Enhanced Cycleability of Amorphous MnO2 by Covering on α-MnO2 Needles in an Electrochemical Capacitor
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of α-MnO2@amorphous MnO2
2.2. Characterizations
2.3. Electrochemical Measurements
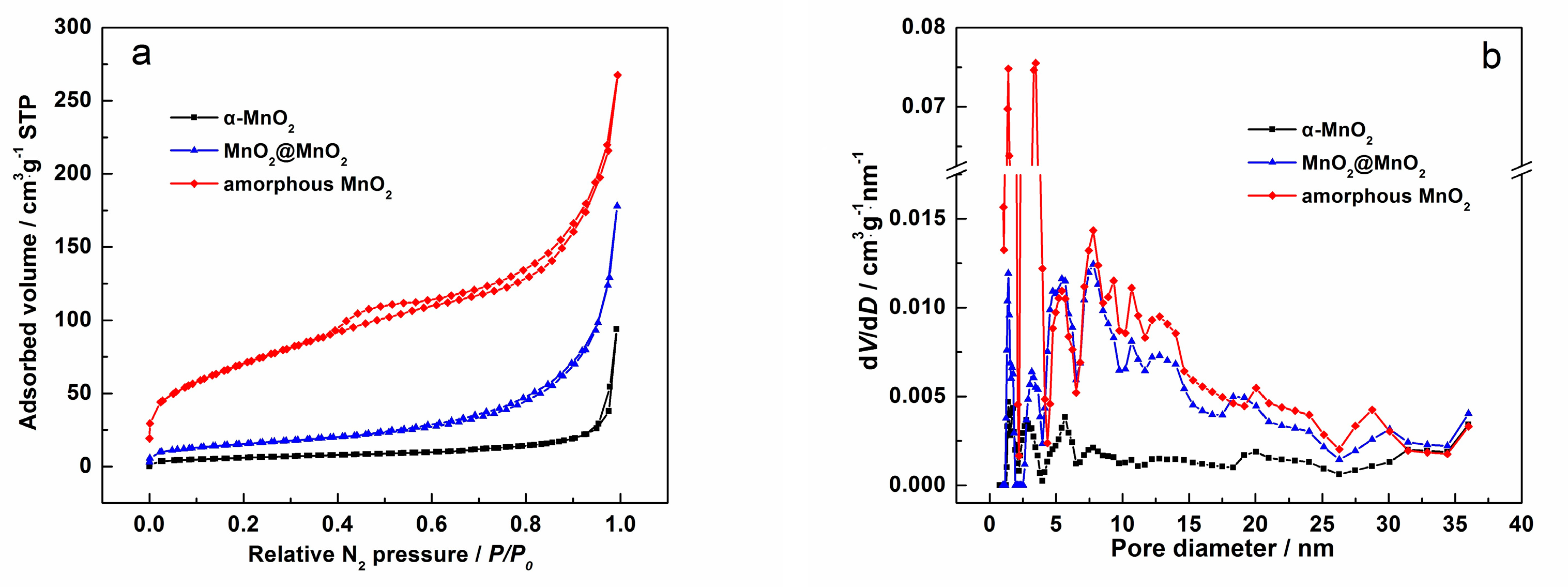
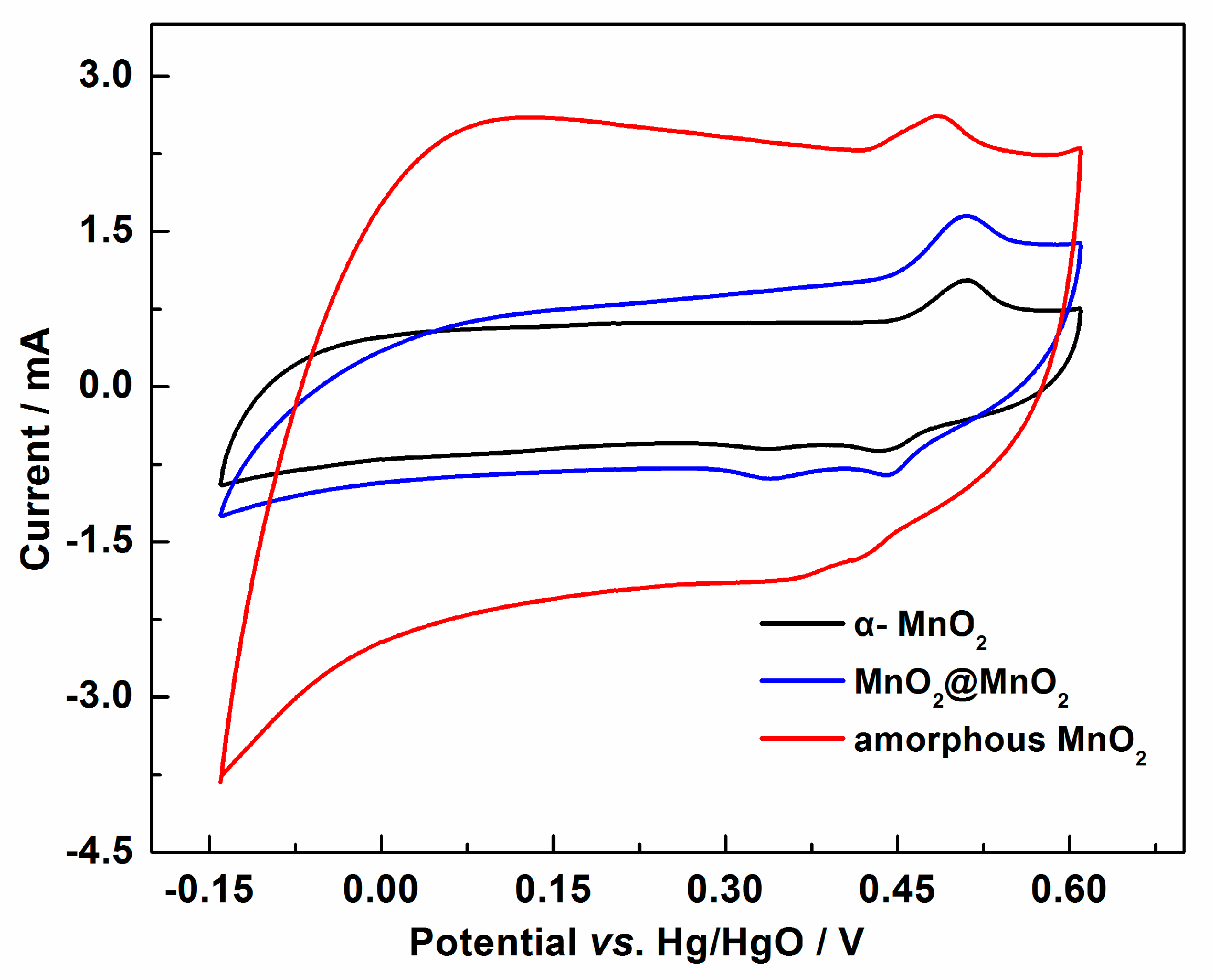
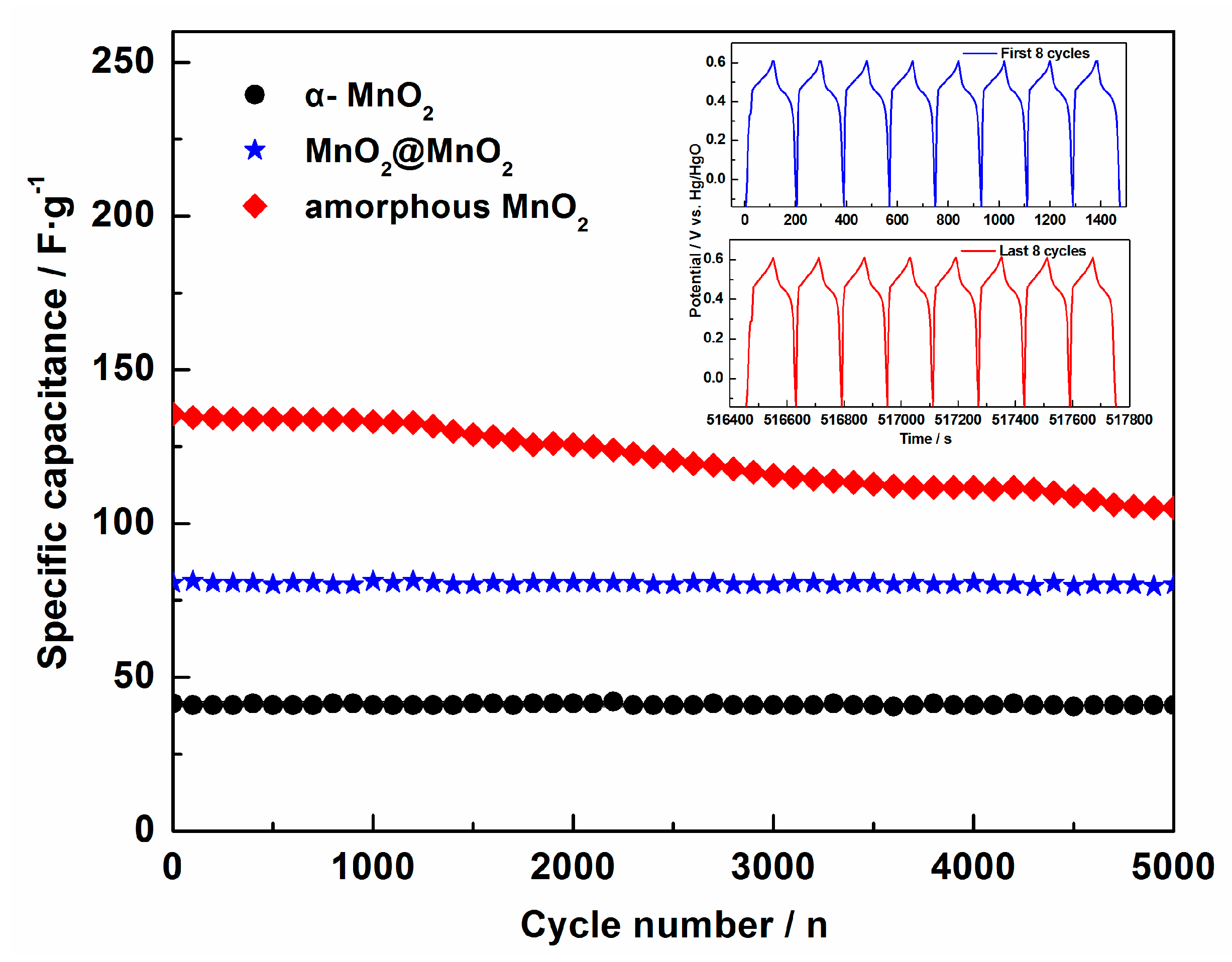
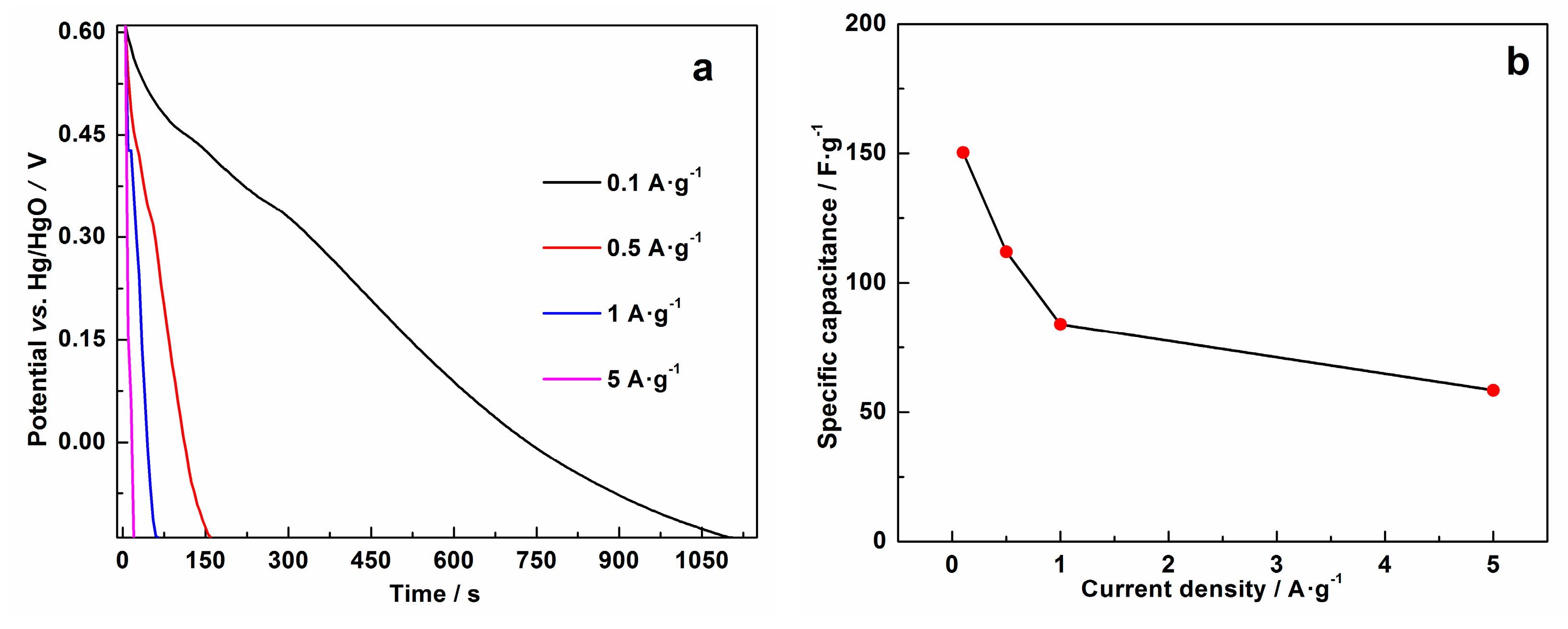
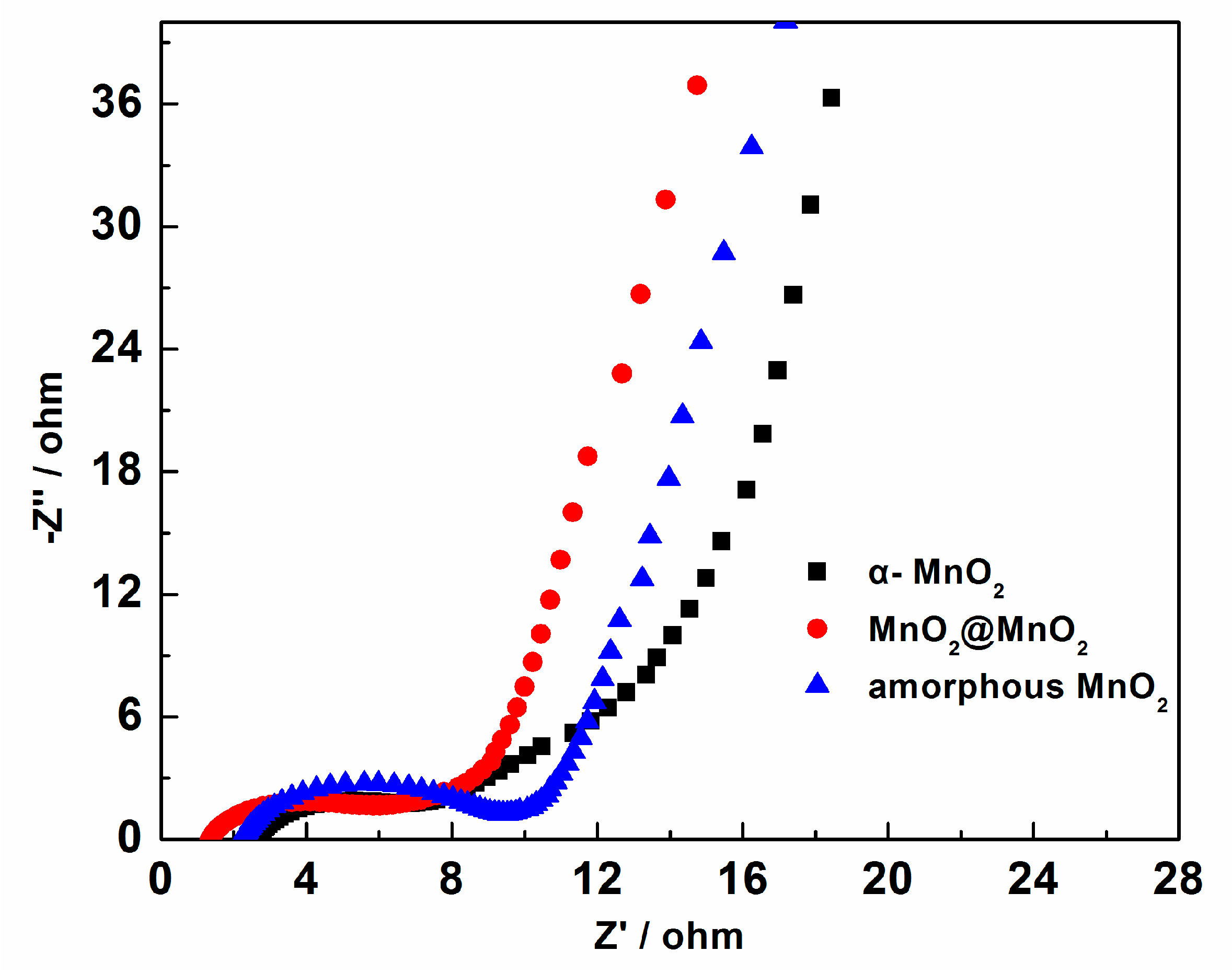
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jiang, R.; Huang, T.; Liu, J.; Zhuang, J.; Yu, A. A novel method to prepare nanostructured manganese dioxide and its electrochemical properties as a supercapacitor electrode. Electrochim. Acta 2009, 54, 3047–3052. [Google Scholar] [CrossRef]
- Wang, J.-G.; Yang, Y.; Huang, Z.-H.; Kang, F. Interfacial synthesis of mesoporous MnO2/polyaniline hollow spheres and their application in electrochemical capacitors. J. Power Sources 2012, 204, 236–243. [Google Scholar] [CrossRef]
- Li, S.-H.; Liu, Q.-H.; Qi, L.; Lu, L.-H.; Wang, H.-Y. Progress in research on manganese dioxide electrode materials for electrochemical capacitors. Chin. J. Anal. Chem. 2012, 40, 339–346. [Google Scholar] [CrossRef]
- Toupin, M.; Brousse, T.; Be’langer, D. Influence of microstucture on the charge storage properties of chemically synthesized manganese dioxide. Chem. Mater. 2002, 14, 3946–3952. [Google Scholar] [CrossRef]
- Wang, R.; Ma, Y.; Wang, H.; Key, J.; Brett, D.; Ji, S.; Yin, S.; Shen, P.K. A cost effective, highly porous, manganese oxide/carbon supercapacitor material with high rate capability. J. Mater. Chem. A 2016, 4, 5390–5394. [Google Scholar] [CrossRef]
- Minakshi, M.; Mitchell, D.R.G.; Prince, K. Incorporation of TiB2 additive into MnO2 cathode and its influence on rechargeability in an aqueous battery system. Solid State Ion. 2008, 179, 355–361. [Google Scholar] [CrossRef]
- Minakshi, M. Examining manganese dioxide electrode in KOH electrolyte using TEM technique. J. Electroanal. Chem. 2008, 616, 99–106. [Google Scholar] [CrossRef]
- Bao, S.-J.; He, B.-L.; Liang, Y.-Y.; Zhou, W.-J.; Li, H.-L. Synthesis and electrochemical characterization of amorphous MnO2 for electrochemical capacitor. Mater. Sci. Eng. A 2005, 397, 305–309. [Google Scholar] [CrossRef]
- Reddy, R.N.; Reddy, R.G. Synthesis and electrochemical characterization of amorphous MnO2 electrochemical capacitor electrode material. J. Power Sources 2004, 132, 315–320. [Google Scholar] [CrossRef]
- Xu, C.; Li, B.; Du, H.; Kang, F.; Zeng, Y. Supercapacitive studies on amorphous MnO2 in mild solutions. J. Power Sources 2008, 184, 691–694. [Google Scholar] [CrossRef]
- Zhan, H.; Zhou, Y.H.; Wang, C.G.; Guo, W.Y.; Peng, Z.H. Improved electrochemical performance of amorphous MnO2 modified by a novel supermolecular compound. Mater. Chem. Phys. 2005, 90, 353–360. [Google Scholar] [CrossRef]
- Shao, J.; Zhou, X.; Liu, Q.; Zou, R.; Li, W.; Yang, J.; Hu, J. Mechanism analysis of the capacitance contributions and ultralong cycling-stability of the isomorphous MnO2@MnO2 core/shell nanostructures for supercapacitors. J. Mater. Chem. A 2015, 3, 6168–6176. [Google Scholar] [CrossRef]
- Ma, Z.; Shao, G.; Fan, Y.; Wang, G.; Song, J.; Shen, D. Construction of hierarchical α-MnO2 Nanowires@Ultrathin δ-MnO2 nanosheets core-shell nanostructure with excellent cycling stability for high-power asymmetric supercapacitor electrodes. ACS Appl. Mater. Interfaces 2016, 8, 9050–9058. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zhang, Y.; Li, F.; Zhang, L.; Wen, Z.; Liu, Q. Facile synthesis of hierarchical Co3O4@MnO2 core-shell arrays on Ni foam for asymmetric supercapacitors. J. Power Sources 2014, 252, 98–106. [Google Scholar] [CrossRef]
- Yang, W.; Gao, Z.; Ma, J.; Zhang, X.; Wang, J. Controlled synthesis of Co3O4 and Co3O4@MnO2 nanoarchitectures and their electrochemical capacitor application. J. Alloys Compd. 2014, 611, 171–178. [Google Scholar] [CrossRef]
- Wang, K.; Shi, Z.; Wang, Y.; Ye, Z.; Xia, H.; Liu, G.; Qiao, G. Co3O4 nanowires@MnO2 nanolayer or nanoflakes core-shell arrays for high-performance supercapacitors: The influence of morphology on performance. J. Alloys Compd. 2015, 624, 85–93. [Google Scholar] [CrossRef]
- Yan, J.; Khoo, E.; Sumboja, A.; Lee, P.S. Facile coating of manganese oxide on tin oxide nanowires with high-performance capacitive behavior. ACS Nano 2010, 4, 4247–4255. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, J.; Bosman, M.; Fan, H.J. Three-dimensional tubular arrays of MnO2–NiO nanoflakes with high areal pseudocapacitance. J. Mater. Chem. 2012, 22, 2419–2426. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, G.; Yuan, C.; Lou, X.W.D. Hierarchical NiCo2O4@MnO2 core-shell heterostructured nanowire arrays on Ni foam as high-performance supercapacitor electrodes. Chem. Commun. 2013, 49, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, R.; Wang, H.; Key, J.; Ji, S. Control of MnO2 nanocrystal shape from tremella to nanobelt for ehancement of the oxygen reduction reaction activity. J. Power Sources 2015, 280, 526–532. [Google Scholar] [CrossRef]
- Brunauer, S.; Demine, L.S.; Deming, W.E.; Teller, E. On a theory of the van der Waals adsorption of gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, H.; Ji, S.; Linkov, V.; Wang, R. Soybean-derived mesoporous carbon as an effective catalyst support for electrooxidation of methanol. J. Power Sources 2014, 248, 427–433. [Google Scholar] [CrossRef]
- Qu, Q.; Zhang, P.; Wang, B.; Chen, Y.; Tian, S.; Wu, Y.; Holze, R. Electrochemical performance of MnO2 nanorods in neutral aqueous electrolytes as a cathode for asymmetric supercapacitors. J. Phys. Chem. C 2009, 113, 14020–14027. [Google Scholar] [CrossRef]
- Sodtipinta, J.; Pon-On, W.; Veerasai, W.; Smith, S.M.; Pakawatpanurut, P. Chelating agent- and surfactant-assisted synthesis of manganese oxide/carbon nanotube composite for electrochemical capacitors. Mater. Res. Bull. 2013, 48, 1204–1212. [Google Scholar] [CrossRef]
- Pang, M.; Long, G.; Jiang, S.; Ji, Y.; Han, W.; Wang, B.; Liu, X.; Xi, Y. Rapid synthesis of graphene/amorphous α-MnO2 composite with enhanced electrochemical performance for electrochemical capacitor. Mater. Sci. Eng. B 2015, 194, 41–47. [Google Scholar] [CrossRef]
- Zhao, Y.; Meng, Y.; Jiang, P. Carbon@MnO2 core-shell nanospheres for flexible high-performance supercapacitor electrode materials. J. Power Sources 2014, 259, 219–226. [Google Scholar] [CrossRef]
- Han, D.; Jing, X.; Xu, P.; Ding, Y.; Liu, J. Facile synthesis of hierarchical hollow ε-MnO2 spheres and their application in supercapacitor electrodes. J. Solid State Chem. 2014, 218, 178–183. [Google Scholar] [CrossRef]
- Lee, T.T.; Hong, J.R.; Lin, W.C.; Hu, C.C.; Wu, P.W.; Li, Y.Y. Synthesis of petal-like carbon Nanocapsule@MnO2 core-shell particles and their application in supercapacitors. J. Electrochem. Soc. 2014, 161, H598–H605. [Google Scholar] [CrossRef]
- Min, S.; Zhao, C.; Zhang, Z.; Wang, K.; Chen, G.; Qiana, X.; Guo, Z. Hydrothermal growth of MnO2/RGO/Ni(OH)2 on nickel foam with superior supercapacitor performance. RSC Adv. 2015, 5, 62571–62576. [Google Scholar] [CrossRef]
- Ren, Q.; Wang, R.; Wang, H.; Key, J.; Brett, D.J.L.; Ji, S.; Yin, S.; Shen, P.K. Ranunculus flower-like Ni(OH)2@Mn2O3 as a high specific capacitance cathode material for alkaline supercapacitors. J. Mater. Chem. A 2016, 4, 7591–7595. [Google Scholar] [CrossRef]
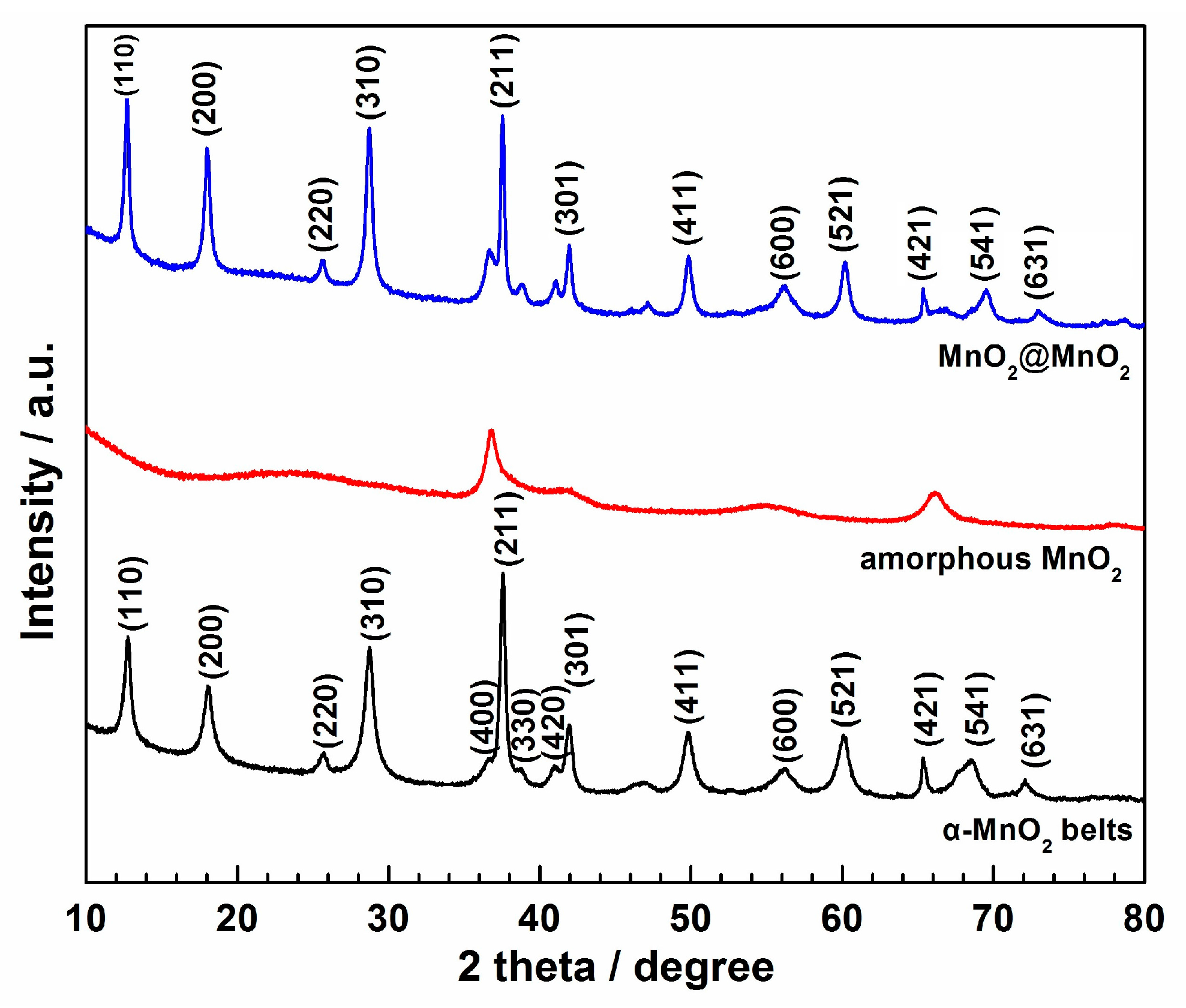
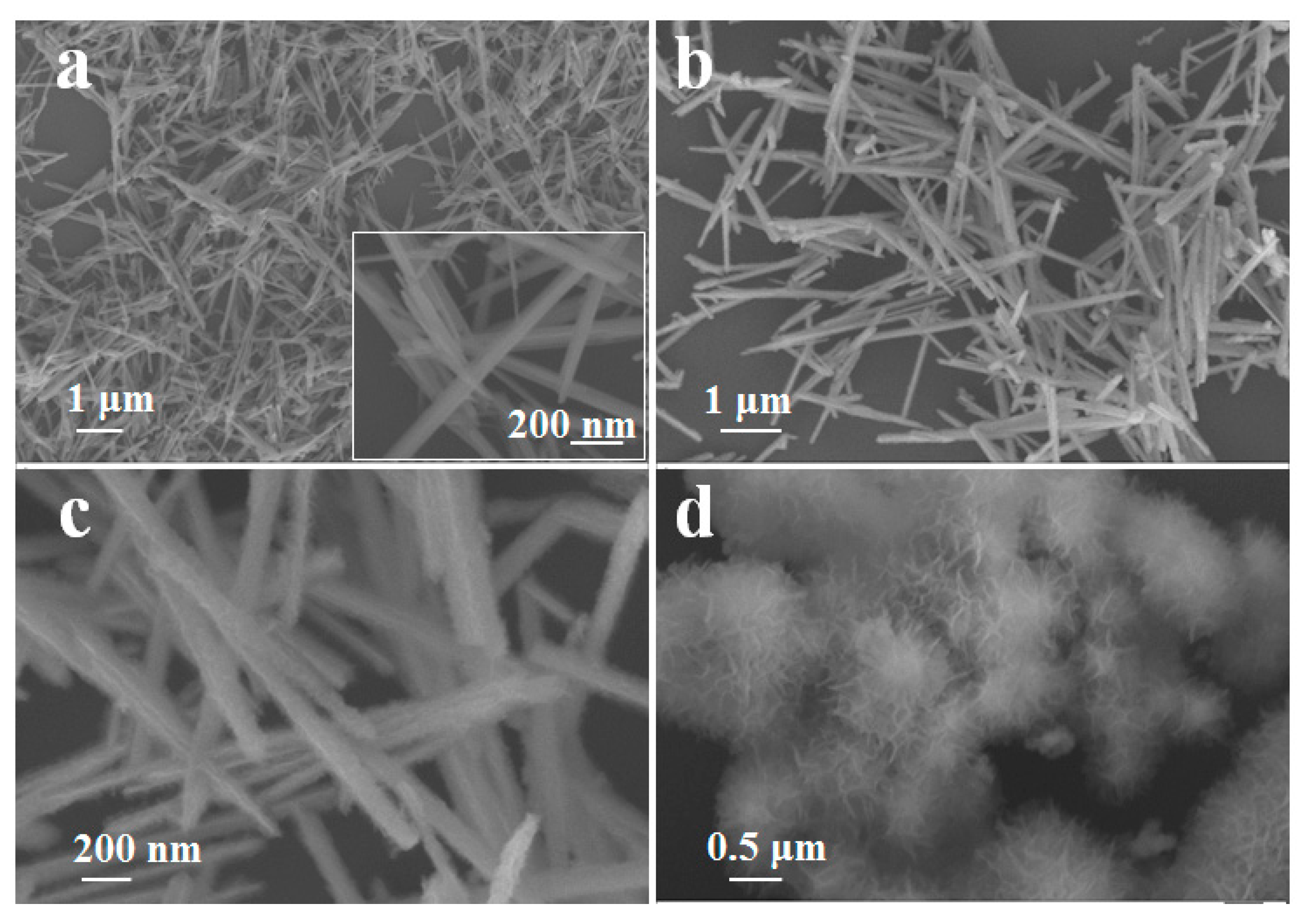
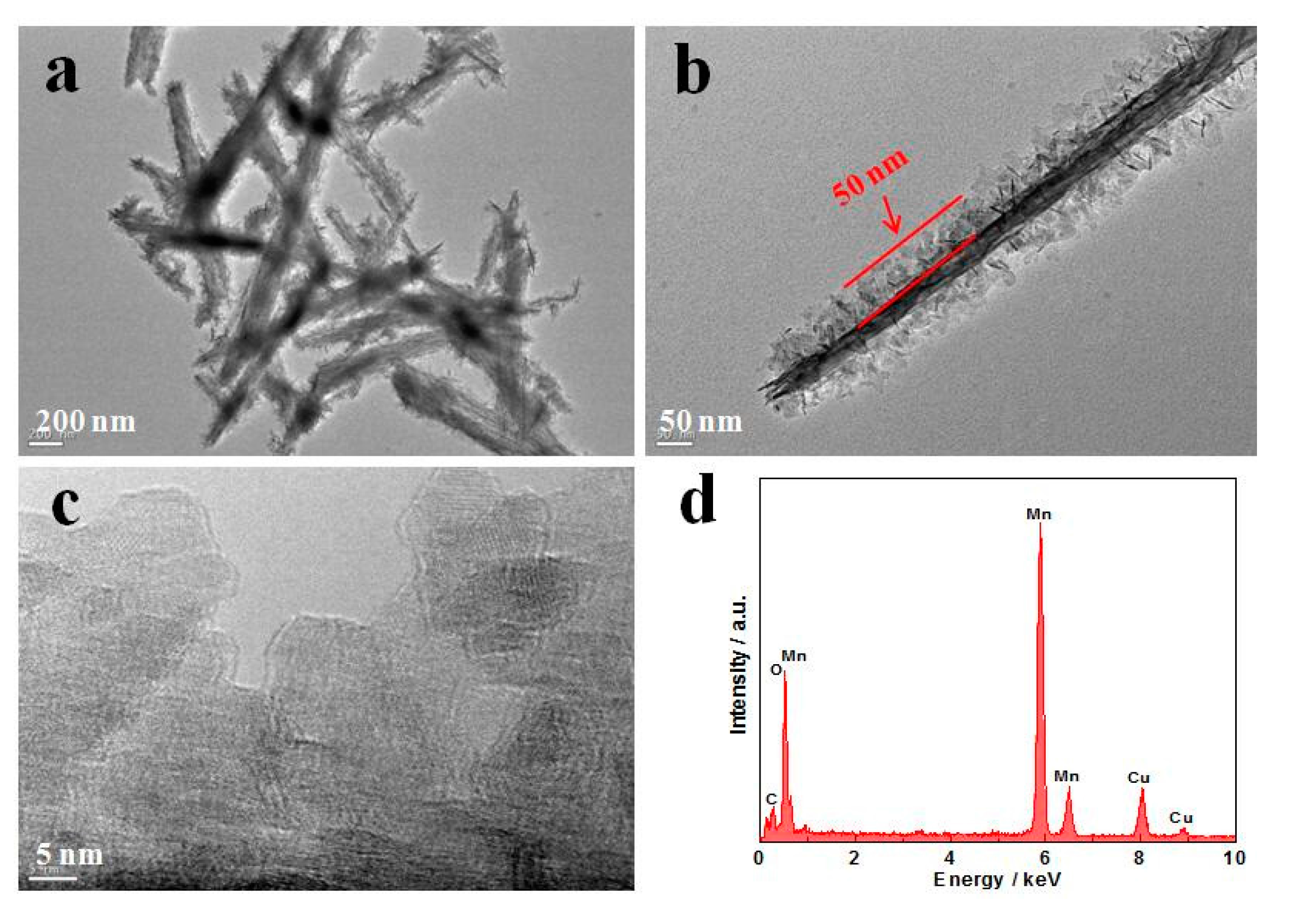

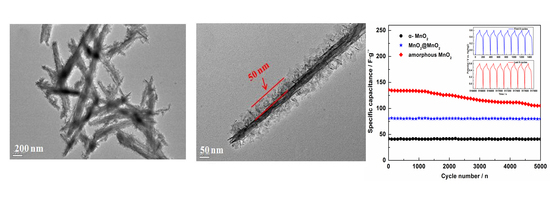
| Samples | [Ref.] | The specific Capacitance (F·g−1) | Electrolyte Type & Concentration | Scan Rate or Current Density |
|---|---|---|---|---|
| Amorphous MnO2 | herein | 247 | 1.0 M LiOH | 0.1 A·g−1 |
| MnO2@MnO2 | herein | 150 | 1.0 M LiOH | 0.1 A·g−1 |
| Amorphous MnO2 | [8] | 242 | 0.5 M Na2SO4 | 2 mA·cm−2 |
| Amorphous MnO2 | [9] | 110 | 2 M NaCl | 5 mV·s−1 |
| Amorphous MnO2 | [10] | 225 | 1.0 M Li2SO4 | 2 mV·s−1 |
| 242 | 1.0 M Na2SO4 | |||
| 194 | 0.5 M K2SO4 | |||
| Amorphous MnO2 | [25] | 79 | 1.0 M Na2SO4 | 1 A·g−1 |
| MnO2@MnO2 | [12] | 108 | 0.5 M Na2SO4 | 5 A·g−1 |
| MnO2@MnO2 | [13] | 310 | 6.0 M KOH | 0.5 A·g−1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Ji, S.; Yang, J.; Wang, H.; Pollet, B.G.; Wang, R. Enhanced Cycleability of Amorphous MnO2 by Covering on α-MnO2 Needles in an Electrochemical Capacitor. Materials 2017, 10, 988. https://doi.org/10.3390/ma10090988
Liu Q, Ji S, Yang J, Wang H, Pollet BG, Wang R. Enhanced Cycleability of Amorphous MnO2 by Covering on α-MnO2 Needles in an Electrochemical Capacitor. Materials. 2017; 10(9):988. https://doi.org/10.3390/ma10090988
Chicago/Turabian StyleLiu, Quanbing, Shan Ji, Juan Yang, Hui Wang, Bruno G. Pollet, and Rongfang Wang. 2017. "Enhanced Cycleability of Amorphous MnO2 by Covering on α-MnO2 Needles in an Electrochemical Capacitor" Materials 10, no. 9: 988. https://doi.org/10.3390/ma10090988