Effect of Novel Quercetin Titanium Dioxide-Decorated Multi-Walled Carbon Nanotubes Nanocomposite on Bacillus subtilis Biofilm Development
Abstract
:1. Introduction
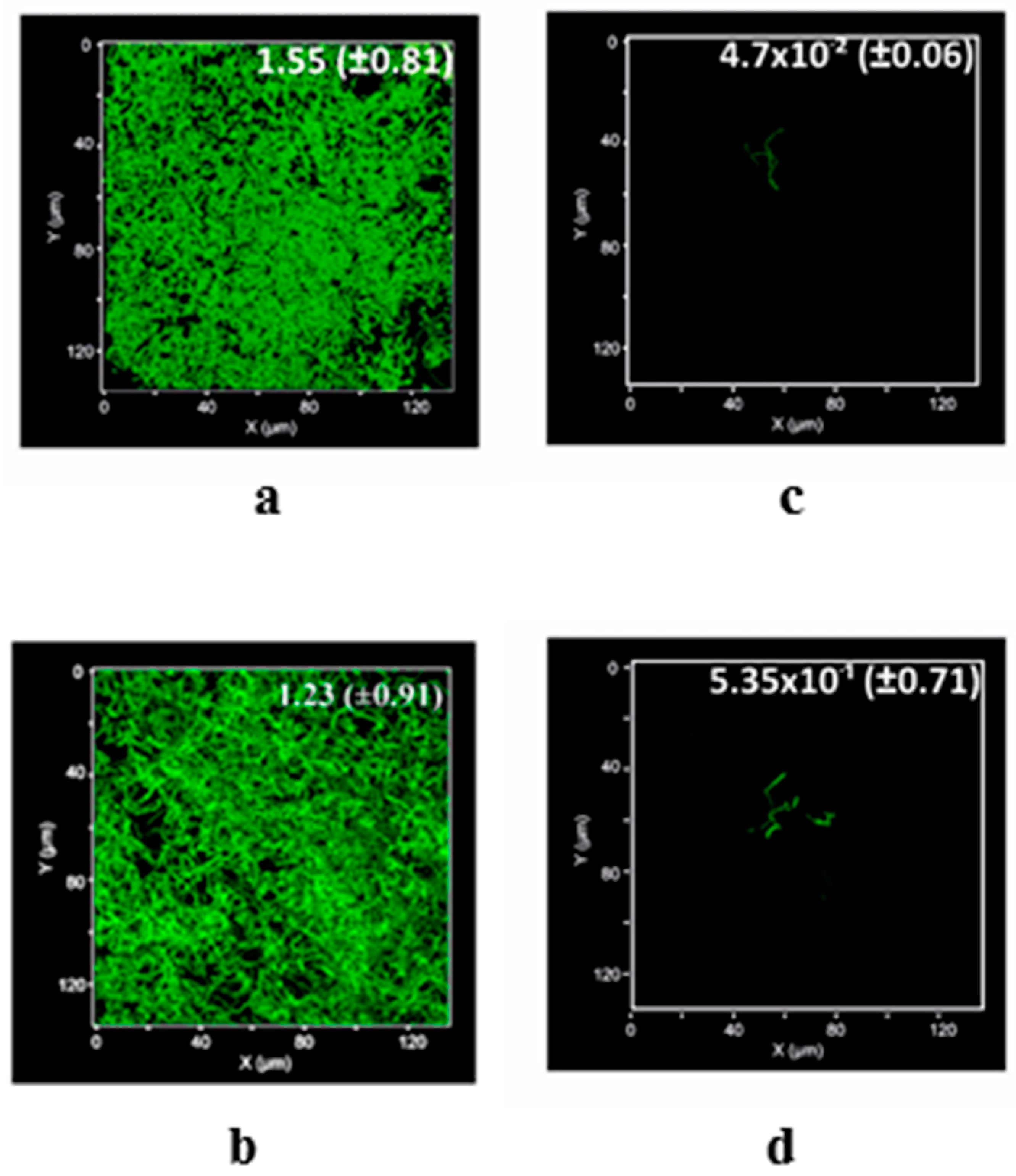
2. Results
3. Discussion
4. Materials and Methods
4.1. Preparation of Nanomaterials
4.2. Characterization of Nanomaterials
4.3. Coating Glass Surface and Characterization
4.4. Induced Biofilm Development Test
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dragoš, A.; Kovács, Á.T. The Peculiar Functions of the Bacterial Extracellular Matrix. Trends Microbiol. 2017, 25, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Vlamakis, H.; Chai, Y.; Beauregard, P.; Losick, R.; Kolter, R. Sticking together: Building a biofilm the Bacillus subtilis way. Nat. Rev. Microbiol. 2013, 11, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Seiffert, F.; Bandow, N.; Bouchez, J.; Von Blanckenburg, F.; Gorbushina, A.A. Microbial colonization of bare rocks: Laboratory biofilm enhances mineral weathering. Procedia Earth Planet. Sci. 2014, 10, 123–129. [Google Scholar] [CrossRef]
- Lan, S.; Wu, L.; Yang, H.; Zhang, D.; Hu, C. A new biofilm based microalgal cultivation approach on shifting sand surface for desert cyanobacterium Microcoleus vaginatus. Bioresour. Technol. 2017, 238, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Peng, D.; Walker, S.L.; Cao, B.; Gao, C.H.; Huang, Q.; Cai, P. Bacillus subtilis biofilm development in the presence of soil clay minerals and iron oxides. NPJ Biofilms Microbiomes 2017, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Labena, A.; Hegazy, M.A.; Horn, H.; Muller, E. The biocidal effect of a novel synthesized gemini surfactant on environmental sulfidogenic bacteria: Planktonic cells and biofilms. Mater. Sci. Eng. C 2015, 47, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Beloin, C.; Fernández-Hidalgo, N.; Lebeaux, D. Understanding biofilm formation in intravascular device-related infections. Intensive Care Med. 2017, 43, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Besseling, E.; Quik, J.T.K.; Sun, M.; Koelmans, A.A. Fate of nano- and microplastic in freshwater systems: A modeling. Environ. Pollut. 2017, 220, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Raie, D.S.; Mhatre, E.; Thiele, M.; Labena, A.; El-Ghannam, G.; Farahat, L.A.; Youssef, T.; Fritzsche, W.; Kovács, Á.T. Application of quercetin and its bio-inspired nanoparticles as anti-adhesive agents against Bacillus subtilis attachment to surface. Mater. Sci. Eng. C 2017, 70, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.K.; Byun, S.H.; Woo, J.M.; Kim, S.M.; Lee, S.M.; Kim, B.J.; Kim, H.E.; Lee, J.W.; Kim, S.M.; Lee, J.H. Biocompatibility and Biocorrosion of Hydroxyapatite-Coated Magnesium Plate: Animal Experiment. Materials 2017, 10, 1149. [Google Scholar] [CrossRef] [PubMed]
- Labena, A.; Hegazy, M.A.; Horn, H.; Müller, E. Sulfidogenic-corrosion inhibitory effect of cationic monomeric and gemini surfactants: Planktonic and sessile diversity. RSC Adv. 2016, 6, 42263–42278. [Google Scholar] [CrossRef]
- Ramesh, T.; Nayak, B.; Amirbahman, A.; Tripp, C.P.; Mukhopadhyay, S. Application of ultraviolet light assisted titanium dioxide photocatalysis for food safety: A review. Innov. Food Sci. Emerg. Technol. 2016, 38, 105–115. [Google Scholar] [CrossRef]
- Wang, J.; Liu, M.; Xiao, H.; Wu, W.; Xie, M.; Sun, M.; Zhu, C.; Li, P. Bacterial community structure in cooling water and biofilm in an industrial recirculating cooling water system. Water Sci. Technol. 2013, 68, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, J.; Chen, H.; Chen, D. Progress of marine biofouling and antifouling technologies. Chin. Sci. Bull. 2011, 56, 598–612. [Google Scholar] [CrossRef]
- Shah, R. The Antibacterial Properties of Brookite Phase Titanium Dioxide Nanoparticles against Methicillin-Resistant Staphylococcus aureus. Ph.D. Thesis, Clemson University, Clemson, SC, USA, 2007. [Google Scholar]
- Schneider, I.; Topalova, Y. Microbial Structure and Functions of Biofilm during Wastewater Treatment in the Dairy Industry. Biotechnol. Biotechnol. Equip. 2013, 27, 3782–3786. [Google Scholar] [CrossRef]
- Cresson, R.; Carr, H.; Delgen, J.P.; Bernet, N. Biofilm formation during the start-up period of an anaerobic biofilm reactor—Impact of nutrient complementation. Biochem. Eng. J. 2006, 30, 55–62. [Google Scholar] [CrossRef]
- Demirel, B.; Yenigun, O.; Onay, T.T. Anaerobic treatment of dairy wastewaters: A review. Process Biochem. 2005, 40, 2583–2595. [Google Scholar] [CrossRef]
- Gajda, I.; Stinchcombe, A.; Greenman, J.; Melhuish, C.; Ieropoulos, I. Microbial fuel cell—A novel self-powered wastewater electrolyser for electrocoagulation of heavy metals. Int. J. Hydrogen Energy 2017, 42, 1813–1819. [Google Scholar] [CrossRef]
- Srinophakun, P.; Thanapimmetha, A.; Plangsri, S.; Vetchayakunchai, S.; Saisriyoot, M. Application of modified chitosan membrane for microbial fuel cell: Roles of proton carrier site and positive charge. J. Clean. Prod. 2017, 142, 1274–1282. [Google Scholar] [CrossRef]
- Tamilarasan, K.; Banu, J.R.; Jayashree, C.; Yogalakshmi, K.N.; Gokulakrishnan, K. Effect of organic loading rate on electricity generating potential of upflow anaerobic microbial fuel cell treating surgical cotton industry wastewater. J. Environ. Chem. Eng. 2017, 5, 1021–1026. [Google Scholar] [CrossRef]
- Park, Y.; Park, S.; Nguyen, V.K.; Yu, J.; Torres, C.I.; Rittmann, B.E.; Lee, T. Complete nitrogen removal by simultaneous nitrification and denitrification in flat-panel air-cathode microbial fuel cells treating domestic wastewater. Chem. Eng. J. 2017, 316, 673–679. [Google Scholar] [CrossRef]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Feng, G.; Cheng, Y.; Wang, S.Y.; Borca-Tasciuc, D.A.; Worobo, R.W.; Moraru, C.I. Bacterial attachment and biofilm formation on surfaces are reduced by small-diameter nanoscale pores: How small is small enough? NPJ Biofilms Microbiomes 2015, 1, 15022–15030. [Google Scholar] [CrossRef] [PubMed]
- Sotiri, I.; Overton, J.C.; Waterhouse, A.; Howell, C. Immobilized liquid layers: A new approach to anti-adhesion surface for medical applications. Exp. Biol. Med. 2016, 241, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, T.; Cui, T. Wettability conversion from superoleophobic to superhydrophilic on titania/single-walled carbon nanotube composite coatings. Langmuir 2011, 27, 9295–9301. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Hwang, H.M.; Wang, L.; Kim, I.; Yoon, Y.; Lee, H. Solar-light photocatalytic disinfection using crystalline/amorphous low energy bandgap reduced TiO2. Sci. Rep. 2016, 6, 25212. [Google Scholar] [CrossRef] [PubMed]
- Rabie, G.H.; Hegazy, H.S.; Shaban, L.D.; Raie, D.S. Extracellular Bio-synthesis of Bio-active Nano-silver Using Alfalfa Seedling. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 87–93. [Google Scholar]
- Halo, M.; Ferrari, A.M.; Berlier, G.; Miletto, I.; Casassa, S. Experimental and first-principles IR characterization of quercetin adsorbed on a silica surface. Theor. Chem. Acc. 2016, 135, 123. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, M.; Li, Y.; He, X. A molecularly imprinted polymer with incorporated Graphene oxide for electrochemical determination of quercetin. Sensors 2013, 13, 5493–5506. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific Opinion on the substantiation of health claims related to quercetin and protection of DNA, proteins and lipids from oxidative damage (ID 1647), “cardiovascular system” (ID 1844), “mental state and performance” (ID 1845), and “liver, kidneys” (ID 1846) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2067. [Google Scholar]
- Chen, Y.; Yan, F.; Chai, Y.; Liu, H.; Kolter, R.; Losick, R.; Guo, J.H. Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environ. Microbiol. 2013, 15, 848–864. [Google Scholar] [CrossRef] [PubMed]
- Awadallah, A.E.; Abdel-Hamid, S.M.; El-Desouki, D.S.; Aboul-Enein, A.A.; Aboul-Gheit, A.K. Synthesis of carbon nanotubes by CCVD of natural gas using hydrotreating catalysts. Egypt. J. Pet. 2012, 21, 101–107. [Google Scholar] [CrossRef]
- Wen, Z.; Ci, S.; Mao, S.; Cui, S.; Lu, G.; Yu, K.; Luo, S.; He, Z.; Chen, J. TiO2 nanoparticles-decorated carbon nanotubes for significantly improved bioelectricity generation in microbial fuel cells. J. Power Sources 2013, 234, 100–106. [Google Scholar] [CrossRef]
- Niederberger, M. Nonaqueous Sol–Gel Routes to Metal Oxide Nanoparticles. Acc. Chem. Res. 2007, 40, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Derjaguin, B.; Landau, L. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Prog. Surf. Sci. 1941, 14, 633–662. [Google Scholar] [CrossRef]
- Pina-Salazar, E.Z.; Kaneko, K. Adsorption of water vapor on mesoporosity-controlled singe wall carbon nanohorn. Colloids Interface Sci. Commun. 2015, 5, 8–11. [Google Scholar] [CrossRef]
- Lin, X.Q.; He, J.B.; Zha, Z.G. Simultaneous determination of quercetin and rutin at a multi-wall carbon-nanotube paste electrodes by reversing differential pulse voltammetry. Sens. Actuators B 2006, 119, 608–614. [Google Scholar] [CrossRef]
- Suttiponparnit, K.; Jiang, J.; Sahu, M.; Suvachittanont, S.; Charinpanitkul, T.; Biswas, P. Role of Surface Area, Primary Particle Size, and Crystal Phase on Titanium Dioxide Nanoparticle Dispersion Properties. Nanoscale Res. Lett. 2011, 6, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, H.S.; Shabaan, L.D.; Rabie, G.H.; Raie, D.S. Biosynthesis of silver nanoparticles using cell free callus exudates of Medicago sativa L. Pak. J. Bot. 2015, 47, 1825–1829. [Google Scholar]
- Sumner, A.L.; Menke, E.J.; Dubowski, Y.; Newberg, J.T.; Penner, R.M.; Hemminger, J.C.; Wingen, L.M.; Brauers, T.; Finlayson-Pitts, B.J. The nature of water on surfaces of laboratory systems and implications for heterogeneous chemistry in the troposphere. Phys. Chem. Chem. Phys. 2004, 6, 604–613. [Google Scholar] [CrossRef]
- Mattia, D.; Rossi, M.P.; Kim, B.M.; Korneva, G.; Bau, H.H.; Gogotsi, Y. Effect of graphitization on the wettability and electrical conductivity of CVD-carbon nanotubes and films. J. Phys. Chem. B 2006, 110, 9850–9855. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.A.; Bharatiya, B.; Casas, M.; Lage, E.V.; Sandez-Macho, I.; Pal, H.; Bahadur, P. A multitechnique approach on adsorption, self-assembly and quercetin solubilization by Tetronics® micelles in aqueous solutions modulated by glycine. Colloids Surf. B 2016, 148, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Malanovic, N.; Lohner, K. Gram-positive bacterial cell envelopes: The impact on the activity of antimicrobial peptides. Biochim. Biophys. Acta 2016, 1858, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Thwala, J.M.; Li, M.; Wong, M.C.; Kang, S.; Hoek, E.M.; Mamba, B.B. Bacteria-polymeric membrane interactions: Atomic force microscopy and XDLVO predictions. Langmuir 2013, 29, 13773–13782. [Google Scholar] [CrossRef] [PubMed]
- Lorenzetti, M.; Dogša, I.; Stošicki, T.; Stopar, D.; Kalin, M.; Kobe, S.; Novak, S. The Influence of Surface Modi fi cation on Bacterial Adhesion to Titanium-Based Substrates. ACS Appl. Mater. Interfaces 2015, 7, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Subbiahdoss, G.; Swartjes, J.; van der Mei, H.C.; Busscher, H.J.; Libera, M. Length-scale mediated differential adhesion of mammalian cells and microbes. Adv. Funct. Mater. 2011, 21, 3916–3923. [Google Scholar] [CrossRef]
- Wang, L.; Li, G.; Lin, Y.; Zhang, Z.; Chen, Z.; Wu, S. Polymer Chemistry based on interfacial thiol—Ene photoclick catechol anchor group and zwitterionic betaine. Polym. Chem. 2016, 7, 4964–4974. [Google Scholar] [CrossRef]
- Nimje, V.R.; Chen, C.Y.; Chen, C.C.; Jean, J.S.; Reddy, A.S.; Fan, C.W.; Pan, K.Y.; Liu, H.T.; Chen, J.L. Stable and high energy generation by a strain of Bacillus subtilis in a microbial fuel cell. J. Power Sources 2009, 190, 258–263. [Google Scholar] [CrossRef]
- Chumsakul, O.; Anantsri, D.P.; Quirke, T.; Oshima, T.; Nakamura, K.; Ishikawa, S.; Nakano, M.M. Genome-Wide Analysis of ResD, NsrR, and Fur Binding in Bacillus subtilis during Anaerobic Fermentative Growth by In Vivo Footprinting. J. Bacteriol. 2017, 199, e00086-17. [Google Scholar] [CrossRef] [PubMed]
- Awadallah, A.E.; Aboul-Enein, A.A.; Yonis, M.M.; Aboul-Gheit, A.K. Effect of structural promoters on the catalytic performance of cobalt based catalysts during natural gas decomposition to hydrogen and carbon nanotubes. Fuller. Nanotub. Carbon Nanostruct. 2015, 24, 181–189. [Google Scholar] [CrossRef]
- Zhang, Y.; Guerra-Nuñez, C.; Li, M.; Michler, J.; Park, H.G.; Rossell, M.D.; Erni, R.; Utke, I. High Conformity and Large Domain Monocrystalline Anatase on Multiwall Carbon Nanotube Core-Shell Nanostructure: Synthesis, Structure, and Interface. Chem. Mater. 2016, 28, 3488–3496. [Google Scholar] [CrossRef]
- Grassi, G.; Scala, A.; Piperno, A.; Iannazzo, D.; Lanza, M.; Milone, C.; Pistone, A.; Galvagno, S. A facile and ecofriendly functionalization of multiwalled carbon nanotubes by an old mesoionic compound. Chem. Commun. (Camb.) 2012, 48, 6836–6838. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Meissner, G.; Kim, D.H. Effects of quercetin on single Ca(2+) release channel behavior of skeletal muscle. Biophys. J. 2002, 82, 1266–1277. [Google Scholar] [CrossRef]
- Pemberton, J.E.; Wood, L.L.; Ghoman, G.S. Determination of Surface Coverage of an Adsorbate on Silica Using FTIR Spectroscopy. J. Chem. Educ. 1999, 76, 253. [Google Scholar] [CrossRef]
- Van Gestel, J.; Weissing, F.J.; Kuipers, O.P.; Kovács, A.T. Density of founder cells affects spatial pattern formation and cooperation in Bacillus subtilis biofilms. ISME J. 2014, 8, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Mhatre, E.; Troszok, A.; Gallegos-Monterrosa, R.; Lindstädt, S.; Hölscher, T.; Kuipers, O.P.; Kovács, Á.T. The impact of manganese on biofilm development of Bacillus subtilis. Microbiology 2016, 162, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Heydorn, A.; Nielsen, A.T.; Hentzer, M.; Sternberg, C.; Givskov, M.; Ersbøll, B.K.; Molin, S. Quantication of biofilm structures by the novel computer program. Microbiology 2000, 146, 2395–2407. [Google Scholar] [CrossRef] [PubMed]

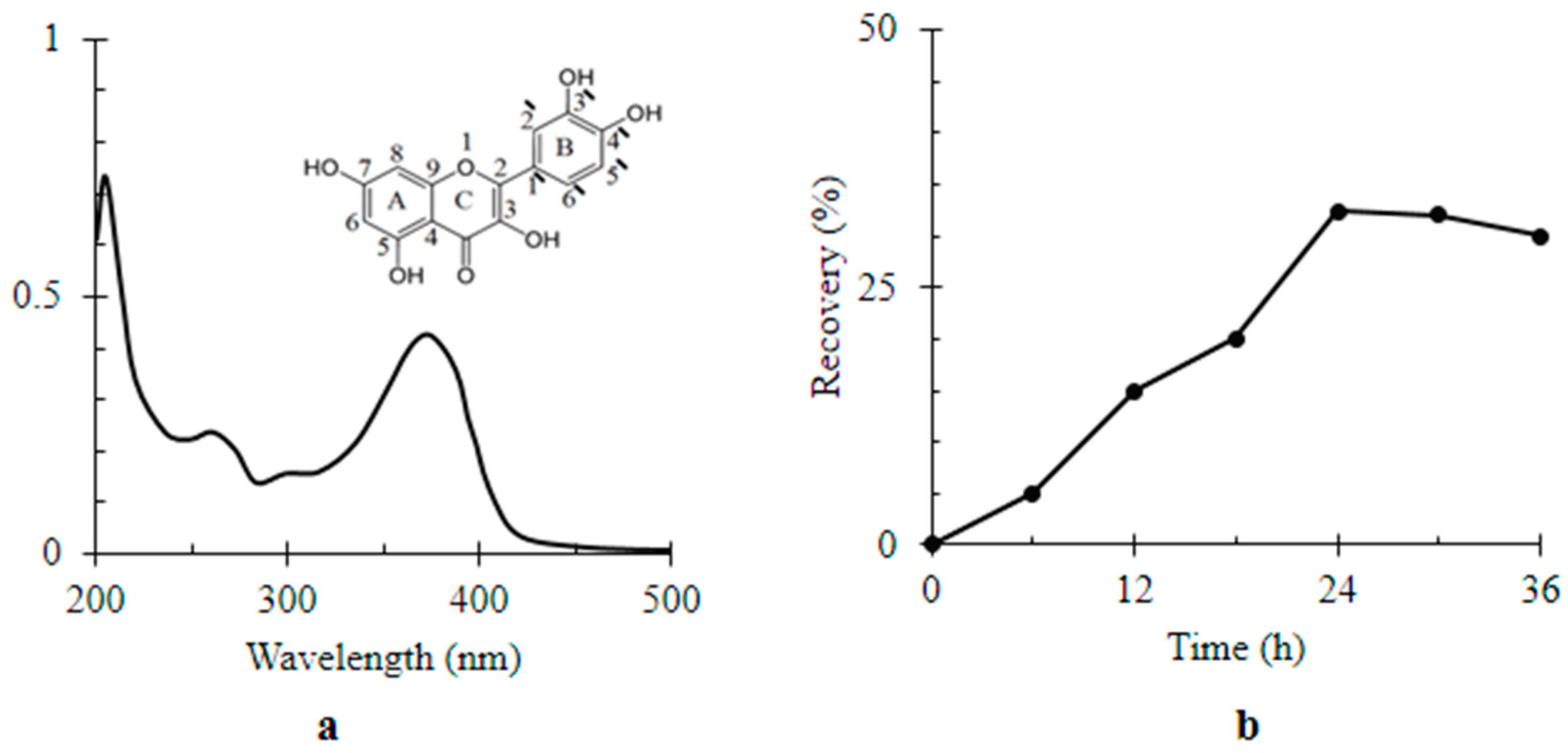
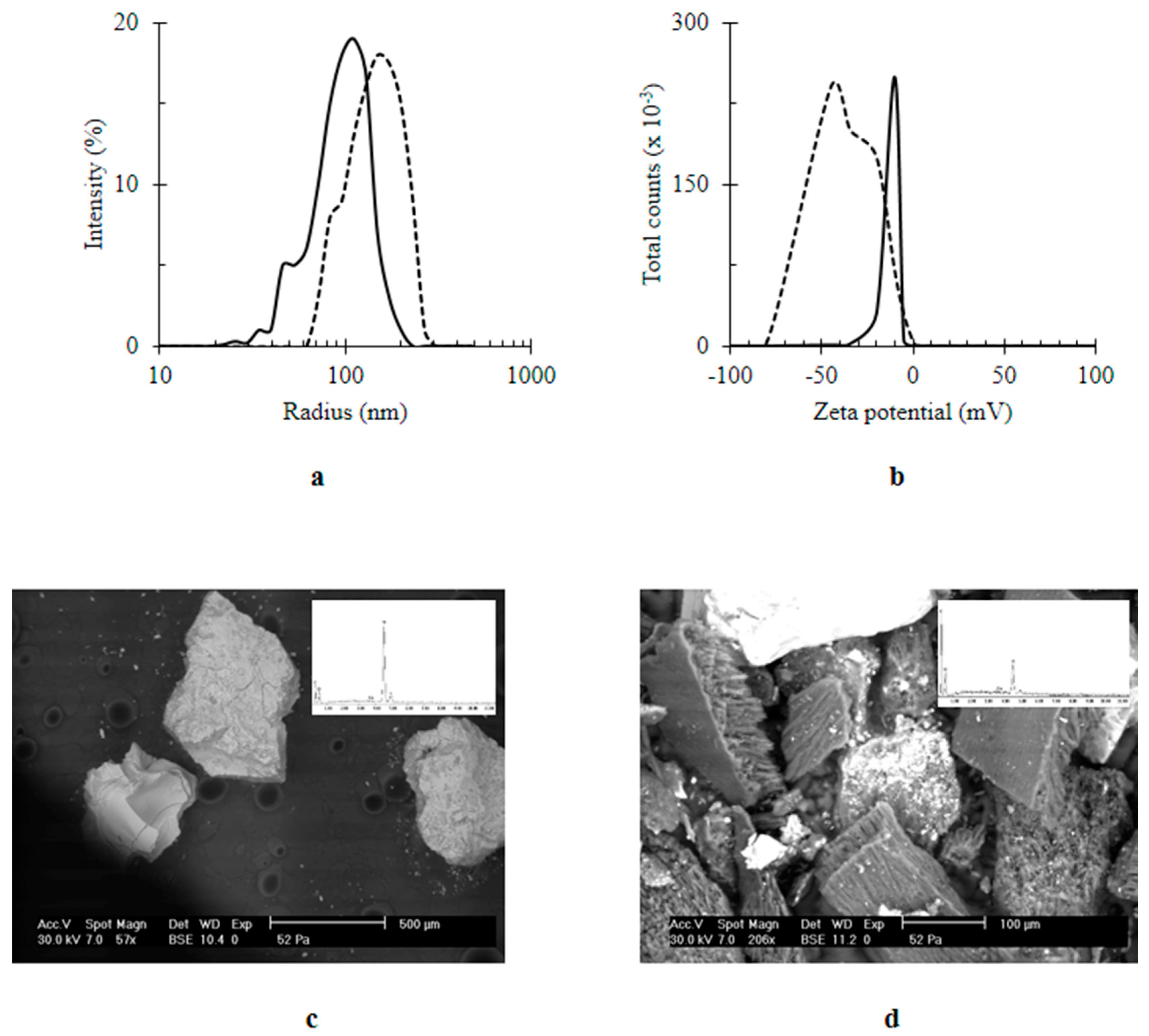
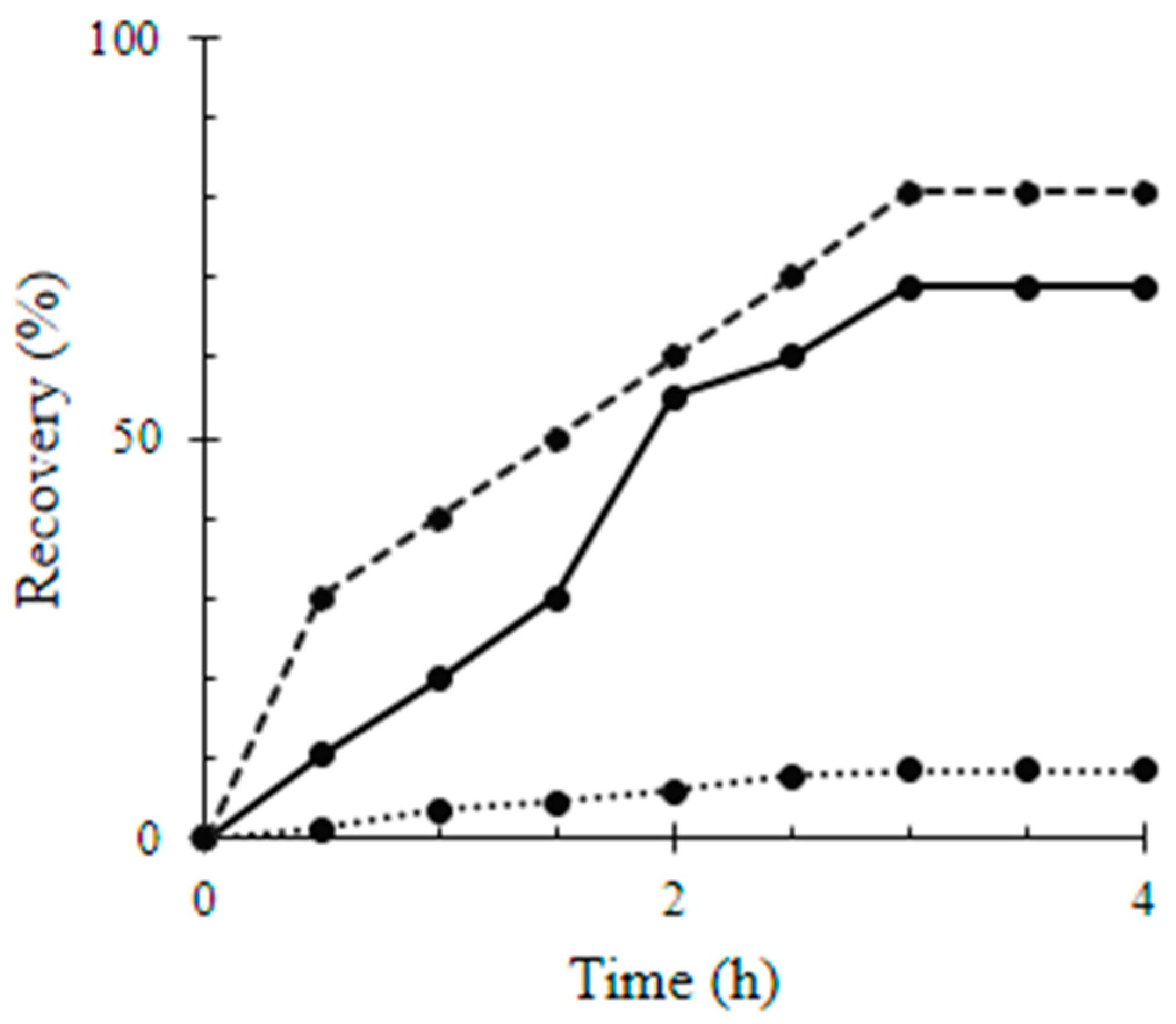
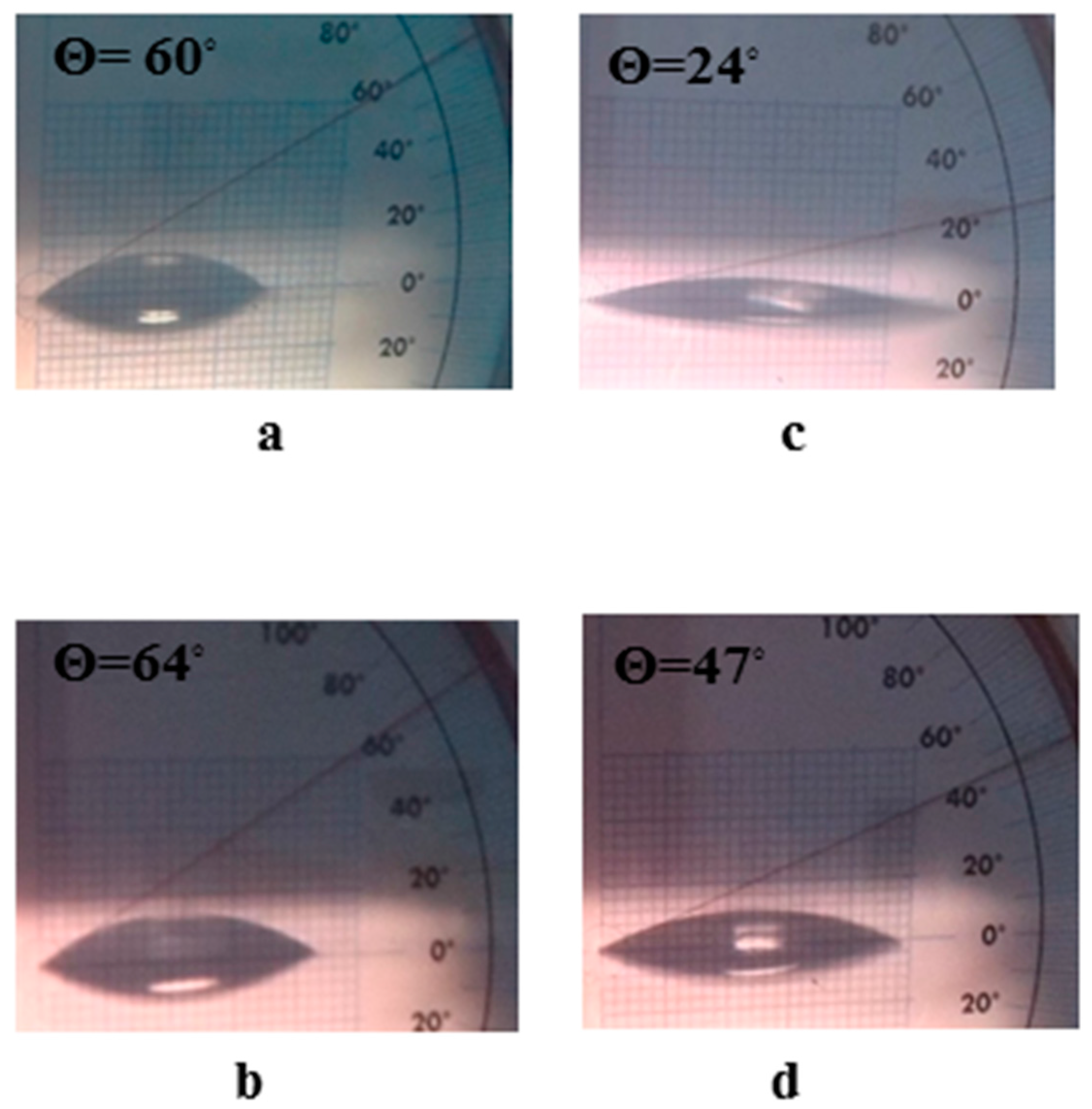
| Material | Surface Coverage (mg mm−2) | Recovery Percentage (%) |
|---|---|---|
| Q | 27.26 ± 0.22 | 8.45 ± 0.72 |
| MWCNTs/TiO2 | 30.33 ± 0.07 × 10−2 | 68.75 ± 0.01 |
| Q/MWCNTs/TiO2 | 35.63 ± 1.13 × 10−2 | 80.63 ± 0.38 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raie, D.S.; Mhatre, E.; El-Desouki, D.S.; Labena, A.; El-Ghannam, G.; Farahat, L.A.; Youssef, T.; Fritzsche, W.; Kovács, Á.T. Effect of Novel Quercetin Titanium Dioxide-Decorated Multi-Walled Carbon Nanotubes Nanocomposite on Bacillus subtilis Biofilm Development. Materials 2018, 11, 157. https://doi.org/10.3390/ma11010157
Raie DS, Mhatre E, El-Desouki DS, Labena A, El-Ghannam G, Farahat LA, Youssef T, Fritzsche W, Kovács ÁT. Effect of Novel Quercetin Titanium Dioxide-Decorated Multi-Walled Carbon Nanotubes Nanocomposite on Bacillus subtilis Biofilm Development. Materials. 2018; 11(1):157. https://doi.org/10.3390/ma11010157
Chicago/Turabian StyleRaie, Diana S., Eisha Mhatre, Doaa S. El-Desouki, Ahmed Labena, Gamal El-Ghannam, Laila A. Farahat, Tareq Youssef, Wolfgang Fritzsche, and Ákos T. Kovács. 2018. "Effect of Novel Quercetin Titanium Dioxide-Decorated Multi-Walled Carbon Nanotubes Nanocomposite on Bacillus subtilis Biofilm Development" Materials 11, no. 1: 157. https://doi.org/10.3390/ma11010157




