Preparation of 1D Hierarchical Material Mesosilica/Pal Composite and Its Performance in the Adsorption of Methyl Orange
Abstract
:1. Introduction
2. Experimental Section
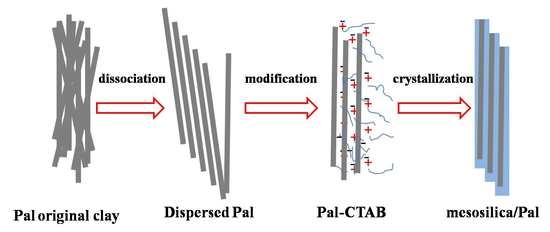
2.1. Preparation of Mesosilica/Pal Composite
2.1.1. Purifying and Dissociation of Pal Original Clay
2.1.2. Fabrication of Dual-Template Pal-CTAB
2.1.3. Preparation of Mesosilica/Pal Composite
2.2. Characterization
2.3. Evaluation of Adsorption Performance
3. Result and Discussion
3.1. Characterization of Mesosilica/Pal Composite
3.2. Methyl Orange Adsorption Property
3.2.1. MO Adsorption with Different Materials
3.2.2. Effect of Contact Time on the Uptake of MO by Mesosilica/Pal Composite
3.2.3. Effect of pH on MO Removal by Mesosilica/Pal Composite
3.2.4. The Regenerability of Mesosilica/Pal Composite
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ni, Z.-M.; Xia, S.-J.; Wang, L.-G.; Xing, F.-F.; Pan, G.-X. Treatment of methyl orange by calcined layered double hydroxides in aqueous solution: Adsorption property and kinetic studies. J. Colloid Interface Sci. 2007, 316, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Li, Y.; Yu, Y.; Li, Y.; Xia, K.; Wang, Y.; Li, S. Synthesis of mesoporous carbon nanospheres for highly efficient adsorption of bulky dye molecules. J. Mater. Sci. 2016, 51, 7016–7028. [Google Scholar] [CrossRef]
- Yokoi, T.; Kubota, Y.; Tatsumi, T. Amino-functionalized mesoporous silica as base catalyst and adsorbent. Appl. Catal. Gen. 2012, 421, 14–37. [Google Scholar] [CrossRef]
- Lee, C.-K.; Liu, S.-S.; Juang, L.-C.; Wang, C.-C.; Lin, K.-S.; Lyu, M.-D. Application of mcm-41 for dyes removal from wastewater. J. Hazard. Mater. 2007, 147, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Ma, J.; Liu, K. Adsorption of anionic dyes on ammonium-functionalized mcm-41. J. Hazard. Mater. 2009, 162, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Li, F.; Gu, Q.; Liang, C.; Chen, J. Heavy metal-contaminated groundwater treatment by a novel nanofiber membrane. Desalination 2008, 223, 349–360. [Google Scholar] [CrossRef]
- Jafarzadeh, A.; Sohrabnezhad, S.; Zanjanchi, M.A.; Arvand, M. Synthesis and characterization of thiol-functionalized mcm-41 nanofibers and its application as photocatalyst. Microporous Mesoporous Mater. 2016, 236, 109–119. [Google Scholar] [CrossRef]
- Haider, S.; Park, S.-Y. Preparation of the electrospun chitosan nanofibers and their applications to the adsorption of cu(ii) and pb(ii) ions from an aqueous solution. J. Membr. Sci. 2009, 328, 90–96. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, H.; Chen, M.; Song, Z.; Zhao, Y. Absorbents based on maleic anhydride-modified cellulose fibers/diatomite for dye removal. J. Mater. Sci. 2014, 49, 6696–6704. [Google Scholar] [CrossRef]
- Lin, K.-S.; Cheng, H.-W.; Chen, W.-R.; Wu, C.-F. Synthesis, characterization, and adsorption kinetics of titania nanotubes for basic dye wastewater treatment. Adsorption 2010, 16, 47–56. [Google Scholar] [CrossRef]
- Shokouhimehr, M. Magnetically separable and sustainable nanostructured catalysts for heterogeneous reduction of nitroaromatics. Catalysts 2015, 5, 534–560. [Google Scholar] [CrossRef]
- Patil, J.V.; Mali, S.S.; Kamble, A.S.; Hong, C.K.; Kim, J.H.; Patil, P.S. Electrospinning: A versatile technique for making of 1d growth of nanostructured nanofibers and its applications: An experimental approach. Appl. Surf. Sci. 2017, 423, 641–674. [Google Scholar] [CrossRef]
- Lu, Q.; Gao, F.; Komarneni, S.; Mallouk, T.E. Ordered sba-15 nanorod arrays inside a porous alumina membrane. J. Am. Chem. Soc. 2004, 126, 8650–8651. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, P.J.; Kim, A.Y.; Liu, J.; Baskaran, S. Mesoporous silica synthesized by solvent evaporation: Spun fibers and spray-dried hollow spheres. Chem. Mater. 1997, 9, 2507–2512. [Google Scholar] [CrossRef]
- Julian-Lopez, B.; Boissiere, C.; Chaneac, C.; Grosso, D.; Vasseur, S.; Miraux, S.; Duguet, E.; Sanchez, C. Mesoporous maghemite-organosilica microspheres: A promising route towards multifunctional platforms for smart diagnosis and therapy. J. Mater. Chem. 2007, 17, 1563–1569. [Google Scholar] [CrossRef]
- Pega, S.; Boissière, C.; Grosso, D.; Azaïs, T.; Chaumonnot, A.; Sanchez, C. Direct aerosol synthesis of large-pore amorphous mesostructured aluminosilicates with superior acid-catalytic properties. Angew. Chem. Int. Ed. 2009, 48, 2784–2787. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Kang, F.; Huang, Z.; Guo, Z.; Chuan, X. Synthesis of mesoporous carbon nanosheets using tubular halloysite and furfuryl alcohol by a template-like method. Microporous Mesoporous Mater. 2008, 108, 318–324. [Google Scholar] [CrossRef]
- Sun, L.; Yan, C.; Chen, Y.; Wang, H.; Wang, Q. Preparation of amorphous carbon nanotubes using attapulgite as template and furfuryl alcohol as carbon source. J. Non-Cryst. Solids 2012, 358, 2723–2726. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, Z.; Duanmu, C.; Gu, Y.; Chen, J.; Ni, L. Economical synthesis of amorphous carbon nanotubes and sba-15 mesoporous materials using palygorskite as a template and silica source. Mater. Lett. 2014, 132, 425–427. [Google Scholar] [CrossRef]
- Yang, R.; Li, D.; Li, A.; Yang, H. Adsorption properties and mechanisms of palygorskite for removal of various ionic dyes from water. Appl. Clay Sci. 2018, 151, 20–28. [Google Scholar] [CrossRef]
- Sohrabnezhad, S.; Jafarzadeh, A.; Pourahmad, A. Synthesis and characterization of mcm-41 ropes. Mater. Lett. 2018, 212, 16–19. [Google Scholar] [CrossRef]
- Shang, F.; Sun, J.; Wu, S.; Yang, Y.; Kan, Q.; Guan, J. Direct synthesis of acid–base bifunctional mesoporous mcm-41 silica and its catalytic reactivity in deacetalization–knoevenagel reactions. Microporous Mesoporous Mater. 2010, 134, 44–50. [Google Scholar] [CrossRef]
- Chuah, G.K.; Hu, X.; Zhan, P.; Jaenicke, S. Catalysts from mcm-41: Framework modification, pore size engineering, and organic–inorganic hybrid materials. J. Mol. Catal. Chem. 2002, 181, 25–31. [Google Scholar] [CrossRef]
- Grün, M.; Unger, K.K.; Matsumoto, A.; Tsutsumi, K. Novel pathways for the preparation of mesoporous mcm-41 materials: Control of porosity and morphology. Microporous Mesoporous Mater. 1999, 27, 207–216. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, A.; Wu, J.; Zhao, J.; Yan, H. Adsorption behaviors and mechanisms of methyl orange on heat-treated palygorskite clays. Ind. Eng. Chem. Res. 2012, 51, 14026–14036. [Google Scholar] [CrossRef]
- Darmograi, G.; Prelot, B.; Layrac, G.; Tichit, D.; Martin-Gassin, G.; Salles, F.; Zajac, J. Study of adsorption and intercalation of orange-type dyes into Mg–Al layered double hydroxide. J. Phys. Chem. C 2015, 119, 23388–23397. [Google Scholar] [CrossRef]
- Anbia, M.; Hariri, S.A.; Ashrafizadeh, S.N. Adsorptive removal of anionic dyes by modified nanoporous silica sba-3. Appl. Surf. Sci. 2010, 256, 3228–3233. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chatterjee, S.; Chatterjee, B.P.; Das, A.R.; Guha, A.K. Adsorption of a model anionic dye, eosin y, from aqueous solution by chitosan hydrobeads. J. Colloid Interface Sci. 2005, 288, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Bing, H.; Feifei, X.; Xiaofeng, C. Equilibrium and kinetic studies of methyl orange adsorption on multiwalled carbon nanotubes. Chem. Eng. J. 2011, 170, 82–89. [Google Scholar] [CrossRef]
- Mohammadi, N.; Khani, H.; Gupta, V.K.; Amereh, E.; Agarwal, S. Adsorption process of methyl orange dye onto mesoporous carbon material–kinetic and thermodynamic studies. J. Colloid Interface Sci. 2011, 362, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Ulibarri, M.A.; Pavlovic, I.; Barriga, C.; Hermosı́n, M.C.; Cornejo, J. Adsorption of anionic species on hydrotalcite-like compounds: Effect of interlayer anion and crystallinity. Appl. Clay Sci. 2001, 18, 17–27. [Google Scholar] [CrossRef]


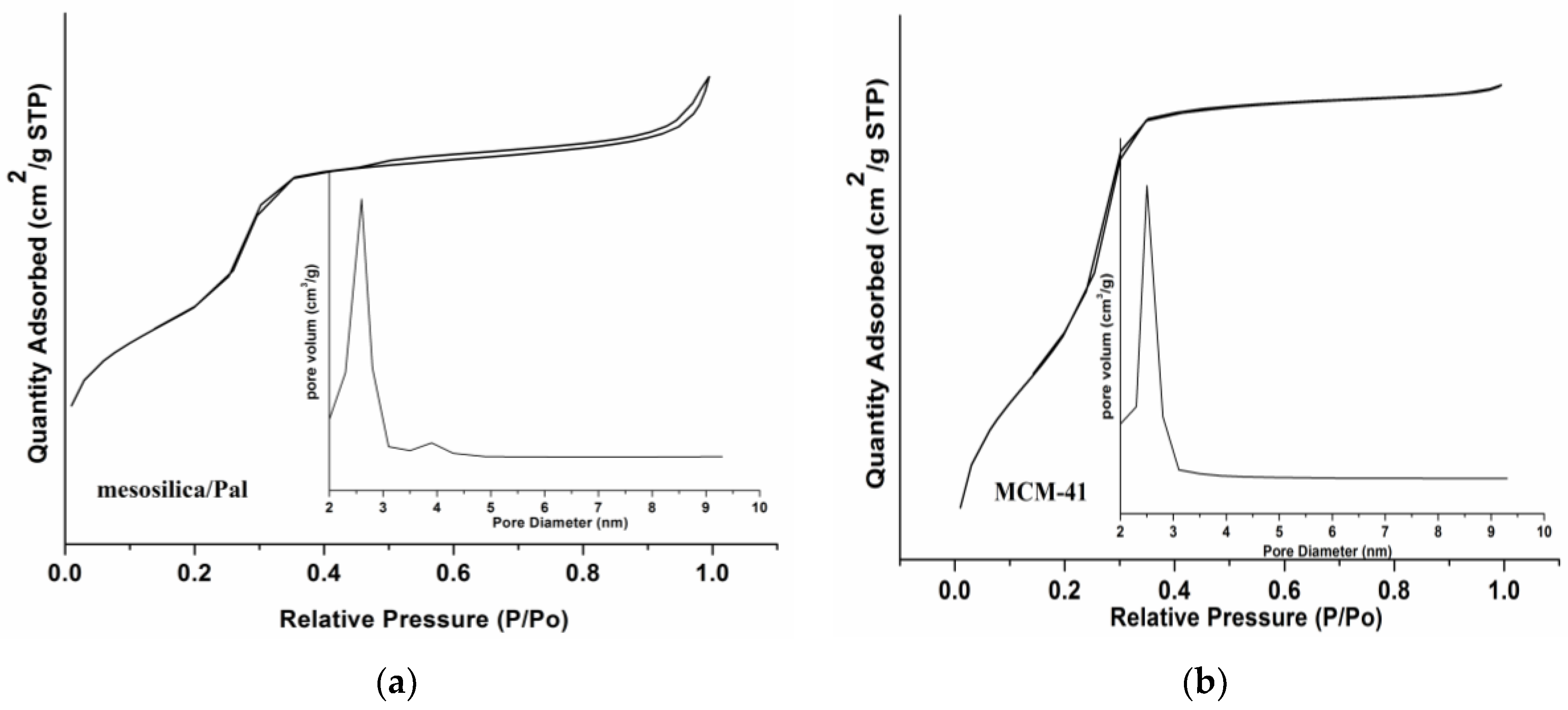







| Material | Pal | MCM-41 | Mesosilica/Pal |
|---|---|---|---|
| BET surface area (cm2/g) | 169.2 | 937.2 | 533.1 |
| Pore volume (cm3/g) | - | 0.77 | 0.60 |
| Pore diameter (nm) | <2 | 2.6 | 2.6, 3.9 |
| Materials | Zeta Potential (mV) | * AMO | * CMO (mg/L) | * qe (%) | Qads (mg/g) |
|---|---|---|---|---|---|
| Pal | −20.8 | 0.089 | 178 | 35.0 | 88.80 |
| MCM-41 | −24.4 | 0.135 | 269 | 1.8 | 4.56 |
| mesosilica/Pal | −4.38 | 0.040 | 81 | 70.4 | 178.61 |
| Heat treated Pal | - | - | - | - | 97.80 [25] |
| Pal-O | - | - | - | - | 188.38 [26] |
| Mg-Al-LDHs | - | - | - | - | 1471.00 [27] |
| Materials | Al (ppm) | Ca (ppm) | Fe (ppm) | K (ppm) | Mg (ppm) | Na (ppm) | Ti (ppm) |
|---|---|---|---|---|---|---|---|
| mesosilica/Pal | 15,287.64 | 3877.91 | 12,035.59 | 3038.33 | 29,620.82 | 6110.50 | 5334.48 |
| Pal | 23,241.40 | 8315.25 | 21,271.64 | 11,360.13 | 68,391.34 | 9435.50 | 3790.80 |
| MCM-41 | 9726.40 | 23.40 | −38.51 | 11.74 | 16.40 | 444.50 | 796.30 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Han, H.; Ni, L.; Song, D.; Li, S.; Hu, T.; Jiang, J.; Chen, J. Preparation of 1D Hierarchical Material Mesosilica/Pal Composite and Its Performance in the Adsorption of Methyl Orange. Materials 2018, 11, 164. https://doi.org/10.3390/ma11010164
Wu M, Han H, Ni L, Song D, Li S, Hu T, Jiang J, Chen J. Preparation of 1D Hierarchical Material Mesosilica/Pal Composite and Its Performance in the Adsorption of Methyl Orange. Materials. 2018; 11(1):164. https://doi.org/10.3390/ma11010164
Chicago/Turabian StyleWu, Mei, Haifeng Han, Lingli Ni, Daiyun Song, Shuang Li, Tao Hu, Jinlong Jiang, and Jing Chen. 2018. "Preparation of 1D Hierarchical Material Mesosilica/Pal Composite and Its Performance in the Adsorption of Methyl Orange" Materials 11, no. 1: 164. https://doi.org/10.3390/ma11010164