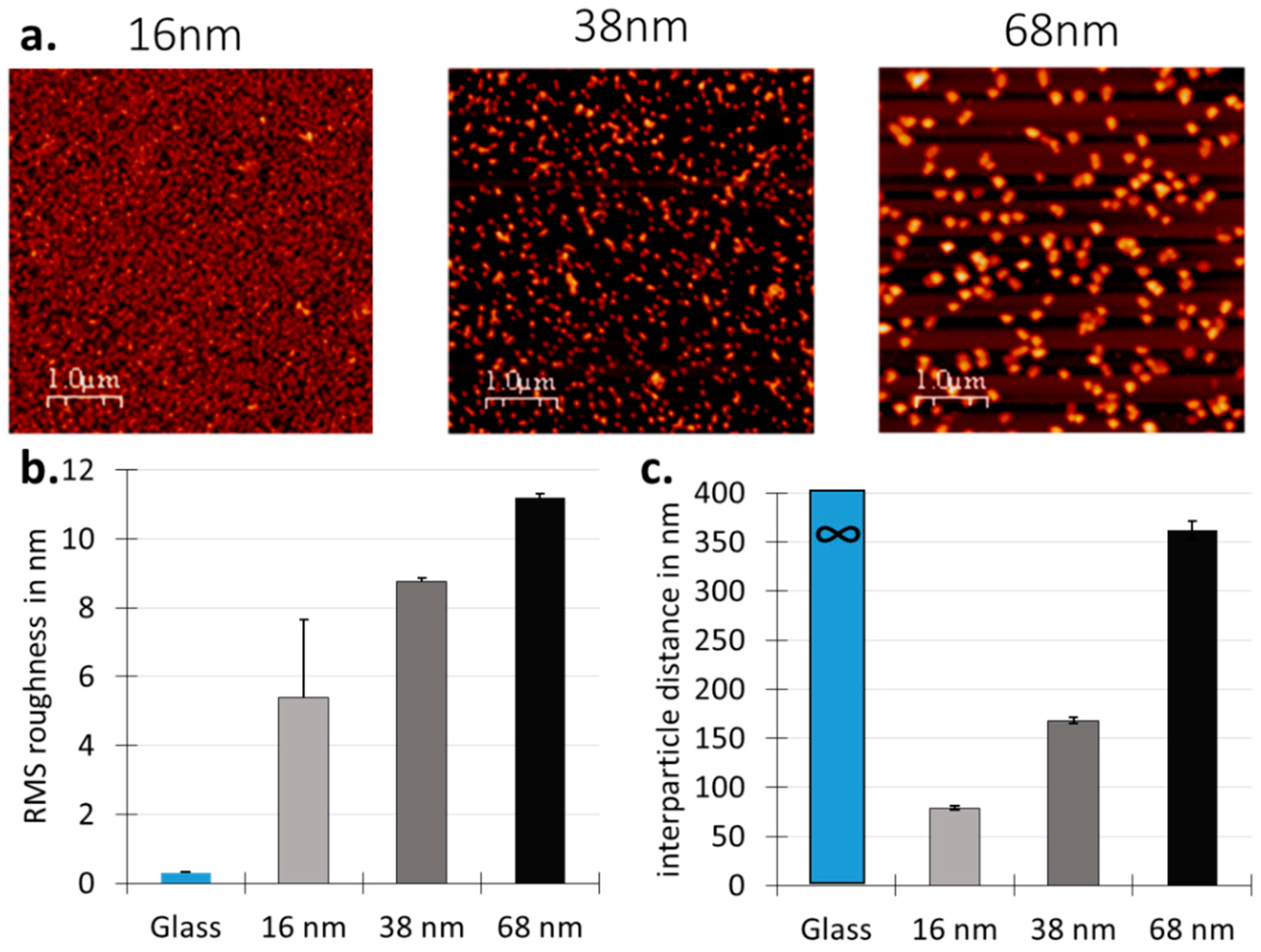
3.1. Surface Design
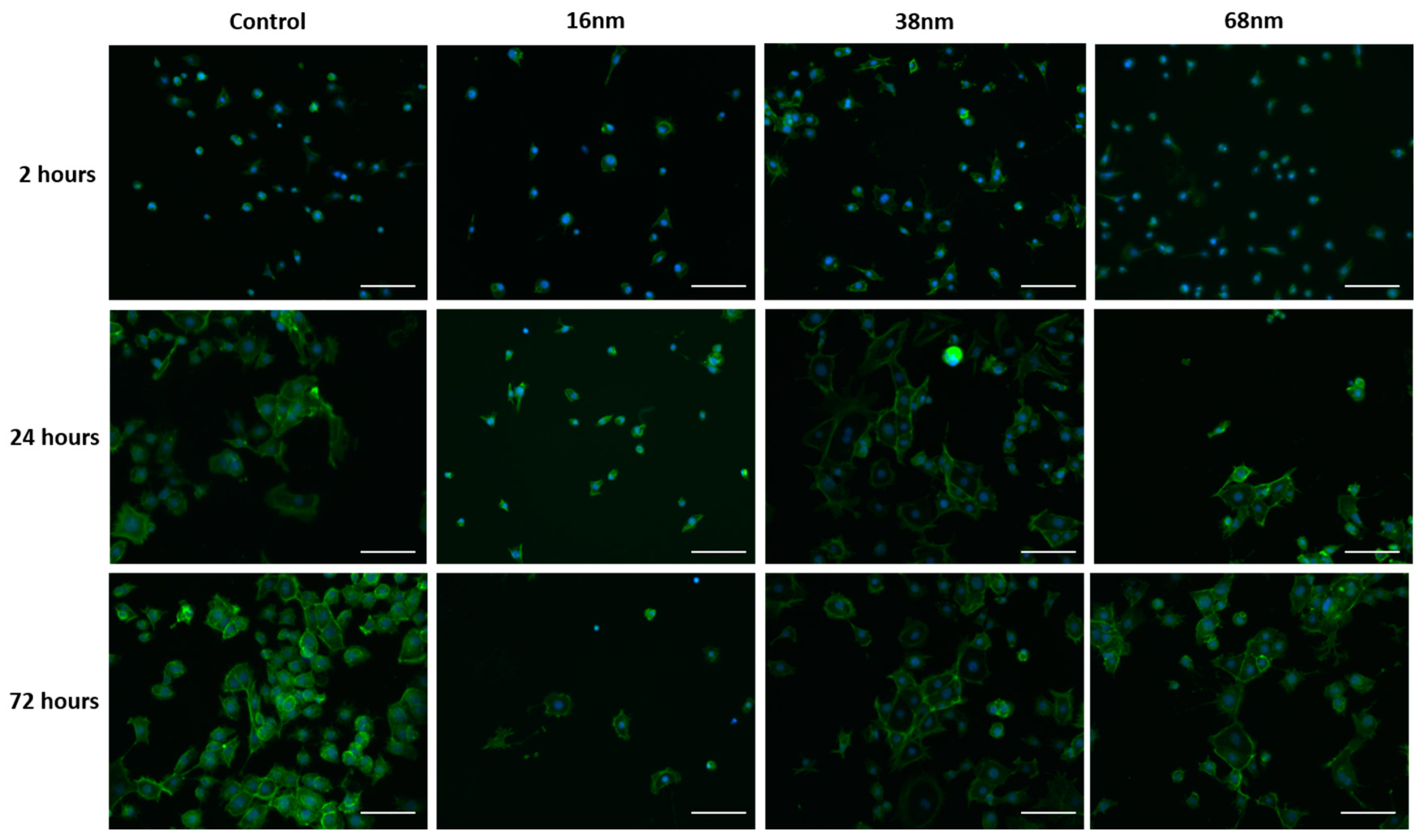
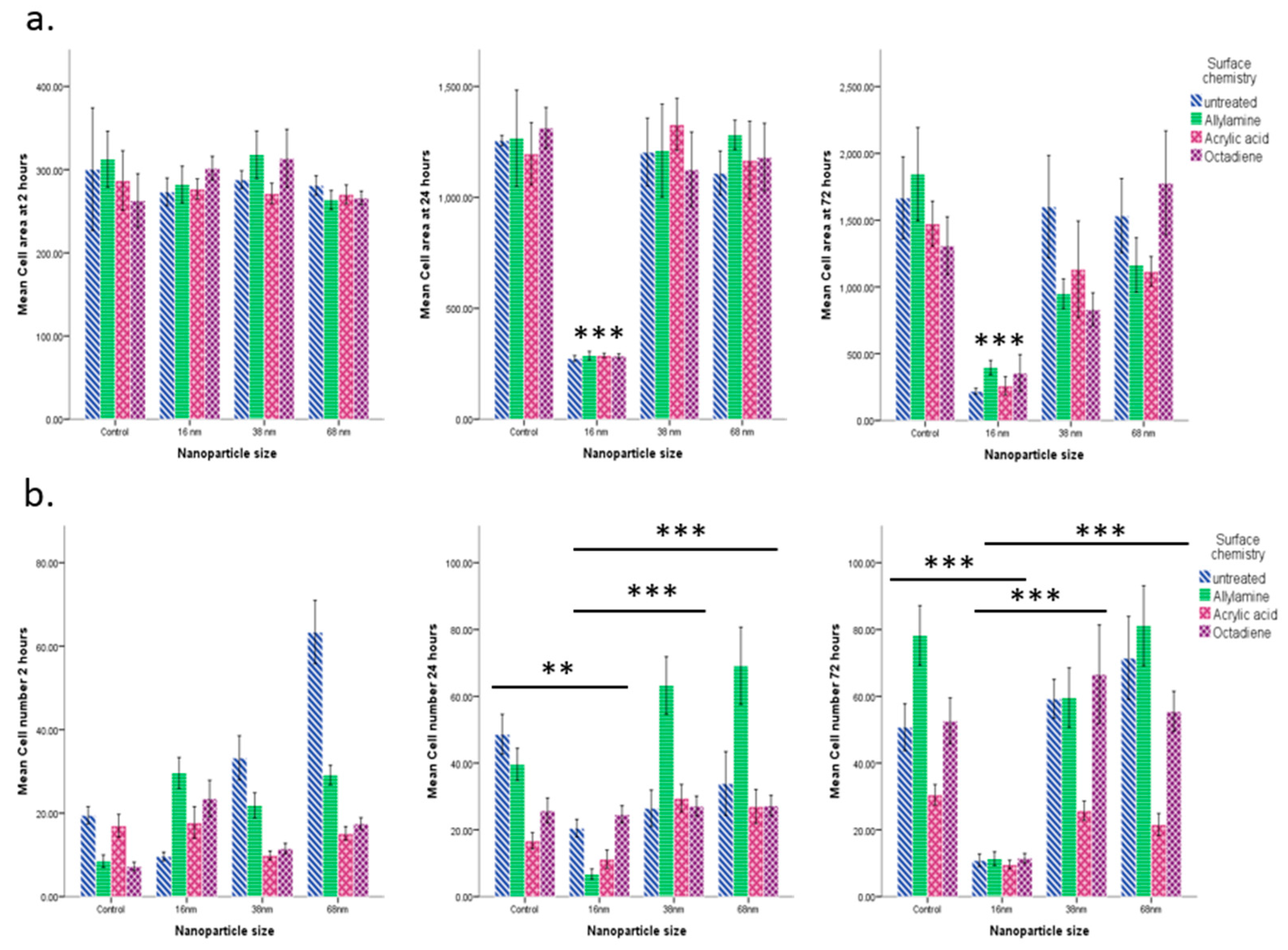
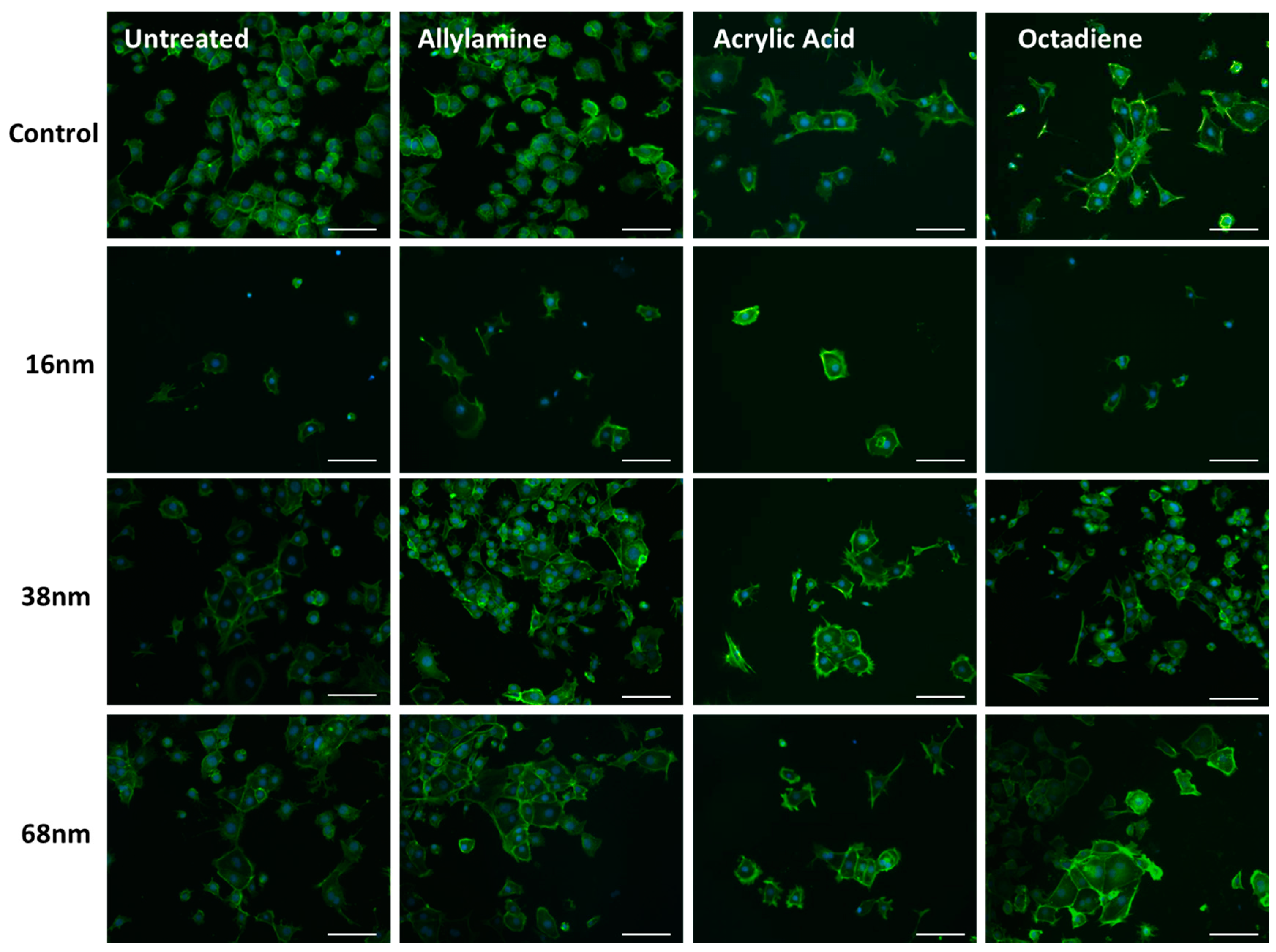
3.2. Cell Behaviour
Cell Adhesion and Spreading
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tabar, V.; Studer, L. Pluripotent stem cells in regenerative medicine: Challenges and recent progress. Nat. Rev. Genet. 2014, 15, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.; Tobita, M.; Uysal, A.C. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells 2012, 30, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Jaklenec, A.; Stamp, A.; Deweerd, E.; Sherwin, A.; Langer, R. Progress in the tissue engineering and stem cell industry “are we there yet?”. Tissue Eng. Part B Rev. 2012, 18, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.; Jones, D.L.; Visvader, J.; Weissman, I.L.; Wahl, G.M. Cancer stem cells—Perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006, 66, 9339–9344. [Google Scholar] [CrossRef] [PubMed]
- Rezania, A.; Bruin, J.E.; Riedel, M.J.; Mojibian, M.; Asadi, A.; Xu, J.; Gauvin, R.; Narayan, K.; Karanu, F.; O’Neil, J.J.; et al. Maturation of human embryonic stem cell—Derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes 2012, 61, 2016–2029. [Google Scholar] [CrossRef] [PubMed]
- Tyndall, A. Successes and Failures of Stem Cell Transplantation in Autoimmune Diseases. ASH Educ. Book 2011, 2011, 280–284. [Google Scholar] [CrossRef]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth Factors, Matrices, and Forces Combine and Control Stem Cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef] [PubMed]
- Crowder, S.W.; Leonardo, V.; Whittaker, T.; Papathanasiou, P.; Stevens, M.M. Material Cues as Potent Regulators of Epigenetics and Stem Cell Function. Cell Stem Cell 2016, 18, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.; Prewitz, M.; Hopp, I.; Wells, N.; Zhang, H.; Cooper, A.; Parry, K.L.; Short, R.; Antoine, D.J.; Edgar, D. The self-renewal of mouse embryonic stem cells is regulated by cell-substratum adhesion and cell spreading. Int. J. Biochem. Cell Biol. 2013, 45, 2698–2705. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Gadegaard, N.; Oreffo, R.O.C. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat. Mater. 2014, 13, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of Stem Cell Fate by Physical Interactions with the Extracellular Matrix. Cell Stem Cell 2009, 5, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Feng, Q.; Bachhuka, A.; Vasilev, K. Surface chemical functionalities affect the behavior of human adipose-derived stem cells in vitro. Appl. Surf. Sci. 2013, 270, 473–479. [Google Scholar] [CrossRef]
- Liu, X.; Feng, Q.; Bachhuka, A.; Vasilev, K. Surface modification by allylamine plasma polymerization promotes osteogenic differentiation of human adipose-derived stem cells. ACS Appl. Mater. Interfaces 2014, 6, 9733–9741. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, S.; Feng, Q.; Bachhuka, A.; He, W.; Huang, Q.; Zhang, R.; Yang, X.; Vasilev, K. Surface Chemical Gradient Affects the Differentiation of Human Adipose-Derived Stem Cells via ERK1/2 Signaling Pathway. ACS Appl. Mater. Interfaces 2015, 7, 18473–18482. [Google Scholar] [CrossRef] [PubMed]
- Wells, N.; Baxter, M.A.; Turnbull, J.E.; Murray, P.; Edgar, D.; Parry, K.L.; Steele, D.A.; Short, R.D. The geometric control of E14 and R1 mouse embryonic stem cell pluripotency by plasma polymer surface chemical gradients. Biomaterials 2009, 30, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Pollock, J.F.; Schaffer, D.V.; Healy, K.E. Designing synthetic materials to control stem cell phenotype. Curr. Opin. Chem. Biol. 2007, 11, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Gilbert, P.M.; Blau, H.M. Designing materials to direct stem-cell fate. Nature 2009, 462, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Borghi, F.F.; Rider, A.E.; Kumar, S.; Han, Z.J.; Haylock, D.; Ostrikov, K. Emerging Stem Cell Controls: Nanomaterials and Plasma Effects. J. Nanomater. 2013, 2013, 15. [Google Scholar] [CrossRef]
- MacGregor-Ramiasa, M.; Hopp, I.; Bachhuka, A.; Murray, P.; Vasilev, K. Surface nanotopography guides kidney-derived stem cell differentiation into podocytes. Acta Biomater. 2017. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.K.; Chen, J.Y.; Wang, L.P.; Huang, N. Plasma-surface modification of biomaterials. Mater. Sci. Eng. R Rep. 2002, 36, 143–206. [Google Scholar] [CrossRef]
- Goreham, R.V.; Mierczynsk, A.; Smith, L.E.; Sedev, R.; Vasilev, K. Small surface nanotopography encourages fibroblast and osteoblast cell adhesion. RSC Adv. 2013, 3, 10309–10317. [Google Scholar] [CrossRef]
- Ramiasa, M.N.; Cavallaro, A.A.; Mierczynska, A.; Christo, S.N.; Gleadle, J.M.; Hayball, J.D.; Vasilev, K. Plasma polymerised polyoxazoline thin films for biomedical applications. Chem. Commun. 2015, 51, 4279–4282. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, K. Nanoengineered plasma polymer films for biomaterial applications. Plasma Chem. Plasma Process. 2013, 34, 545–558. [Google Scholar] [CrossRef]
- Ramiasa-MacGregor, M.; Mierczynska, A.; Sedev, R.; Vasilev, K. Tuning and predicting the wetting of nanoengineered material surface. Nanoscale 2016, 8, 4635–4642. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Lopez, J.L.; Bauer, R.E.; Chang, W.S.; Glasser, G.; Grebel-Koehler, D.; Klapper, M.; Kreiter, M.; Leclaire, J.; Majoral, J.P.; Mittler, S.; et al. Functional polymers as nanoscopic building blocks. Mater. Sci. Eng. C 2003, 23, 267–274. [Google Scholar] [CrossRef]
- Vasilev, K.; Michelmore, A.; Griesser, H.J.; Short, R.D. Substrate influence on the initial growth phase of plasma-deposited polymer films. Chem. Commun. 2009, 3600–3602. [Google Scholar] [CrossRef] [PubMed]
- Goreham, R.V.; Mierczynska, A.; Pierce, M.; Short, R.D.; Taheri, S.; Bachhuka, A.; Cavallaro, A.; Smith, L.; Vasilev, K. A substrate independent approach for generation of surface gradients. Thin Solid Films 2013, 528, 106–110. [Google Scholar] [CrossRef]
- Macgregor-Ramiasa, M.N.; Cavallaro, A.A.; Vasilev, K. Properties and reactivity of polyoxazoline plasma polymer films. J. Mater. Chem. B 2015, 3, 6327–6337. [Google Scholar] [CrossRef]
- Zhu, T.; Vasilev, K.; Kreiter, M.; Mittler, S.; Knoll, W. Surface Modification of Citrate-Reduced Colloidal Gold Nanoparticles with 2-Mercaptosuccinic Acid. Langmuir 2003, 19, 9518–9525. [Google Scholar] [CrossRef]
- Ostrikov, K.; Macgregor-Ramiasa, M.; Cavallaro, A.; Ostrikov, K.K.; Vasilev, K. Bactericidal effects of plasma-modified surface chemistry of silicon nanograss. J. Phys. D Appl. Phys. 2016, 49, 304001. [Google Scholar] [CrossRef]
- Gonzalez Garcia, L.E.; MacGregor-Ramiasa, M.; Visalakshan, R.M.; Vasilev, K. Protein Interactions with Nanoengineered Polyoxazoline Surfaces Generated via Plasma Deposition. Langmuir 2017, 33, 7322–7331. [Google Scholar] [CrossRef] [PubMed]
- Ramiasa, M.; Ralston, J.; Fetzer, R.; Sedev, R.; Fopp-Spori, D.M.; Morhard, C.; Pacholski, C.; Spatz, J.P. Contact Line Motion on Nanorough Surfaces: A Thermally Activated Process. J. Am. Chem. Soc. 2013, 135, 7159–7171. [Google Scholar] [CrossRef] [PubMed]
- Ramiasa, M.; Ralston, J.; Fetzer, R.; Sedev, R. Nanoroughness Impact on Liquid–Liquid Displacement. J. Phys. Chem. C 2012, 116, 10934–10943. [Google Scholar] [CrossRef]
- Chowdhury, N.R.; Ramiasa, M.; Majewski, P.; Vasilev, K. Influence of nanotopography and chemistry on bacterial surface colonisation. In Chemeca 2016: Chemical Engineering-Regeneration, Recovery and Reinvention; Engineers Australia: Melbourne, Australia, 2016; pp. 745–755. [Google Scholar]
- Gengenbach, T.R.; Chatelier, R.C.; Griesser, H.J. Characterization of the Ageing of Plasma-deposited Polymer Films: Global Analysis of X-ray Photoelectron Spectroscopy Data. Surf. Interface Anal. 1996, 24, 271–281. [Google Scholar] [CrossRef]
- Saboohi, S.; Jasieniak, M.; Coad, B.R.; Griesser, H.J.; Short, R.D.; Michelmore, A. Comparison of Plasma Polymerization under Collisional and Collision-Less Pressure Regimes. J. Phys. Chem. B 2015, 119, 15359–15369. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E.; Pierschbacher, M.D. New perspectives in cell adhesion: RGD and integrins. Science 1987, 238, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Karim, A.; Bates, F.S.; Tirrell, M.; Furusawa, K. Adsorption of End-Functionalized Polystyrene on Model Textured Surfaces. Macromolecules 1994, 27, 2586–2594. [Google Scholar] [CrossRef]
- Deng, Z.J.; Liang, M.; Toth, I.; Monteiro, M.J.; Minchin, R.F. Molecular Interaction of Poly(acrylic acid) Gold Nanoparticles with Human Fibrinogen. ACS Nano 2012, 6, 8962–8969. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.J.; Liang, M.; Monteiro, M.; Toth, I.; Minchin, R.F. Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation. Nat. Nano 2011, 6, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Lord, M.S.; Cousins, B.G.; Doherty, P.J.; Whitelock, J.M.; Simmons, A.; Williams, R.L.; Milthorpe, B.K. The effect of silica nanoparticulate coatings on serum protein adsorption and cellular response. Biomaterials 2006, 27, 4856–4862. [Google Scholar] [CrossRef] [PubMed]
- Lord, M.S.; Whitelock, J.M.; Simmons, A.; Williams, R.L.; Milthorpe, B.K. Fibrinogen adsorption and platelet adhesion to silica surfaces with stochastic nanotopography. Biointerphases 2014, 9, 041002. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Li, K.; Xie, Y.; Cao, Y.; Zheng, X. Enhanced osteogenic activity of anatase TiO2 film: Surface hydroxyl groups induce conformational changes in fibronectin. Mater. Sci. Eng. C 2017, 78, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Stirnemann, G.; Kang, S.-G.; Zhou, R.; Berne, B.J. How force unfolding differs from chemical denaturation. Proc. Natl. Acad. Sci. USA 2014, 111, 3413–3418. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, G.A.P.; Silva, J.L. A hypothesis to reconcile the physical and chemical unfolding of proteins. Proc. Natl. Acad. Sci. USA 2015, 112, E2775–E2784. [Google Scholar] [CrossRef] [PubMed]
- Levato, R.; Planell, J.A.; Mateos-Timoneda, M.A.; Engel, E. Role of ECM/peptide coatings on SDF-1α triggered mesenchymal stromal cell migration from microcarriers for cell therapy. Acta Biomater. 2015, 18, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Ramasamy, K.; Leena, M.; Jiménez, C.; Campos, J.; Ibarra, P.; Haidar, Z.S.; Ramalingam, M. Surface functionalization of nanobiomaterials for application in stem cell culture, tissue engineering, and regenerative medicine. Biotechnol. Prog. 2016, 32, 554–567. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macgregor, M.; Williams, R.; Downes, J.; Bachhuka, A.; Vasilev, K. The Role of Controlled Surface Topography and Chemistry on Mouse Embryonic Stem Cell Attachment, Growth and Self-Renewal. Materials 2017, 10, 1081. https://doi.org/10.3390/ma10091081
Macgregor M, Williams R, Downes J, Bachhuka A, Vasilev K. The Role of Controlled Surface Topography and Chemistry on Mouse Embryonic Stem Cell Attachment, Growth and Self-Renewal. Materials. 2017; 10(9):1081. https://doi.org/10.3390/ma10091081
Chicago/Turabian StyleMacgregor, Melanie, Rachel Williams, Joni Downes, Akash Bachhuka, and Krasimir Vasilev. 2017. "The Role of Controlled Surface Topography and Chemistry on Mouse Embryonic Stem Cell Attachment, Growth and Self-Renewal" Materials 10, no. 9: 1081. https://doi.org/10.3390/ma10091081





