2. Experimental
FeCoNiNb
0.5Mo
0.5 and FeCoNiNb alloys were prepared by arc melting using appropriate amounts of the constituent elements with purities above 99.9%. The alloys were made by combining the above elements and arc-melting them in an argon atmosphere with a partial pressure of 200 torr.
Table 1 presents the chemical compositions of the alloys. The microstructural evolution of the alloys was observed using a field emission scanning electron microscope with an energy dispersive spectrometer (SEM/EDS, JEOL JSM-6335, JEOL Ltd., Tokyo, Japan), which was operated at 15 kV. Samples for SEM observation were metallographically prepared and chemically etched in Marble’s etching solution (HCl 50 mL + CuSO
4 10 g + H
2O 100 mL). The structures were characterized by X-ray diffraction (XRD) using a Rigaku ME510-FM2 (Rigaku Ltd., Tokyo, Japan) with Cu-Kα (with a wavelength of 1.541 Å) radiation, operated at 30 kV. The microstructures and lattice images of the alloys were obtained using a high-resolution transmission electron microscope (HREM, JEOL JEM-3000F, JEOL Ltd., Tokyo, Japan), which was operated at 300 kV. The corresponding selection area diffraction patterns (SAD) were obtained from the high-resolution lattice images by fast Fourier transformation (FFT) in Gatan digital micrograph software. Thin foil specimens for TEM observation were electrochemically prepared in a digital Fischione twin-jet electropolisher, model 110 (Fischione Instruments Co., Pittsburgh, PA, USA), in a solution of 10 vol.% perchloric acid and 90 vol.% methanol at a potential of 30 V. The hardness of the alloys was measured using both a Mitutoyo Akashi MVK-G1500 microhardness tester (Mitutoyo Co., Kanagawa, Japan) under a load of 10 gf and a Matsuzawa Seiki MV1 Vicker’s hardness tester (Matsuzawa Co., Akita, Japan) under a load of 30 kgf.
Polarization curves of the alloys were obtained in a potentiostat/galvanostat (Autolab PGSTAT302N, Metrohm Autolab B.V., Utrecht, The Netherlands) using a three-electrode system at a scanning rate of 1 mV/s. The polarization data were compared with those of commercial 304 stainless steel (304SS) whose composition was by weight 71.61% Fe, 18.11% Cr, 8.24% Ni, 1.12% Mn, 0.75% Si, 0.05% Co, 0.02% Mo, 0.05% C, 0.03% P, and 0.02% S. The as-cast FeCoNiNb
0.5Mo
0.5 and FeCoNiNb alloys for polarization testing were mounted in epoxy resin, and the exposed surface area of each was fixed at 0.1964 cm
2 (with a diameter of 0.5 cm). The reference electrode was a saturated silver chloride electrode (Ag/AgCl), and the counter electrode was a smooth Pt sheet. All the potentials that are below a saturated silver chloride electrode (SSE), whose reduction potential is 222 mV higher than that of the standard hydrogen electrode (SHE) at 25 °C [
10]. The specimens whose polarization curves were obtained were all mechanically wet-polished using 1200 SiC grit paper. Test solutions with a concentration of 1 M were prepared from reagent-grade sulfuric acid (H
2SO
4) and sodium chloride (NaCl) that were dissolved in distilled water. To eliminate any effect of dissolved oxygen, the solutions were deaerated by bubbling nitrogen gas through them before and during the polarization experiments.
3. Results and Discussion
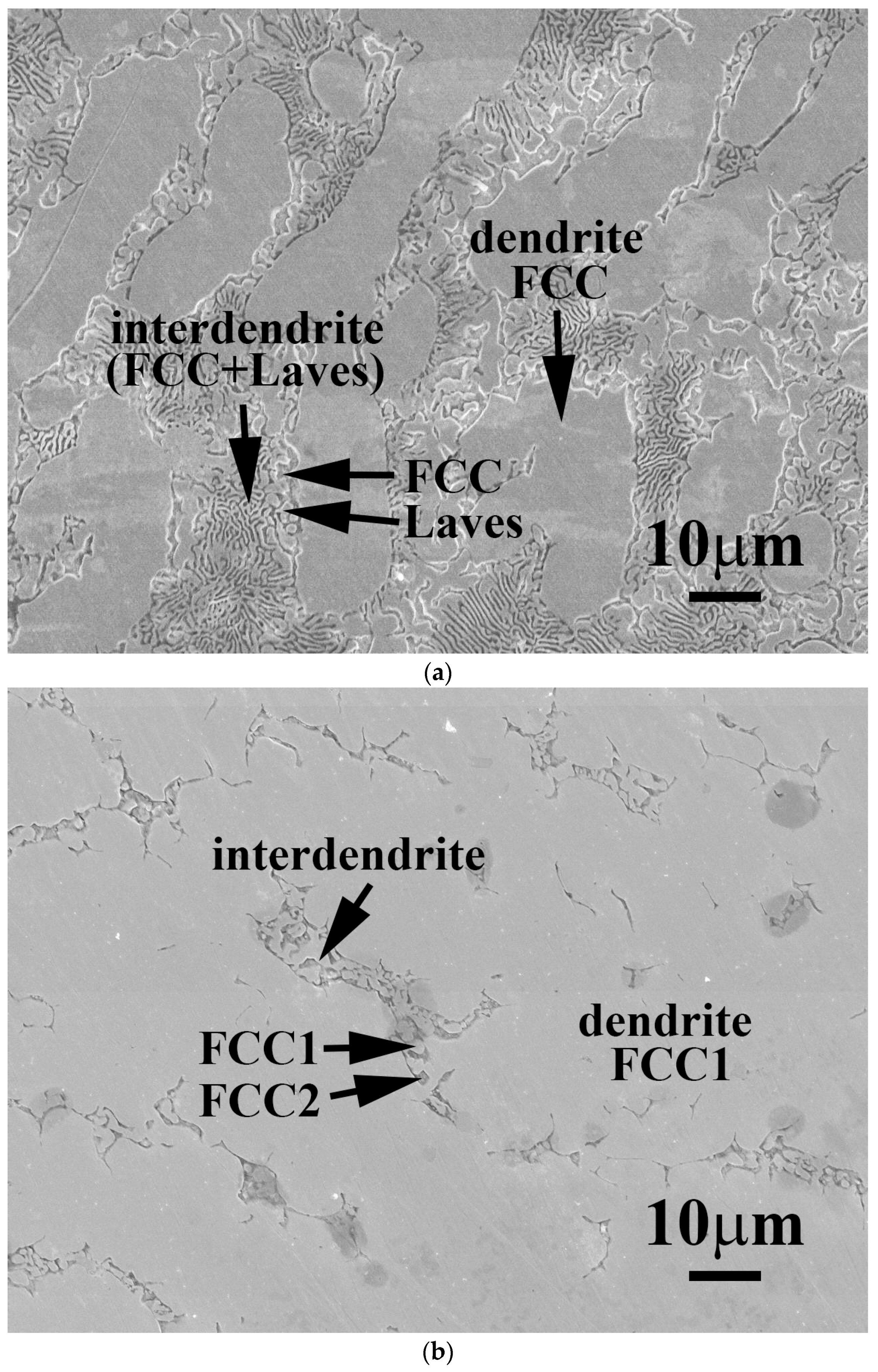
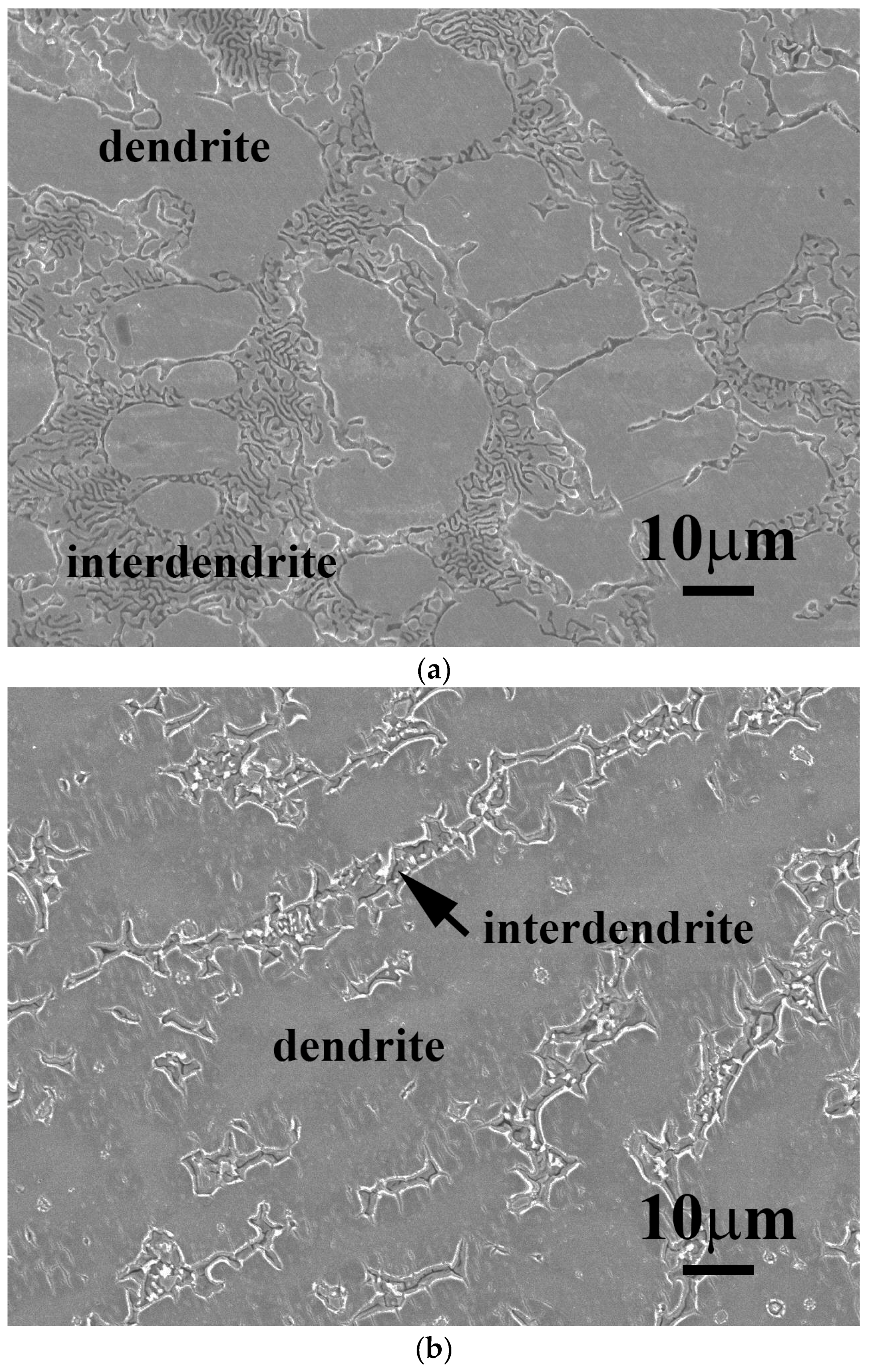
Figure 1 shows as-cast micrographs of FeCoNiNb
0.5Mo
0.5 and FeCoNiNb alloys, which reveal dendritic microstructures. The dendrites exhibit a single phase and the interdendritic regions exhibit a dual-phased eutectic structure. Hence, adding Nb or/and Mo would have changed their microstructures to dendritic, because FeCoNi alloy had a granular structure [
6].
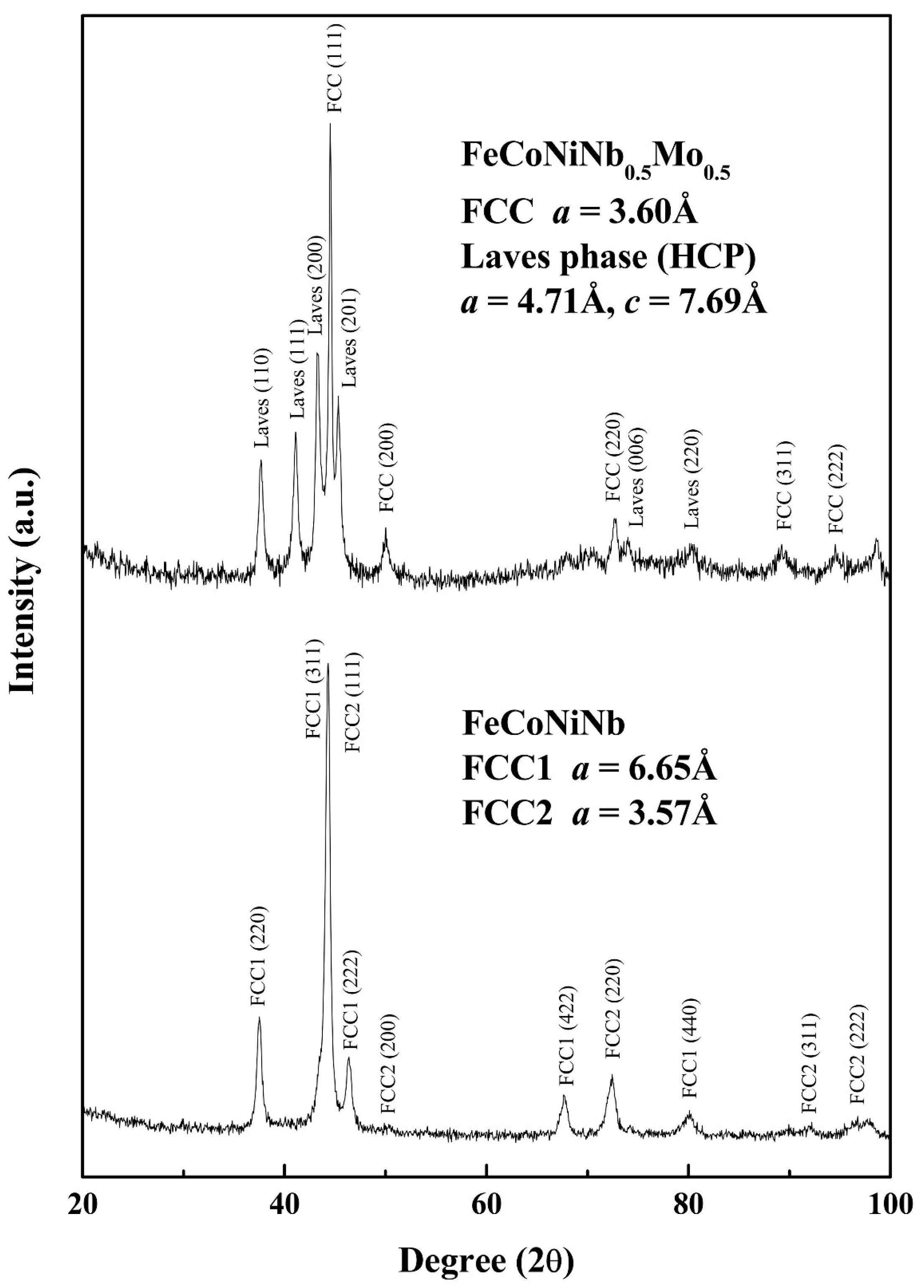
Figure 2 displays the XRD patterns of the FeCoNiNb
0.5Mo
0.5 and FeCoNiNb alloys. The two phases of the FeCoNiNb
0.5Mo
0.5 alloy were the laves phase (HCP) and the FCC phase. The lattice constants of the laves phase were
a0 = 4.71 and
c0 = 7.69 Å; the lattice constant of the FCC phase was 3.60 Å. The two phases of FeCoNiNb alloy are FCC1 and FCC2. The lattice constants of the FCC1 and FCC2 phases herein were 6.65 and 3.57 Å, respectively. The structures of the phases of FeCoNiNb
0.5Mo
0.5 and FeCoNiNb alloys were identified by comparing their SEM micrographs and the intensities of their XRD peaks. The dendrites in each alloy had higher XRD intensities because of their volume fractions. Therefore, the dendrites of FeCoNiNb
0.5Mo
0.5 alloy were an FCC phase, and the dendrites of the FeCoNiNb alloy were an FCC1 phase.
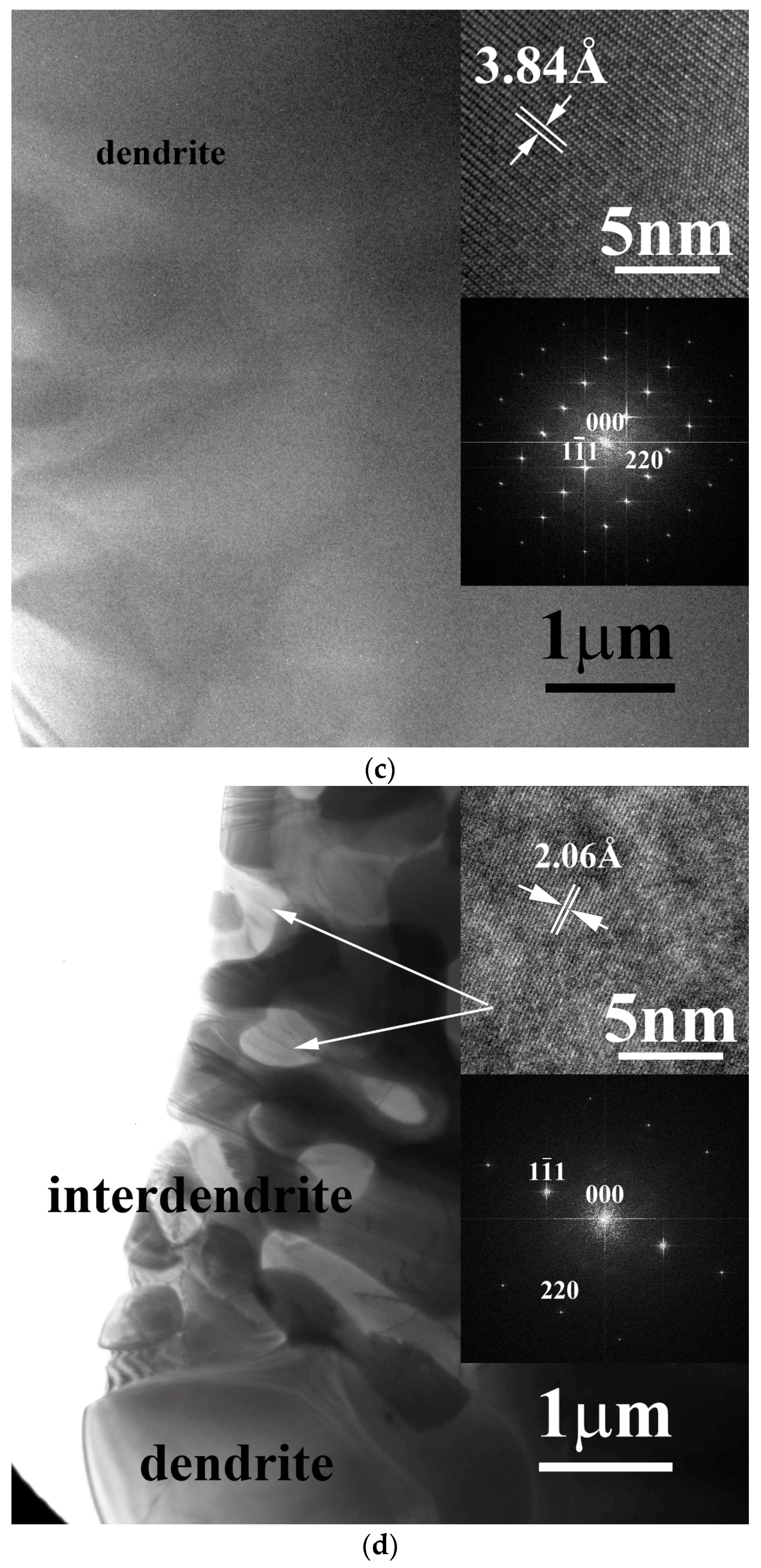
The phases of the FeCoNiNb
0.5Mo
0.5 alloy and FeCoNiNb alloys were all identified by TEM, as shown in
Figure 3. The micrograph of each phase in the FeCoNiNb
0.5Mo
0.5 alloy and FeCoNiNb alloys was examined carefully, and the FFT diffraction pattern of each phase was obtained from its high-resolution lattice images.
Figure 3a shows a TEM image of an FCC dendrite of FeCoNiNb
0.5Mo
0.5 alloy, and the insets present the corresponding lattice image and FFT diffraction pattern, which indicates that the image was taken with a beam direction of [111].
Figure 3b shows a TEM image of a HCP-structured laves phase in the interdendrite of FeCoNiNb
0.5Mo
0.5 alloy, which was obtained with a beam direction of [0001].
Figure 3c shows a TEM image of an FCC1 dendrite of FeCoNiNb alloy, which was obtained with a beam direction of [
12].
Figure 3d shows a TEM image of an FCC2 phase in the interdendrite of FeCoNiNb alloy, which was obtained with beam direction of [
12].
Table 2 presents the chemical compositions of the phases in the alloys. The Nb and Mo contents of the FCC-dendrite of FeCoNiNb
0.5Mo
0.5 alloy exceed those in the laves phase in the interdendritic region. The Nb content of the FCC1 dendrite of FeCoNiNb alloy exceeds that of the FCC2 phase in the interdendrite. The melting points of Fe, Co, Ni, Nb and Mo are 1536, 1498, 1453, 2415 and 2610 °C, respectively [
10]. The elements Nb and Mo have higher melting points than the others. Therefore, a melt that contained more Nb and Mo solidified first to form the dendrites.
Table 3 presents the hardness of the FeCoNiNb
0.5Mo
0.5, FeCoNiNb, FeCoNi and 304SS alloys. FeCoNi alloy is very soft and has an HV of only 112, making it even softer than commercial 304 stainless steel, which has a hardness of HV 185. However, adding Nb/Mo to FeCoNi alloy significantly increased its hardness. FeCoNiNb
0.5Mo
0.5 and FeCoNiNb alloys had higher hardness values of HV 629 and HV 798, respectively. The microhardness values of the FCC-dendrite and eutectic-interdendrite of the FeCoNiNb
0.5Mo
0.5 alloy were HV 677 and HV 317, respectively. The microhardness values of the FCC1-dendrite and eutectic-interdendrite of FeCoNiNb alloy were HV 840 and HV 233, respectively, to which the solid solution effect contributed. The atomic radii of Fe, Co, Ni, Nb and Mo are 0.124, 0.125, 0.125, 0.143 and 0.140 nm, respectively [
11]. The radii of Nb and Mo (exceeds OR are larger than) those of the other elements. No superlattice spot was observed in the TEM diffraction patterns indicating that all of the phases in FeCoNiNb
0.5Mo
0.5 and FeCoNiNb alloys were solid solution phases; a large lattice distortion was thus expected due to the presence of elements with larger radii, Nb and Mo. Therefore, the solid solution effect was the cause of the increased hardness.
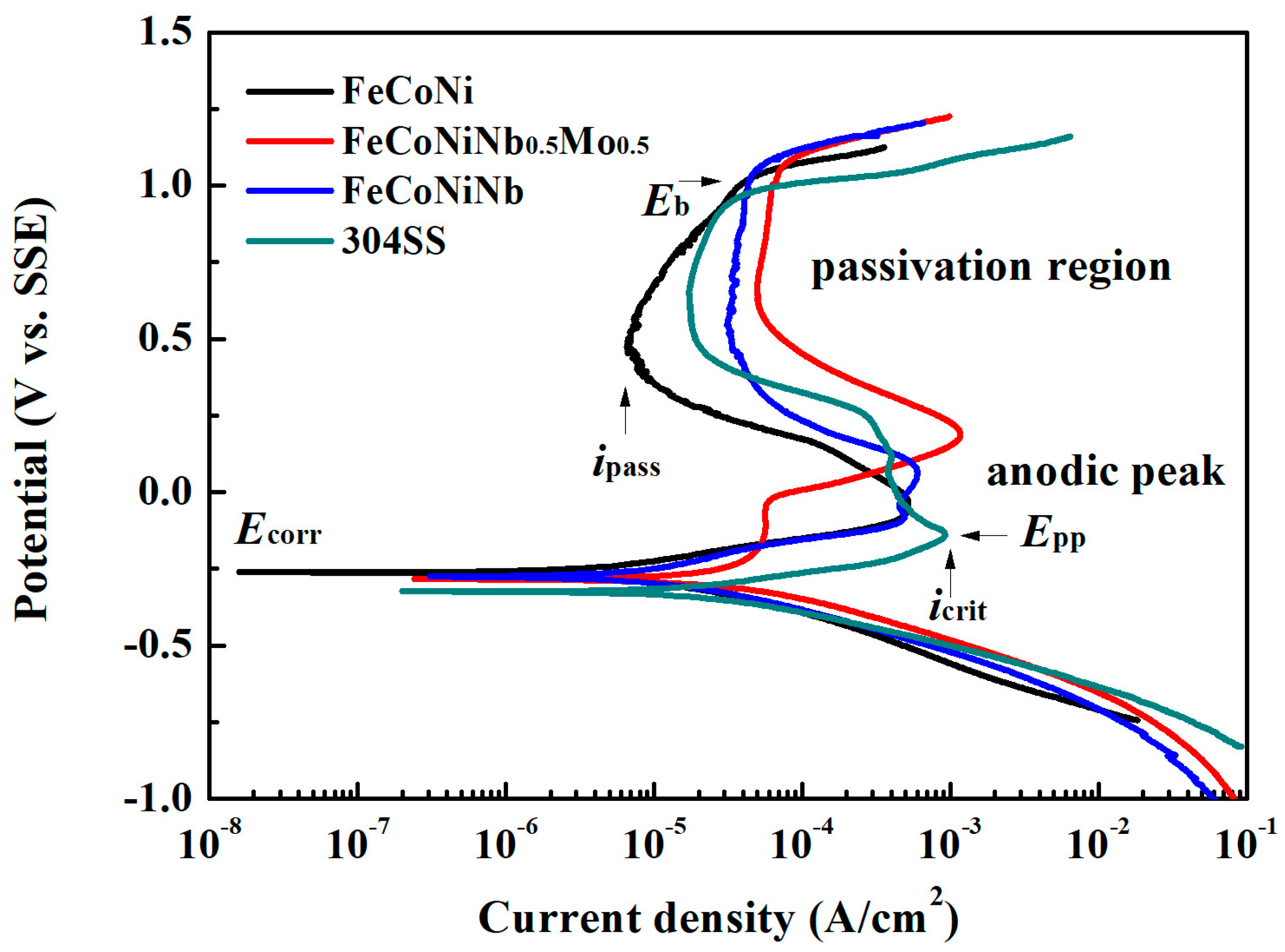
Figure 4 plots the polarization curves of FeCoNiNb
0.5Mo
0.5, FeCoNiNb, FeCoNi and 304SS alloys in 1 M deaerated H
2SO
4 solution and indicates important characteristics, such as corrosion potential (
Ecorr), passivation potential (
Epp, the potential of the anodic peak), critical current density of the anodic peak (
icrit), passive current density (
ipass) and breakdown potential (
Eb).
Table 4 presents the data that are obtained from the polarization curves. The corrosion current density (
icorr) associated with each polarization curve was obtained from the intersection of the free corrosion potential and the cathodic Tafel line. The corrosion current densities of FeCoNiNb
0.5Mo
0.5 and FeCoNiNb alloys exceeded the
icorr of the single FCC-phased FeCoNi alloy, because they both had a dual-phased microstructure and thus easily formed local cells between the anodic and cathodic regions. However, the corrosion current densities of FeCoNiNb
0.5Mo
0.5 and FeCoNiNb alloys were smaller than the
icorr of 304 stainless steel. Therefore, the corrosion rates of FeCoNiNb
0.5Mo
0.5 and FeCoNiNb alloys were smaller than that of 304 stainless steel in 1 M deaerated H
2SO
4 solution at 30 °C. The corrosion potentials of FeCoNiNb
0.5Mo
0.5 and FeCoNiNb alloys were only slightly lower than the
Ecorr of FeCoNi alloy, but these alloys were still nobler than 304 stainless steel. However, the passive current densities of FeCoNiNb
0.5Mo
0.5 and FeCoNiNb alloys exceed those of 304 stainless steel and FeCoNi alloy.
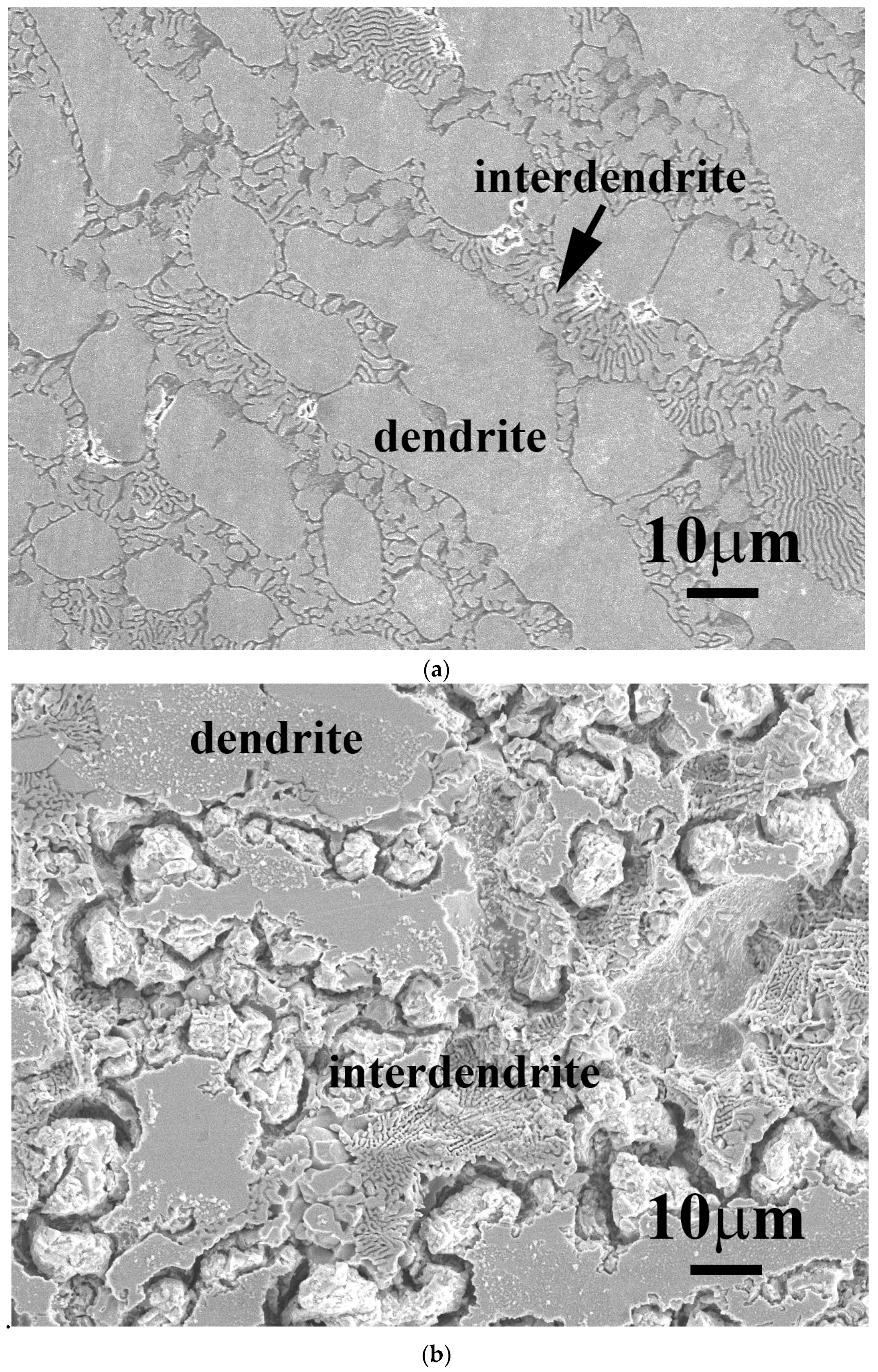
Figure 5 shows the SEM micrographs of FeCoNiNb
0.5Mo
0.5 and FeCoNiNb alloys after the polarization test in 1 M deaerated H
2SO
4 solution at 30 °C. The dendrites of FeCoNiNb
0.5Mo
0.5 alloy were an FCC phase and the interdendrites were a eutectic structure of FCC and laves phases. The laves phase was the matrix of the interdendrite, which was corroded during the polarization test in 1 M deaerated H
2SO
4 solution at 30 °C, as shown in
Figure 5a. In contrast, the morphology of the FCC phase of FeCoNiNb
0.5Mo
0.5 alloy was almost unchanged after the polarization test.
Figure 5b shows a micrograph of FeCoNiNb alloy after a polarization test in 1 M deaerated H
2SO
4 solution at 30 °C. The dendrites of FeCoNiNb alloy were an FCC1 phase, and the interdendrites were a eutectic of FCC1 and FCC2 phases. The FCC2 phase in the interdendrite of FeCoNiNb alloy was the main corroded phase in the polarization test in 1 M deaerated H
2SO
4 solution at 30 °C. The FCC1 phase of FeCoNiNb alloy was only slightly corroded after the polarization test. Therefore, the main corroded areas of these two alloys are the interdendrites. Since the interdendrite area of FeCoNiNb alloy was less than that of FeCoNiNb
0.5Mo
0.5 alloy, the corrosion current density of FeCoNiNb alloy was less than that of FeCoNiNb
0.5Mo
0.5 alloy.
Figure 6 plots the polarization curves of FeCoNiNb
0.5Mo
0.5, FeCoNiNb, FeCoNi and 304SS alloys in 1 M deaerated NaCl solution.
Table 5 presents the corrosion potentials and corrosion current densities that are obtained from the curves. The corrosion potentials of FeCoNiNb
0.5Mo
0.5 and FeCoNiNb alloys were close to the
Ecorr of FeCoNi alloy, these alloys were all nobler than 304 stainless steel. This result indicates that adding Nb/Mo to FeCoNi alloy did not significantly change its
Ecorr, although but did change its microstructures to dual-phased ones. However, the dual-phased microstructures of FeCoNiNb
0.5Mo
0.5 and FeCoNiNb alloys significantly influenced on the
icorr of the alloys. The corrosion current densities of FeCoNiNb
0.5Mo
0.5 and FeCoNiNb alloys exceeded the
icorr of FeCoNi alloy; but smaller than the
icorr of 304 stainless steel. FeCoNiNb
0.5Mo
0.5 alloy had a large passivation region because of the addition of molybdenum. Adding Mo can reportedly increase the corrosion resistance of the alloy in a solution that contains chloride ions [
9] because molybdenum can increase the stability of the passivation films of steels. In contrast, the corrosion resistance of FeCoNiNb alloy in 1 M NaCl solution was less than that of FeCoNiNb
0.5Mo
0.5 alloy. The cathodic limiting current densities (
iL) in FeCoNiNb
0.5Mo
0.5 and FeCoNiNb alloys were observed. The cathodic limiting current density corresponds to the maximum reaction rate, which is herein limited by the diffusion rate of hydroxyl ions (OH
−) in solution [
10]. However, no cathodic limiting current density was observed in FeCoNi alloy [
6].
Figure 7 shows SEM micrographs of FeCoNiNb
0.5Mo
0.5 and FeCoNiNb alloys after the polarization test in 1 M deaerated NaCl solution at 30 °C. As for the FeCoNiNb
0.5Mo
0.5 and FeCoNiNb alloys that were tested in 1 M deaerated H
2SO
4 solution, their main corroded areas were the interdendrites.
Figure 7a shows a micrograph of FeCoNiNb
0.5Mo
0.5 alloy after the polarization test. The corroded phase was the laves phase in the interdendrite of FeCoNiNb
0.5Mo
0.5 alloy. The FCC-structured dendrite and FCC phase in the interdendrite were not corroded and so their original morphologies were retained.
Figure 7b shows a micrograph of FeCoNiNb alloy after the polarization test. The FCC2 phase in the interdendrite of FeCoNiNb alloy was the main corroded area. The FCC1 dendrite of FeCoNiNb alloy was also corroded, but to a lesser extent than the FCC2 phase.