Table 4 shows the responses obtained with Vickers hardness and density with respect to different temperature and holding times for each MMC-Al
R as designated in
Table 3. Each of the Vickers hardness values is an average value from 10 indentation readings obtained from each sample. The value shown for the MMC-Al
R density is an average from five repetitions of the density measurement for each specimen. Both results show an enhancement of MMC-Al
R performance for each temperature and holding time increase.
3.1. Vickers Microhardness
The average measurements of the indentations are depicted in
Figure 3. The microhardness value grew as the operating temperature and the holding time were increased. By increasing the operating temperature from 430 to 530 °C at a constant holding time of 90 min, the microhardness increased by 14.39% from 73.367 to 83.921 HV
0.3. Subsequently, a similar trend was identified when studying the effect of holding time at the same temperature (530 °C). The microhardness increased 7.52% from 80.592 HV
0.3 after 60 min, to 86.656 HV
0.3 after 120 min. This positive trend also applies to all the specimens whenever the temperature or the holding time is increased. In short, the operating temperature is the most influential factor for increasing microhardness.
The analysis of grain structure determined that the grain average diameter reduces as the temperature and holding time increase, as shown in
Table 5. Size reduction is one of the factors influencing the increase in hardness. The relationship between grain size and composite performance has been previously discussed [
29].
The grain growth of the composite is suppressed by the ceramic additive which improves the hardness. Several studies have revealed that, according to the Hall–Petch rules, a fine grain size is beneficial for obtaining high microhardness [
29,
30,
31,
32,
33,
34]. In addition, the hardness of Al
2O
3 embedded in soft aluminum can be attributed to the increase in the composite hardness. According to Al-Mosawi et al. [
35], the presence of sub-micrometric Al
2O
3 particles has caused a dispersion strengthening mechanism that may enhance the material toughness. The presence of a reinforcement constituent limited the localized matrix deformation due to increasing the intermetallic phase during the forming process [
36,
37]. Subsequently, the hard ceramic particles of Al
2O
3, which have a higher hardness value compared to the ductile matrix, allowed the matrix to endure large plastic deformation [
38]. Likewise, Rahimian et al. [
39] stated that the greater interfacial area between the hard (Al
2O
3) and soft phase (AR) is the reason for the increase in the composite’s hardness. This was proven through the increase in the hardness of the reinforced aluminum. Moreover, as the temperature and holding time increased, the matrix had high kinetic energy which leads to higher solubility limits and the formation of intermetallic bonding. The matrix itself assisted in retaining the composite hardness.
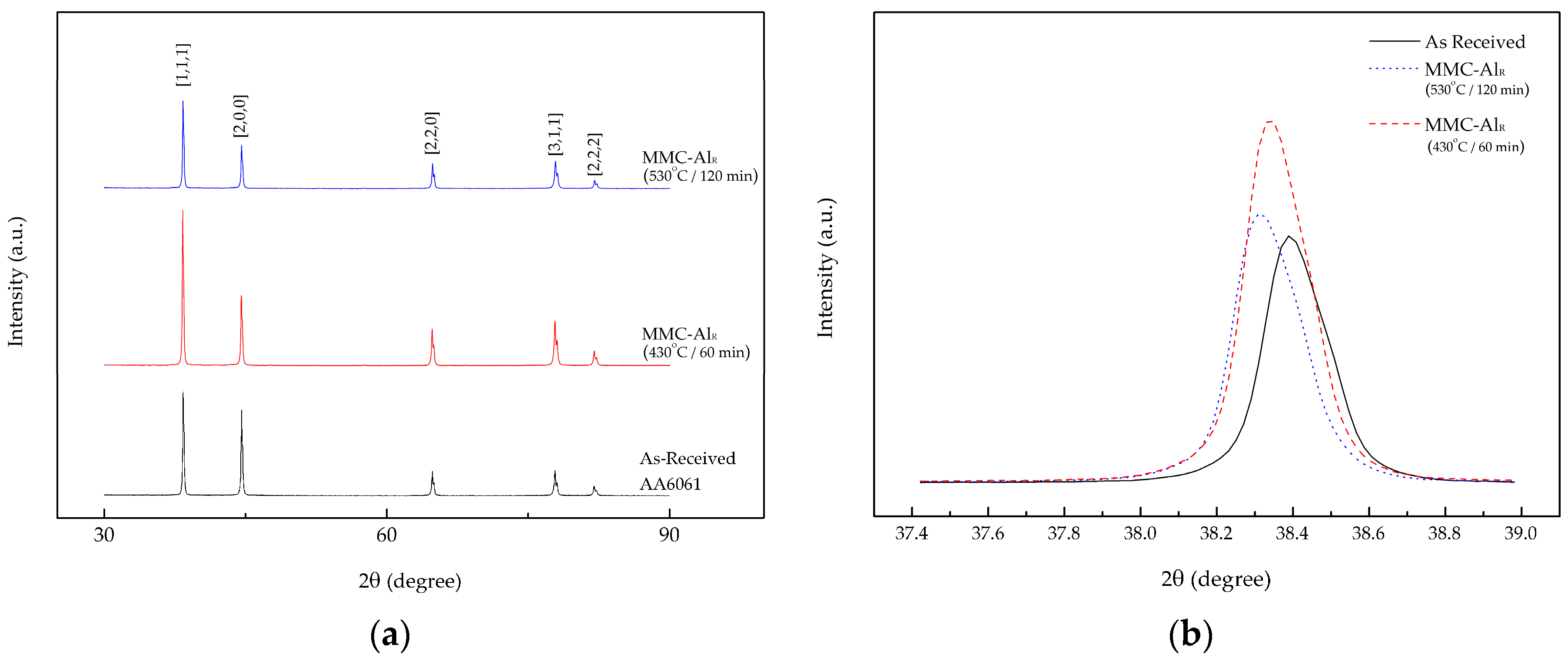
In addition, the XRD analysis showed agreement with the composite behavior.
Figure 4a shows the XRD pattern for the three different materials. All the major peaks in the pattern are identified as belonging to aluminum, with a face-centered cubic crystal structure and a lattice parameter of a = 0.4049 nm. XRD spectra were validated with the standard Aluminum (JCPDS file No. 00-045-1204). The alloy peaks were observed to match along 2-theta of 30 to 90 degrees with the standard, suggesting the alloy is an aluminum series. The peak height for the first peak was reduced when the temperature and holding time increased from 430 to 530 °C and 60 to 120 min, respectively (
Figure 4b,
Table 6). The d-spacing also decreased, which in turn resulted in a tapering line of the peak for the maximum parameter setting. This line tapering of the composite at 530 °C and 120 min resulted in an increase in the crystallite size from 739.9 to 770.6 Å. When compared to the XRD pattern of the maximum parameter setting composite with AR AA6061, the crystallite size is much smaller. This is due to the AR sample is in isotropic condition and the structure does not react to temperature or pressure in comparison to the recycled composite. We also noticed that the position of the peaks shifted slightly (
Figure 4b). The shifted peaks could be related to the dissolution of impurities, particularly iron, in the lattice of aluminum [
27]. The shifted diffraction peaks were also caused by the dissimilar lattice parameter for each of the crystallites present in the composite [
40]. The dominant peak behavior can also be explained by the post-production having shifted the peak to a lower angle [
41]. The internal strain had been unintentionally altered during the production, which may have resulted in a different degree of plastic deformation in the composite grains. High operating temperature and holding time contributed to the increase in the crystalline size. The Al
2O
3 also acted as a catalyst for the structural size change. Hard ceramic particles introduced into the composite caused the matrix to expand in all directions, creating better bonding in the matrix. Previous studies found that the hardness of a material increases as the crystallite size decreases [
30,
42,
43,
44]. However, their studies did not investigate the influence of heat in the process. By assisting the forming process with added heat, the crystallites increase. Kallip et al. [
32] determined that the crystallite size grows when heat is supplied through the hot compaction production of the nanocomposite and that the hardness is higher when compared to cold compaction production. This is due to the pinning effect on reinforcing the constituent at the crystallite boundaries [
45,
46]. In short, the composite crystallite size increases not only due to the heat-assisted process, but due to Al
2O
3 pinned along the crystallite boundaries which yields high composite microhardness.
The residuals for Vickers microhardness, between the minimum and maximum parameter settings, in comparison with our results from unreinforced recycled AA6061 with HPF at 530 °C for 120 min, and the AR AA6061 sample are depicted in
Figure 5. There is an obvious difference between the minimum and maximum parameter settings. The increase from 71.36 to 86.66 HV
0.3 confirms that the temperature and holding time increase yielded better composite hardness. Previously, Yusuf et al. [
20] recorded a hardness of 81.74 HV
0.3 when aluminum chips were pressed at 530 °C with a 120-min holding time [
47]. The increase of 6.02% is mostly due to the presence of Al
2O
3 particles which reinforce and strengthen the matrix and therefore increase the hardness value. As mentioned before, the hardness of AR aluminum was verified as being 95.51 HV
0.3. When comparing the data to our present findings, the residuals were calculated at 10.21%. Initially, the atom structure in pure AL AA6061 is stable, with solid bonding, which leads to high hardness.
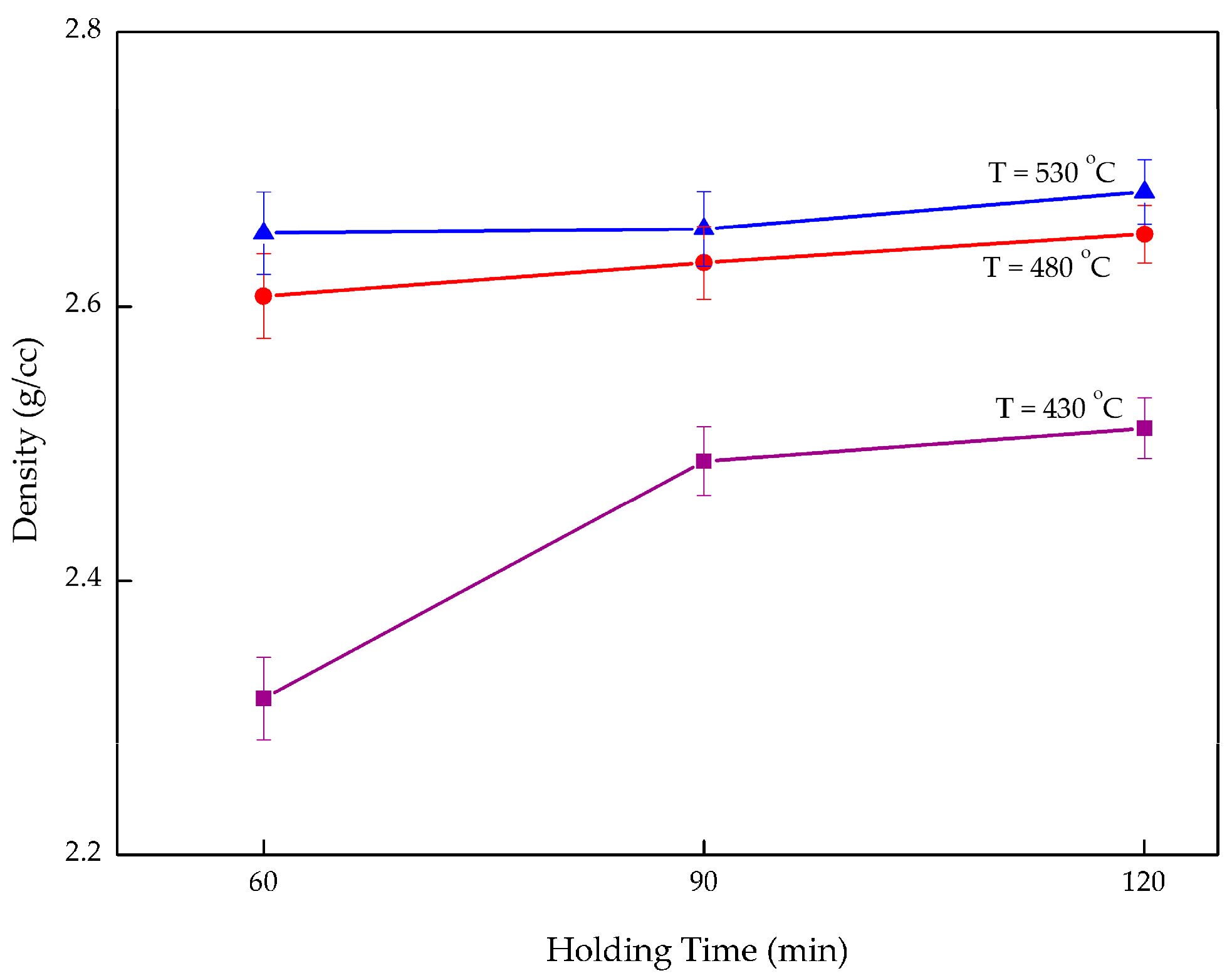
3.2. Density
Overall, density increases with increasing holding time and the operating temperature as exhibited in
Figure 6. When maintaining the temperature at 480 °C and varying holding time, the composite density increased 1.73% from 2.608 g/cc after 60 min to 2.653 g/cc after 120 min. By increasing the temperature but maintaining the holding time at 120 min, the density rose 6.85% from 2.512 g/cc at 430 °C to 2.684 g/cc at 530 °C. This again confirms that operating temperature plays a vital role in enhancing the composite performance. Insufficient temperature and holding time both affected the composite in terms of creating a large void between the chips as well as lowering the density. When exposing composites to a higher temperature, the gaps in the matrix shrink which allows Al
2O
3 to occupy the opening and strengthen the ductile aluminum. Increasing the holding time allowed better bonding between the chips. The right combination of high temperature and longer holding times would eventually lead to high density. In addition, the increase in the composite density may be attributed to the large difference of the initial densities [
38]. The void between the chips decreases when the temperature and holding time increase. This results in it being easier for the Al
2O
3 particles to fill the voids and decrease the porosity, thus enhancing the density of the composite.
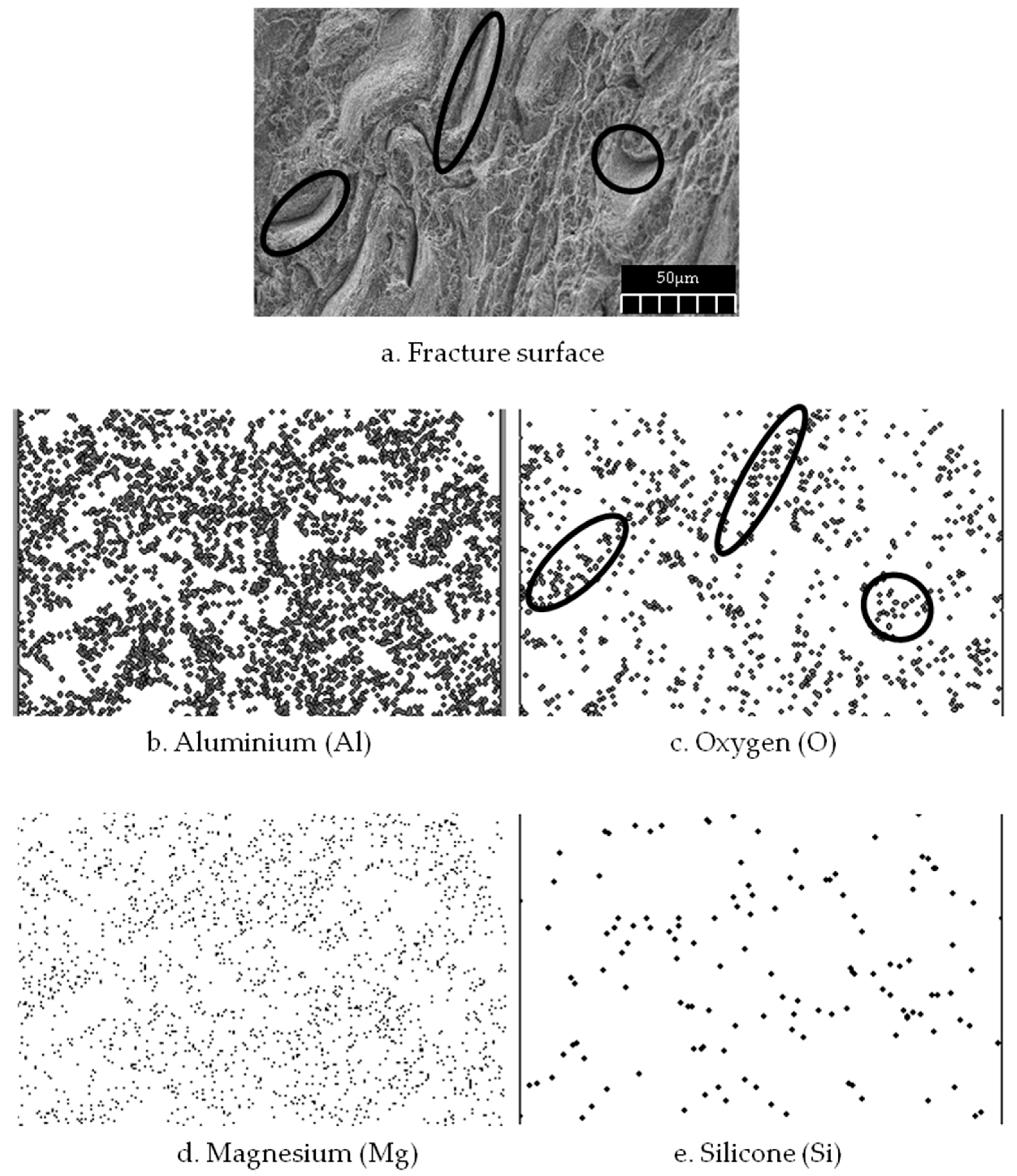
Al
2O
3 contains a higher amount of oxygen compared to the initial matrix element (AA6061), which mostly contains aluminum, magnesium, and silicone. However, to confirm the composite distributions on an atomic scale, X-ray mapping was performed (
Figure 7). The mapping showed that the interface is rich in Mg, Fe, and Si. The reaction between Al
2O
3 and the intermetallic compound leads to the formation of brittle compounds, like the MgAl
2O
4 spinels, which do not dissolve during solution treatment [
48,
49]. A fractograph of the specimen is taken at the gauge area after tensile test (
Figure 7a). The mapping image shows that the mixing activity during the preparation process had uniformly distributed the Al
2O
3 throughout the recycled aluminum chips. In addition, the pressing action assisted by high heat was responsible for the agglomeration of Al
2O
3 in the gaps between the chips. The oxygen controls the distribution which means that the reinforcement particles were allocated within the gaps (
Figure 7c). These void-filling activities reduced the porosity and hence caused high composite density. The Al
2O
3 filling of the gaps within the matrix chips is also responsible for the increase in crystallite size.
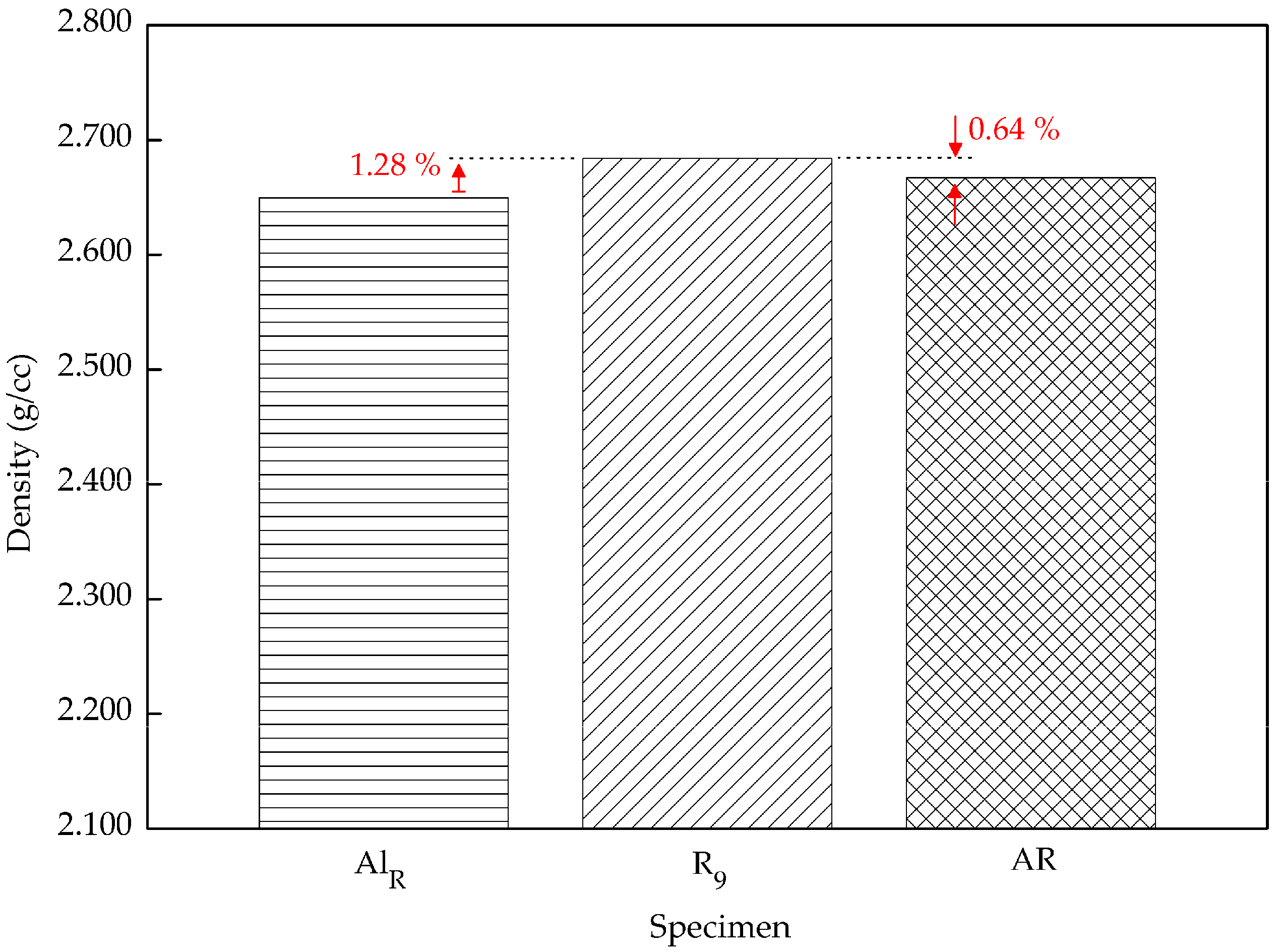
Figure 8 shows that the density has significantly increased from the lowest parameters, 60 min and 430 °C, to highest parameters, 120 min and 530 °C. Compared to the previous study by Yusuf [
47], in our study the reinforcement particle increased the composite density 1.28% higher. The void-filling activities had enhanced the composite density. Furthermore, the density of the AR material is 0.64% lower than the composite. This again proved that particulate Al
2O
3 assists in improving the density. The density of a composite is created by the densities of the two different constituents, which are unalike. Aluminum chips have a density of 2.667 g/cc, which is ~47% lower than Al
2O
3, at 3.9159 g/cc. According to the mixture rule, the theoretical densities were calculated using the weight fraction of the aluminum oxide particles [
22]. From the mixture rule, the calculated theoretical density is 2.692 g/cc. The value from this equation was used as a benchmark in comparing the maximum density with the theoretical density. Subsequently, the composite density was about 0.3% off of the theoretical calculation. This shows that at 530 °C and with a holding time of 120 min, paired with 2 wt % reinforcement, acceptable density can be obtained.
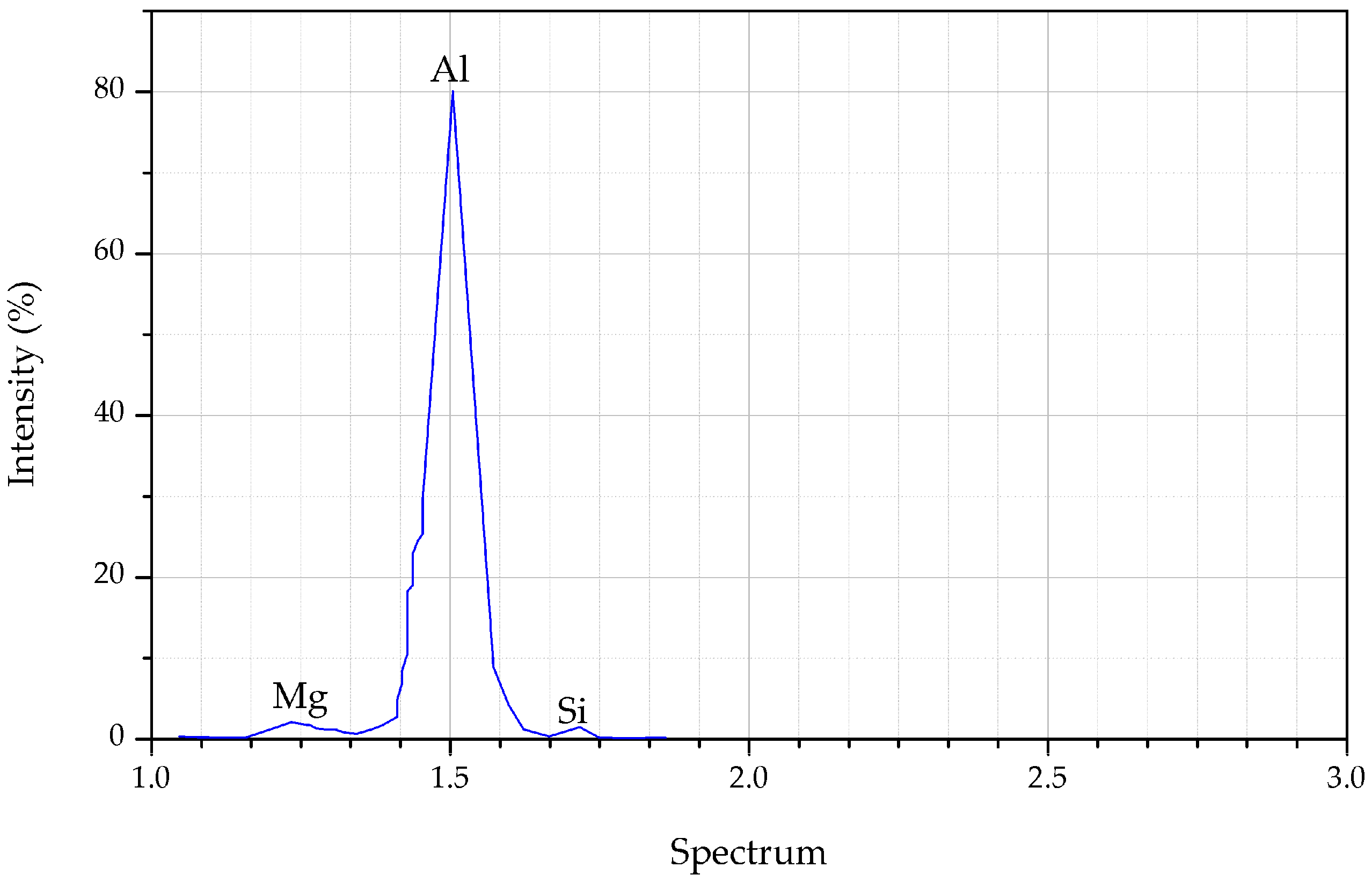
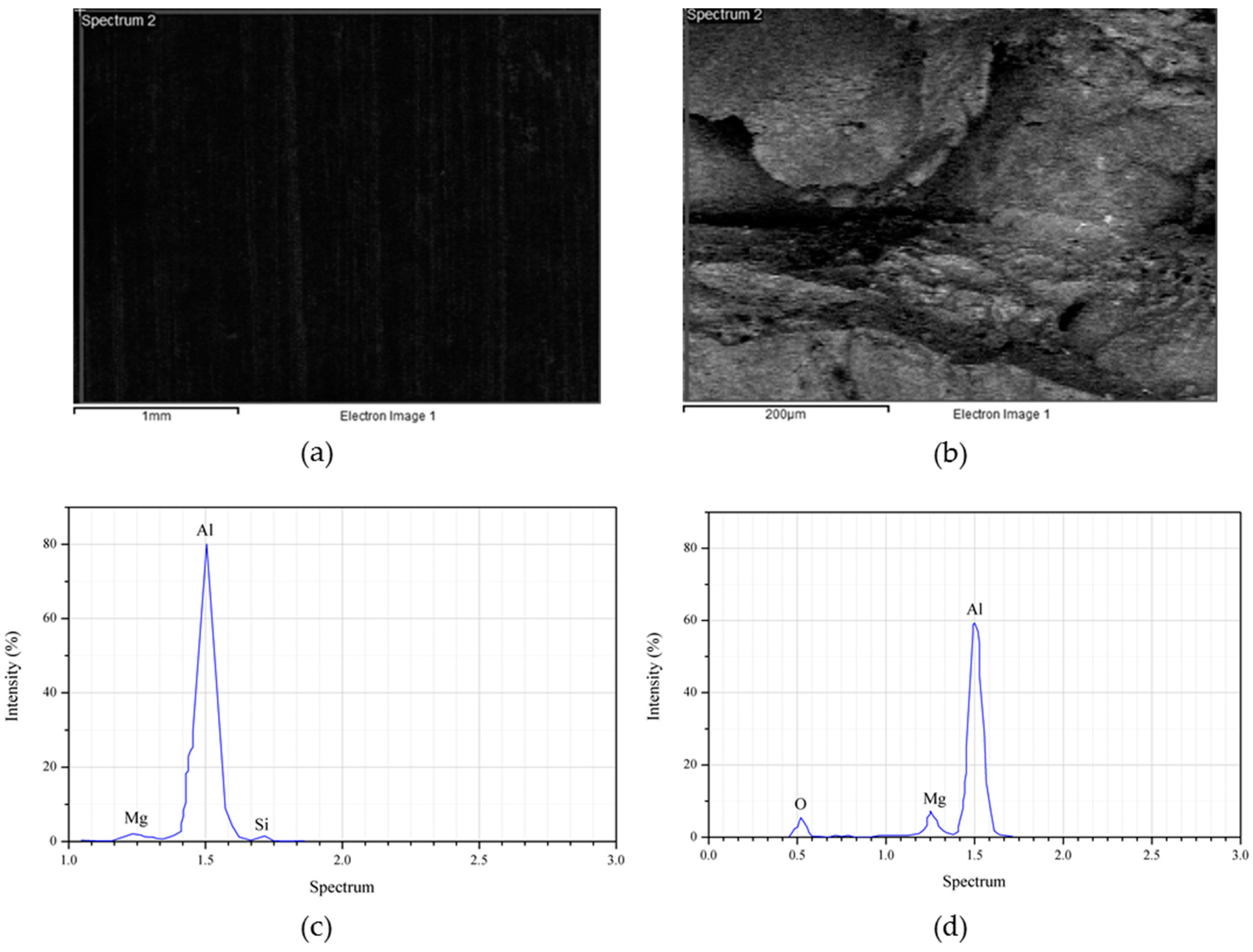
Figure 9 depicted the EDS spectra for AR AA6061 and the reinforced aluminum waste with 2.0 wt % Al
2O
3 particles. The scan of the surface was taken at the cross-section of both the AR and the composite specimen, shown in
Figure 9a,b respectively. The presence of Al, Mg, and Si were noticed from the peak present in the spectrum, confirming no contaminants were present (
Figure 9c). When the reinforcement particle was introduced into the pure alloy, the peak changed. Additionally, the oxygen peak appeared due to high oxygen from the aluminum oxide particles (
Figure 9d). On the other hand, the Si peak was hindered by the composite, which is as a result of high temperature and longer holding time. Previously, a study concluded that temperature increase leads to a refined silicon element [
50]. The fine silicon spread homogeneously in the microstructure and yielded better material strength [
51]. Also, the EDS and mapping analysis of the composite at maximum parameter settings indicated that the interfacial region was enriched with oxygen, especially within the composite gaps. Concisely, the reinforcement constituent helps to improve the composite performance either in terms of mechanical properties or surface integrity.