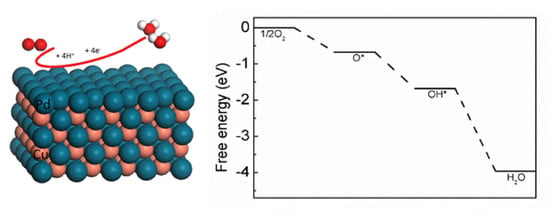
DFT Study on Intermetallic Pd–Cu Alloy with Cover Layer Pd as Efficient Catalyst for Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussion
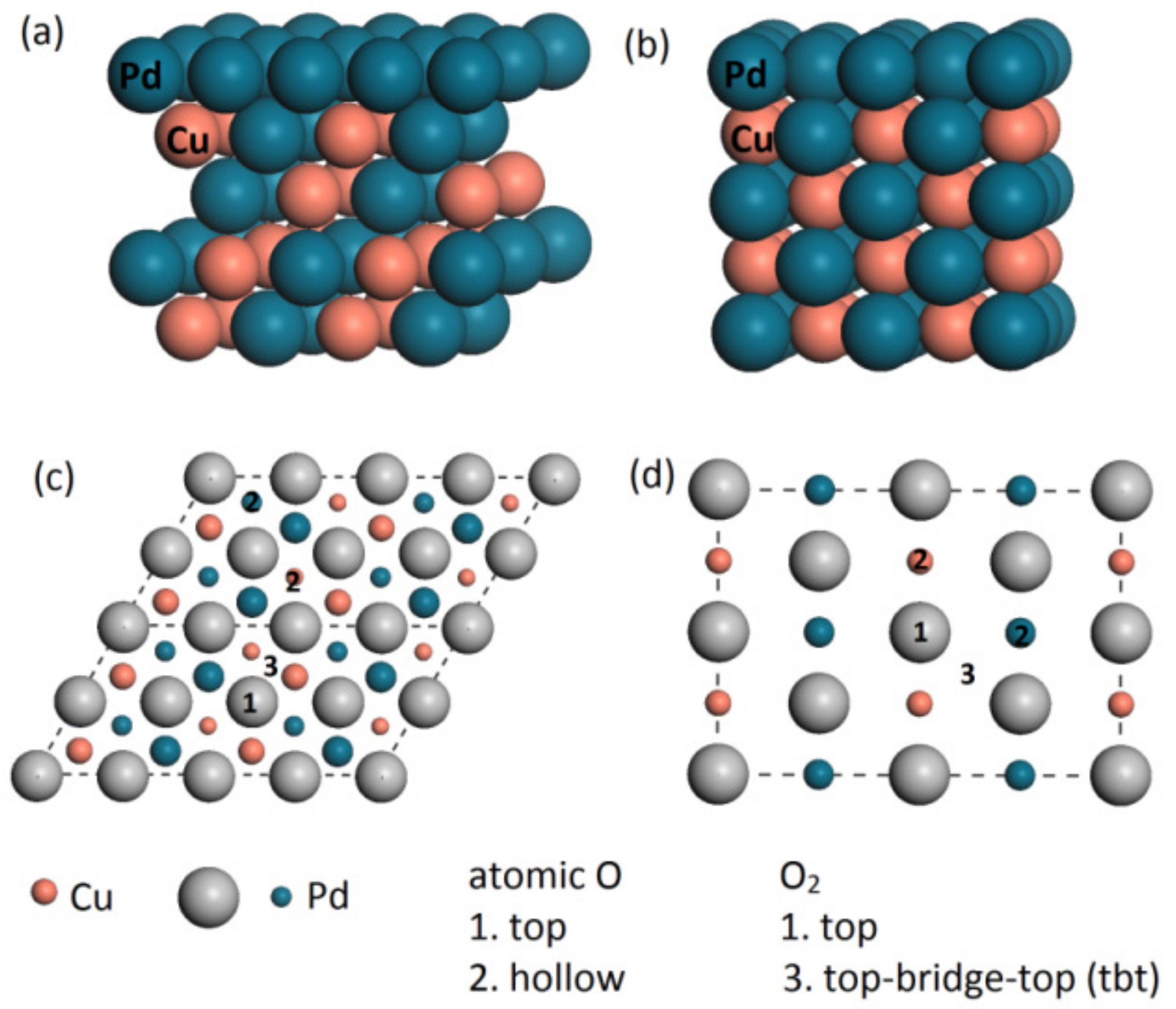
3.1. The Adsorption of O2 on Pd/PdCu Surface
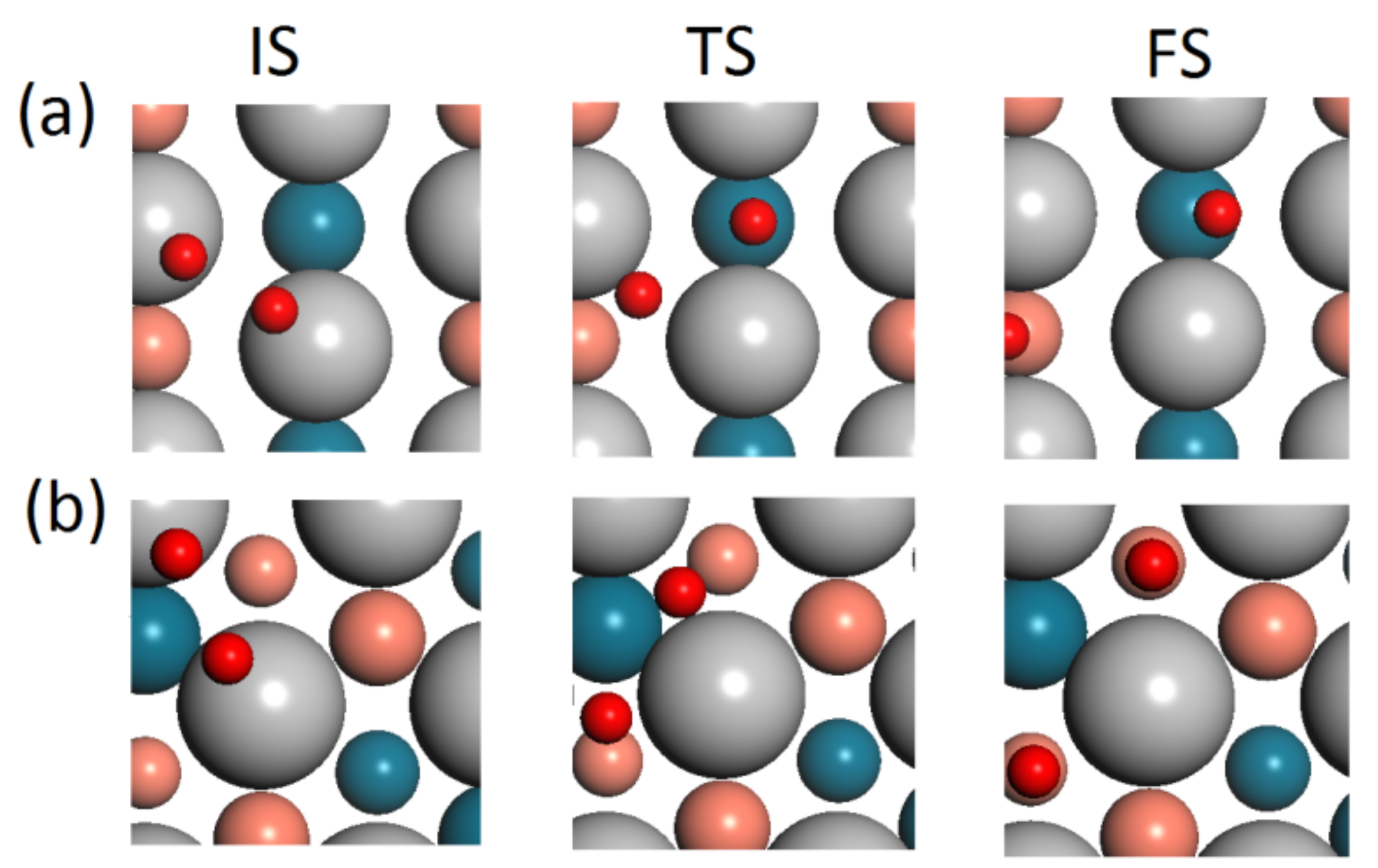
3.2. The Dissociation of O2 on Pd/PdCu Surface
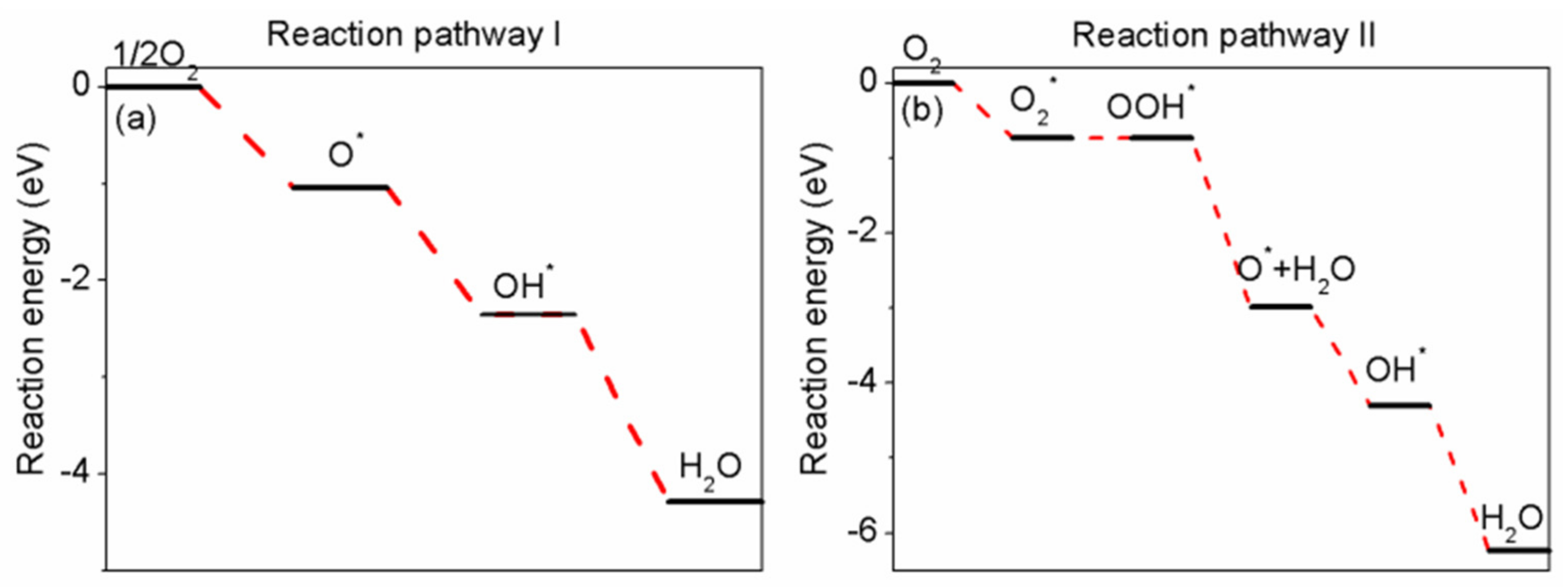
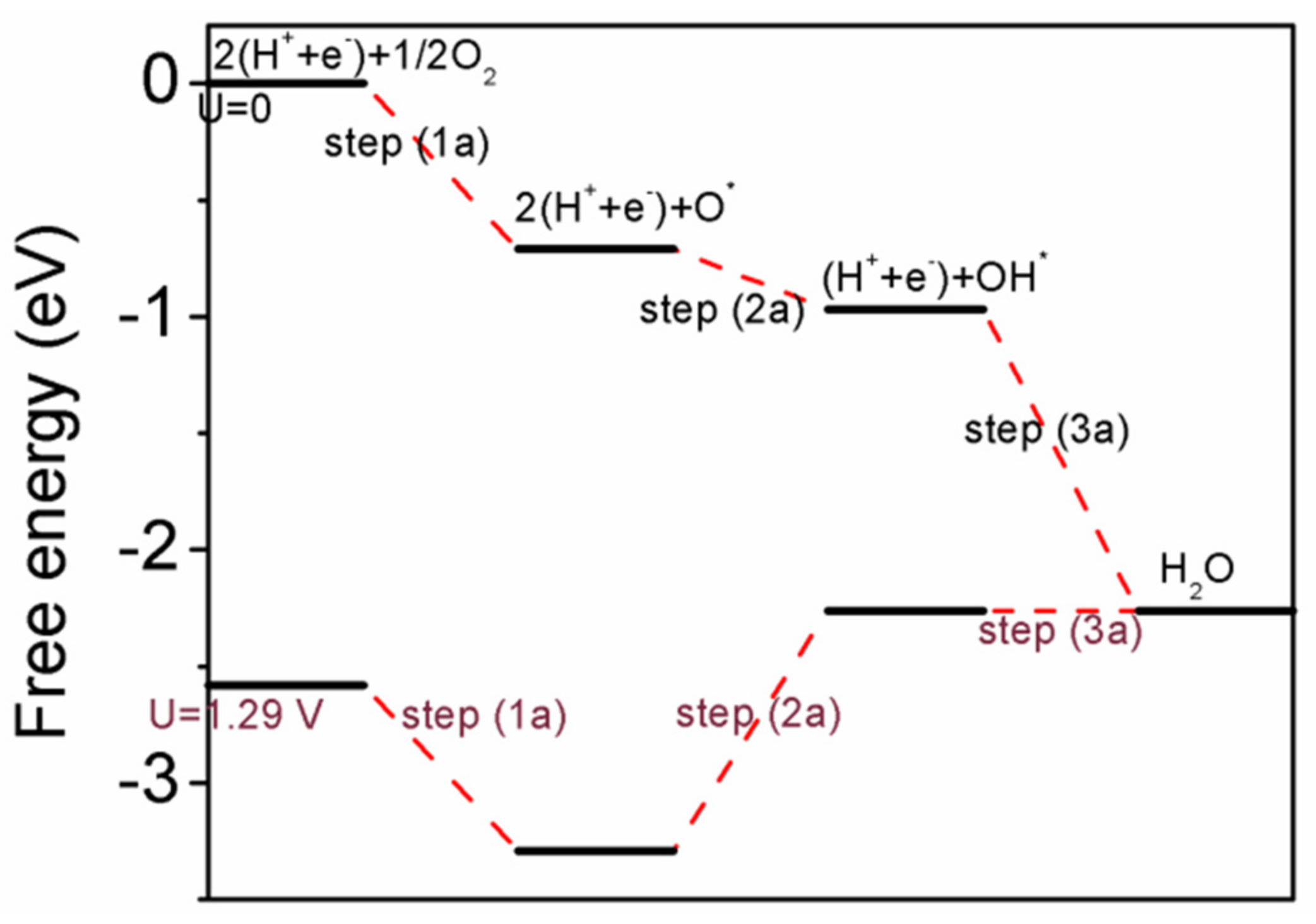
3.3. The Potential Pathway of ORR on Intermetallic Pd/Pd–Cu(110)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Luo, M.; Sun, Y.; Wang, L.; Guo, S. Tuning Multimetallic Ordered Intermetallic Nanocrystals for Efficient Energy Electrocatalysis. Adv. Energy Mater. 2017, 7. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, Z.; Ma, C.; Liu, Y.; Giroux, M.; Chi, M.; Jin, J.; Greeley, J.; Wang, C. Plating Precious Metals on Nonprecious Metal Nanoparticles for Sustainable Electrocatalysts. Nano Lett. 2017, 17, 3391–3395. [Google Scholar] [CrossRef] [PubMed]
- Mahata, A.; Rawat, K.S.; Choudhuri, I.; Pathak, B. Single-layered platinum nanocage: A highly selective and efficient catalyst for fuel cells. J. Mater. Chem. A 2016, 4, 12756–12767. [Google Scholar] [CrossRef]
- Chung, D.Y.; Jun, S.W.; Yoon, G.; Kwon, S.G.; Shin, D.Y.; Seo, P.; Yoo, J.M.; Shin, H.; Chung, Y.-H.; Kim, H. Highly durable and active PtFe nanocatalyst for electrochemical oxygen reduction reaction. J. Am. Chem. Soc. 2015, 137, 15478–15485. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Zhu, H.; Zhang, X.; Shen, B.; Wu, L.; Zhang, S.; Lu, G.; Wu, Z.; Sun, S. Core/shell face-centered tetragonal FePd/Pd nanoparticles as an efficient non-Pt catalyst for the oxygen reduction reaction. ACS Nano 2015, 9, 11014–11022. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.Y.; Han, G.F.; Xiao, B.B.; Gu, L.; Yang, Z.Z.; Wen, Z.; Zhu, Y.F.; Zhao, M.; Li, J.C.; Jiang, Q. Mesostructured Intermetallic Compounds of Platinum and Non-Transition Metals for Enhanced Electrocatalysis of Oxygen Reduction Reaction. Adv. Funct. Mater. 2015, 25, 230–237. [Google Scholar] [CrossRef]
- Shao, M.; Shoemaker, K.; Peles, A.; Kaneko, K.; Protsailo, L. Pt monolayer on porous Pd−Cu alloys as oxygen reduction electrocatalysts. J. Am. Chem. Soc. 2010, 132, 9253–9255. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, V.; Mun, B.S.; Mayrhofer, K.J.; Ross, P.N.; Markovic, N.M.; Rossmeisl, J.; Greeley, J.; Nørskov, J.K. Changing the activity of electrocatalysts for oxygen reduction by tuning the surface electronic structure. Angew. Chem. 2006, 118, 2963–2967. [Google Scholar] [CrossRef]
- Prabhudev, S.; Bugnet, M.; Bock, C.; Botton, G.A. Strained lattice with persistent atomic order in Pt3Fe2 intermetallic core–shell nanocatalysts. ACS Nano 2013, 7, 6103–6110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, X.; Jiang, G.; Zhu, H.; Guo, S.; Su, D.; Lu, G.; Sun, S. Tuning nanoparticle structure and surface strain for catalysis optimization. J. Am. Chem. Soc. 2014, 136, 7734–7739. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yu, Y.; Xin, H.L.; Hovden, R.; Ercius, P.; Mundy, J.A.; Chen, H.; Richard, J.H.; Muller, D.A.; DiSalvo, F.J. Tuning oxygen reduction reaction activity via controllable dealloying: A model study of ordered Cu3Pt/C intermetallic nanocatalysts. Nano Lett. 2012, 12, 5230–5238. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Goswami, A.; Asefa, T.; Guo, H.; Biradar, A.V.; Peng, D.-L.; Zboril, R.; Varma, R.S. Core–shell nanoparticles: Synthesis and applications in catalysis and electrocatalysis. Chem. Soc. Rev. 2015, 44, 7540–7590. [Google Scholar] [CrossRef] [PubMed]
- Kuttiyiel, K.A.; Sasaki, K.; Su, D.; Wu, L.; Zhu, Y.; Adzic, R.R. Gold-promoted structurally ordered intermetallic palladium cobalt nanoparticles for the oxygen reduction reaction. Nat. Commun. 2014, 5, 5185. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Z.; Zhang, X.; Lu, G. Multiscale Computational Design of Core/Shell Nanoparticles for Oxygen Reduction Reaction. J. Phys. Chem. C 2017, 121, 1964–1973. [Google Scholar] [CrossRef]
- Xiao, W.; Cordeiro, M.A.L.; Gong, M.; Han, L.; Wang, J.; Bian, C.; Zhu, J.; Xin, H.L.; Wang, D. Optimizing the ORR activity of Pd based nanocatalysts by tuning their strain and particle size. J. Mater. Chem. A 2017. [Google Scholar] [CrossRef]
- Shao, M.; Liu, P.; Zhang, J.; Adzic, R. Origin of enhanced activity in palladium alloy electrocatalysts for oxygen reduction reaction. J. Phys. Chem. B 2007, 111, 6772–6775. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.; Skriver, H.L.; Nørskov, J.K. Surface segregation energies in transition-metal alloys. Phys. Rev. B 1999, 59, 15990. [Google Scholar] [CrossRef] [Green Version]
- Nørskov, J.K.; Abild-Pedersen, F.; Studt, F.; Bligaard, T. Density functional theory in surface chemistry and catalysis. Proc. Natl. Acad. Sci. USA 2011, 108, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Hammer, B.; Nørskov, J.K. Theoretical surface science and catalysis—Calculations and concepts. Adv. Catal. 2000, 45, 71–129. [Google Scholar]
- Mao, Y.; Wang, H.F.; Hu, P. Theory and applications of surface micro-kinetics in the rational design of catalysts using density functional theory calculations. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2017, 7. [Google Scholar] [CrossRef]
- Wang, C.; Sang, X.; Gamler, J.T.; Chen, D.P.; Unocic, R.R.; Skrabalak, S.E. Facet-Dependent Deposition of Highly Strained Alloyed Shells on Intermetallic Nanoparticles for Enhanced Electrocatalysis. Nano Lett. 2017, 17, 5526–5532. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yu, Y.; Zhu, J.; Liu, S.; Muller, D.A.; Abruña, H.C.D. Morphology and activity tuning of Cu3Pt/C ordered intermetallic nanoparticles by selective electrochemical dealloying. Nano Lett. 2015, 15, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Henkelman, G. Tuning the oxygen reduction activity of Pd shell nanoparticles with random alloy cores. J. Phys. Chem. C 2012, 116, 20860–20865. [Google Scholar] [CrossRef]
- Antolini, E. Alloy vs. intermetallic compounds: Effect of the ordering on the electrocatalytic activity for oxygen reduction and the stability of low temperature fuel cell catalysts. Appl. Catal. B 2017, 217, 201–213. [Google Scholar] [CrossRef]
- Arumugam, B.; Tamaki, T.; Yamaguchi, T. Beneficial role of copper in the enhancement of durability of ordered intermetallic PtFeCu catalyst for electrocatalytic oxygen reduction. ACS Appl. Mater. Interfaces 2015, 7, 16311–16321. [Google Scholar] [CrossRef] [PubMed]
- Du, X.X.; He, Y.; Wang, X.X.; Wang, J.N. Fine-grained and fully ordered intermetallic PtFe catalysts with largely enhanced catalytic activity and durability. Energy Environ. Sci. 2016, 9, 2623–2632. [Google Scholar] [CrossRef]
- Yan, Y.; Du, J.S.; Gilroy, K.D.; Yang, D.; Xia, Y.; Zhang, H. Intermetallic Nanocrystals: Syntheses and Catalytic Applications. Adv. Mater. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Huang, B.; Xiao, L.; Ren, Z.; Chen, H.; Wang, D.; Abruña, H.D.; Lu, J.; Zhuang, L. Pt skin on AuCu intermetallic substrate: A strategy to maximize Pt utilization for fuel cells. J. Am. Chem. Soc. 2014, 136, 9643–9649. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xin, H.L.; Hovden, R.; Wang, H.; Yu, Y.; Muller, D.A.; DiSalvo, F.J.; Abruña, H.D. Structurally ordered intermetallic platinum–cobalt core–shell nanoparticles with enhanced activity and stability as oxygen reduction electrocatalysts. Nat. Mater. 2013, 12, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Gschneidner, K.; Ji, M.; Wang, C.; Ho, K.; Russell, A.; Mudryk, Y.; Becker, A.; Larson, J. Influence of the electronic structure on the ductile behavior of B2 CsCl-type AB intermetallics. Acta Mater. 2009, 57, 5876–5881. [Google Scholar] [CrossRef]
- Chi, M.; Wang, C.; Lei, Y.; Wang, G.; Li, D.; More, K.L.; Lupini, A.; Allard, L.F.; Markovic, N.M.; Stamenkovic, V.R. Surface faceting and elemental diffusion behaviour at atomic scale for alloy nanoparticles during in situ annealing. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, D.P.; Sang, X.; Unocic, R.R.; Skrabalak, S.E. Size-Dependent Disorder–Order Transformation in the Synthesis of Monodisperse Intermetallic PdCu Nanocatalysts. ACS Nano 2016, 10, 6345–6353. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for Ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Mao, J.; Liu, Y.; Chen, Z.; Wang, D.; Li, Y. Bimetallic Pd–Cu nanocrystals and their tunable catalytic properties. Chem. Commun. 2014, 50, 4588–4591. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shan, S.; Luo, J.; Joseph, P.; Petkov, V.; Zhong, C.-J. PdCu nanoalloy electrocatalysts in oxygen reduction reaction: Role of composition and phase state in catalytic synergy. ACS Appl. Mater. Interfaces 2015, 7, 25906–25913. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gong, H. First principles study of thermodynamic and mechanical properties of Pd 50 Cu 50. J. Alloys Compd. 2015, 639, 635–641. [Google Scholar] [CrossRef]
- Bozzolo, G.; Garcés, J.E.; Noebe, R.D.; Abel, P.; Mosca, H.O. Atomistic modeling of surface and bulk properties of Cu, Pd and the Cu–Pd system. Prog. Surf. Sci. 2003, 73, 79–116. [Google Scholar] [CrossRef]
- Wei, C.; Kong, F.; Gong, H. Phase stability and elastic property of PdH and PdCuH phases. Int. J. Hydrogen Energy 2013, 38, 16485–16494. [Google Scholar] [CrossRef]
- Liu, J.; Fan, X.; Sun, C.Q.; Zhu, W. Layer effect on catalytic activity of Pd–Cu bimetal for CO oxidation. Appl. Catal. A 2017, 538, 66–73. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Xu, Y.; Ruban, A.V.; Mavrikakis, M. Adsorption and dissociation of O2 on Pt−Co and Pt−Fe alloys. J. Am. Chem. Soc. 2004, 126, 4717–4725. [Google Scholar] [CrossRef] [PubMed]
- Fouda-Onana, F.; Savadogo, O. Study of O2 and OH adsorption energies on Pd–Cu alloys surface with a quantum chemistry approach. Electrochim. Acta 2009, 54, 1769–1776. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, H.; Sullivan, M.B.; Lim, F.C.H.; Wu, P. Study of Pd–Au bimetallic catalysts for CO oxidation reaction by DFT calculations. Phys. Chem. Chem. Phys. 2009, 11, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhang, L.; Henkelman, G. Catalytic activity of Pd/Cu random alloy nanoparticles for oxygen reduction. J. Phys. Chem. Lett. 2011, 2, 1328–1331. [Google Scholar] [CrossRef] [PubMed]
- Greeley, J. Theoretical heterogeneous catalysis: Scaling relationships and computational catalyst design. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 605–635. [Google Scholar] [CrossRef] [PubMed]
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Bahn, S.; Hansen, L.B.; Bollinger, M.; Bengaard, H.; Hammer, B.; Sljivancanin, Z.; Mavrikakis, M. Universality in heterogeneous catalysis. J. Catal. 2002, 209, 275–278. [Google Scholar] [CrossRef]
- Gómez-Marín, A.M.; Rizo, R.; Feliu, J.M. Oxygen reduction reaction at Pt single crystals: A critical overview. Catal. Sci. Technol. 2014, 4, 1685–1698. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jonsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]


| Composition | Structure | Method | Lattice Constant a = b = c (Å) |
|---|---|---|---|
| Pd | FCC | This work | 3.949 |
| DFT | 3.957 | ||
| Exp. | 3.890 | ||
| Cu | FCC | This work | 3.633 |
| DFT | 3.636 | ||
| Exp. | 3.615 | ||
| PdCu | B2 | This work | 3.021 |
| DFT | 3.026 | ||
| Exp. | 2.958 | ||
| PdCu | FCC | This work | 3.811 |
| DFT | 3.816 | ||
| Exp. | 3.760 |
| Composition | d-Band Center (eV) | Adsorption Energy | Bond Length O–O (Å) | O2 Dissociation (eV) | |||
|---|---|---|---|---|---|---|---|
| EO (eV) | EO2 (eV) | ||||||
| Hollow | Top | tbt | ΔE = EFS − EIS | Ebarrier = ETS − EIS | |||
| Pt/Pd–Cu(110) | −2.66 | −0.63 | Unstable | −0.95 | 1.362 | −0.36 | 1.29 |
| Pd/Pd–Cu(111) | −2.12 | −1.02 | 0.26 | −0.36 | 1.341 | −0.77 | 0.82 |
| Pd/Pd–Cu(110) | −1.93 | −1.18 | 0.31 | −0.73 | 1.340 | −1.34 | 0.60 |
| Pd(111) | −1.86 | −1.23 | - | −1.18 | 1.342 | −0.96 | 0.70 |
| Pt(111)Reference | −2.19 | −0.85 | - | −0.62 | - | −0.86 | 0.77 |
| Reaction Step | Reaction Energy Difference ΔE (eV) |
|---|---|
| O2* + H+ + e−→OOH* | −0.001 |
| OOH*→O* + OH* | −0.011 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Fan, X.; Sun, C.Q.; Zhu, W. DFT Study on Intermetallic Pd–Cu Alloy with Cover Layer Pd as Efficient Catalyst for Oxygen Reduction Reaction. Materials 2018, 11, 33. https://doi.org/10.3390/ma11010033
Liu J, Fan X, Sun CQ, Zhu W. DFT Study on Intermetallic Pd–Cu Alloy with Cover Layer Pd as Efficient Catalyst for Oxygen Reduction Reaction. Materials. 2018; 11(1):33. https://doi.org/10.3390/ma11010033
Chicago/Turabian StyleLiu, Ji, Xiaofeng Fan, Chang Q. Sun, and Weiguang Zhu. 2018. "DFT Study on Intermetallic Pd–Cu Alloy with Cover Layer Pd as Efficient Catalyst for Oxygen Reduction Reaction" Materials 11, no. 1: 33. https://doi.org/10.3390/ma11010033