1. Introduction
Performance and environmental targets for future aero-engines could be met with changes in the propulsive and thermal efficiency of the engines and new materials that have capabilities beyond those of Ni-based superalloys. Industry has set the following property goals for new ultra-high temperature alloys with capabilities beyond those of Ni-based superalloys: the room temperature fracture toughness must be above 20 MPa(m)
1/2, there must be less than 1% creep in 125 h at 1473 K and σ > 170 MPa (alloy density ρ = 7 g/cm
3) and the oxidation life at 1588 K must be equal to that of second generation single crystal Ni-based superalloys at 1423 K, with a short term oxidation goal to have sufficient oxidation resistance in the uncoated condition to survive under typical engine conditions, which requires a loss of material less than 200 μm thickness in 10 h at 1643 K and a long term oxidation goal that requires a loss of material less than 25 μm thickness in 100 h at 1588 K [
1].
Refractory metal intermetallic composites (RMICs) have the potential to offer a balance of properties required in critical applications in future aero-engines. RMICs can be in situ composites. Composite microstructures can be formed in situ with TM
5Si
3 (TM = transition and refractory metal) silicides. Interest in the TM
5Si
3 silicides for structural materials is justified because of their high temperature strength and creep properties, their high melting points, which are in excess of 2273 K and their solubility ranges [
2], which make alloying with different elements possible. The 5-3 silicides of the transition metals of groups IV to VI have the D8
8 (hP16, Mn
5Si
3 prototype), D8
l (tI32, Cr
5B
3 prototype), or D8
m (tI32, W
5Si
3 prototype) structure types. These three structures have different numbers of non-equivalent sites (three for D8
8 and four for D8
l and D8
m). The 5-3 silicides of the group IV elements (Ti, Zr, Hf) have the D8
8 structure. Those of the group V elements Nb and Ta have the D8
m structure at high temperature and the D8
l structure at low temperature. Vanadium and the group VI elements Cr, Mo and W have the D8
m structure.
The motivation for pursuing in situ compositing is the poor toughness of the 5-3 silicide(s) at room temperature and the fact that with 5-3 silicides there are broad alloying opportunities that can lead to suppression of other silicide(s) and the formation of eutectics between 5-3 silicides and refractory metal solid solution(s) [
3,
4,
5,
6]. The properties of 5-3 silicides depend on alloying. For example, the coefficient of thermal expansion (CTE) of 5-3 silicides, including Nb
5Si
3, can be anisotropic and the CTE anisotropy is changed by alloying. Control of the CTE anisotropy of TM
5Si
3 silicides via alloying is important because CTE anisotropy is expected to have a negative effect on the processing of the alloys and a negative effect on the life of components, owing to the residual micro-stresses at grain boundaries at temperatures below the ductile to brittle transition temperature [
7].
The ratio (CTE
c)/(CTE
a) of the coefficients of thermal expansion along the c and a axes of different 5-3 silicides is used to show their CTE anisotropy. This ratio is given in the
Table 1 for different 5-3 silicides. The different experimental data for the CTE anisotropy of Ti
5Si
3, Mo
5Si
3, Zr
5Si
3 and αNb
5Si
3 could be attributed to the difficulties in making arc melted alloys with homogeneous microstructures [
20]. Alloying the Ti
5Si
3 with Nb or Ta or Ge did not change the CTE anisotropy but additions of B, Cr, Hf, V and Zr changed it to about 2 [
9,
10]. Alloying with B had a strong effect on the CTE anisotropy of W
5Si
3 (T2 phase) which was reduced to about 1.1 [
13]. The data in
Table 1 shows that the alloying of Nb
5Si
3 with Ti also changed its CTE anisotropy and that these changes are not as dramatic as those for alloyed Ti
5Si
3. Contamination of the 5-3 silicides by interstitials also can change their CTE anisotropy. For example, in the case of Ti
5Si
3 contamination by C or N changed the anisotropy ratio to about 2 but contamination by O had no effect on the CTE anisotropy [
9].
Nb-silicide based alloys, which are also known as Nb-silicide in situ composites, are RMICs that can surpass the fracture toughness and creep property goals and their oxidation can be close to the oxidation goal [
1,
3,
21]. Reductions in rotor weight of more than 20% could be realized through the substitution of Nb-silicide based aerofoils for Ni-base superalloys aerofoils in present and advanced turbine designs [
1,
21]. The most important phases in the microstructure of Nb-silicide based alloys are the bcc Nb solid solution(s), Nb
ss and the Nb
5Si
3 silicide. The latter can be present as the tetragonal high temperature βNb
5Si
3, or the tetragonal low temperature αNb
5Si
3 [
2], or as the hexagonal γNb
5Si
3 silicide. The γNb
5Si
3 is not desirable owing to its creep properties [
1]. The Nb
ss can be rich in Ti [
4] or free of Si [
22]. Other phases also can be stable in Nb-silicide based alloys, for example the C14-NbCr
2 Laves and A15-Nb
3X (X = Al, Ge, Si, Sn) phases and the tetragonal Nb
3Si [
1,
3,
21]. The Laves phase can be stable in Cr rich alloys and is considered to improve oxidation resistance. The A15 Nb
3X phase(s) also can be stable in the microstructure depending on concentration(s) of element(s) X and can form during oxidation. The tetragonal Nb
3Si can be stable or transform to the low temperature αNb
5Si
3 via the eutectoid reaction Nb
3Si → Nb + αNb
5Si
3 [
2,
4,
5,
23,
24,
25,
26,
27,
28,
29,
30].
The Nb
ss is the key phase for meeting the fracture toughness property goal but has a negative effect on creep and oxidation when present at a high volume fraction. The toughness of the Nb-0.8Si and (Nb,Ti,Cr,Hf,Si,Ge) solid solutions was reported as 17.7 MPa(m)
1/2 and ≥28.7 MPa(m)
1/2 respectively [
31,
32], more than five and nine times the toughness of unalloyed Nb
5Si
3, which is about 3 MPa(m)
1/2 [
21]. The Nb
5Si
3 is the key phase for meeting the creep goal but high volume fractions of the silicide decrease the toughness of the in situ composites. The creep exponent of Nb (≈6) [
21] is six times that of Nb
5Si
3 (≈1) [
33]. The low fracture toughness of tetragonal Nb
5Si
3 is similar to that of Mo
5Si
3 (2–2.5 MPa(m)
1/2, [
34]) and Ti
5Si
3 (2.1 MPa(m)
1/2 [
35] and 2.6 MPa(m)
1/2 [
36]). Alloying improved the toughness of Nb
5Si
3, which was reported to be 7 MPa(m)
1/2 and 13 MPa(m)
1/2 respectively for the (Nb,Ti,Hf,Cr,Fe)
5(Si,Ge,Al,Sn)
3 and (Nb,Ti,Hf,Cr)
5(Si,Ge)
3 silicides [
32]. The compressive fracture strength of Nb
5Si
3 was reported to be 670 MPa at 1773 K [
31]. The compressive creep rate of arc melted αNb
5Si
3 at 1473 K and 69 MPa was 2.23 × 10
−9 s
−1 [
33] compared with 4 × 10
−8 s
−1 of arc melted tetragonal D8
m (tI32, W
5Si
3-type) Mo
5Si
3 [
37] and 2 × 10
−5 s
−1 of hexagonal D8
8 (hP16, Mn
5Si
3-type) Ti
5Si
3 [
38].
Table 1 gives available data for the CTE anisotropy of binary (unalloyed) and ternary 5-3 silicides. Data for creep and toughness of binary 5-3 silicides was given above. Developmental Nb-silicide based alloys can have as many as twelve alloying additions, some of which substitute Nb and others Si in Nb
5Si
3. For example, refractory metals provide solid solution strengthening to the Nb
ss and improve its high temperature strength and simple metal and metalloid element additions improve oxidation. The following composition (at.%) [40.7Nb-12.8Ti-4.7Mo-1.3W-1.5Hf-2.7Cr]-(20.8Si-5.9Ge-4.6Al-5Sn) is an example of a real tetragonal Nb
5Si
3 silicide in a developmental Nb-silicide based alloy, where in parentheses are the elements that substitute Si and in square brackets the elements that substitute Nb. There are four sub-lattices in αNb
5Si
3 (tI32 (D8
l), Cr
5B
3-type) and it is not known which lattice positions are occupied by the different elements.
Data about the alloying and properties of Nb5Si3 is missing in the literature, yet it is crucial for the design of new Nb-silicide based alloys. The motivation for the research presented in this paper was to study the alloying behaviour and properties of Nb5Si3. The alloying and properties of C14-NbCr2 and A15-Nb3X phases that are stable in Nb-silicide based alloys will be the subject of another paper.
Recently, it was shown that the alloying of the Nb
ss in Nb-silicide based alloys depends on composition weighted differences in electronegativity (Δχ) and an average valence electron concentration (VEC) [
22]. Phase stability can be considered in terms of
e/
a (an averaged valence of alloying elements in an alloy) and VEC (number of valence electrons per atom filled into the valence band). The former is the parameter in the Hume-Rothery rules and the latter is key to determining the Fermi level in the valence band. The choice between
e/
a and VEC depends on the stability mechanism involved [
39]. According to Mizutani et al. [
39,
40], the
e/
a is difficult to use as a universal parameter in alloy design because its value cannot be uniquely assigned to a transition metal as it depends on the surrounding environment (the alloying elements in synergy). Instead, VEC is a more important parameter in transition metal alloys.
In this work, the silicide parameters VEC and Δχ were used to study the alloying and properties of tetragonal Nb5Si3. One objective was to find out if there are relationships between solvent and solute additions and between the latter and the silicide parameters VEC and Δχ. Another objective was to find out whether changes in properties of tetragonal Nb5Si3 are related to changes of the silicide parameters VEC and Δχ.
The structure of the paper is as follows. The effects of alloying on the solubility range of X in (Nb,TM)5X3 where X = Al + B + Ge + Si + Sn and TM is a transition and/or refractory metal are discussed first, followed by relationships between solutes and their concentrations in Nb5Si3 and the silicide parameters VEC and Δχ and how alloying influences the hardness of tetragonal Nb5Si3. The latter is discussed further with the help of the silicide parameter VEC using silicides alloyed with Ge as an example. Finally, the alloying and creep of Nb5Si3 is discussed with the help of Δχ versus VEC maps.
2. Methodology, Results and Discussion
Available experimental data for tetragonal Nb
5Si
3 silicides in developmental Nb-silicide based alloys was used to seek out relationships between the silicide parameters Δχ and VEC, the hardness of tetragonal Nb
5Si
3 and changes of the creep of Nb
5Si
3 with alloying. For these tasks, it is necessary to know the actual compositions of the Nb
5Si
3 silicides in studied alloys [
4,
5,
6,
30,
41,
42,
43,
44,
45,
46,
47,
48] in order to calculate the silicide parameters VEC and Δχ. All the tetragonal Nb
5Si
3 silicides studied in this paper were in developmental Nb-silicide based alloys that had been prepared using the same method of arc melting with non-consumable tungsten electrode in an inert atmosphere with water cooled copper crucibles. The phases (Nb
ss, Nb
5Si
3 and others, see introduction) in the cast and heat treated microstructures were identified using XRD (Hiltonbrooks Ltd., Crewe, UK) and JCPDS data (International centre for diffraction data) and quantitative microanalysis [
4,
5,
6,
30,
41,
42,
43,
44,
45,
46,
47,
48]. For the latter, electron probe micro-analysis (EPMA) was used in a JEOL 8600 EPMA (JEOL Ltd., Tokyo, Japan) equipped with energy-dispersive and wavelength-dispersive spectrometers. Standards of high purity elements of Nb, Si and other alloying additions (Al, B, Cr, Ge, Hf, Mo, Si, Sn, Ta, Ti), which had been polished to a finish of 1μm, were used. The operational software was the Oxford Link INCA software (Oxford Instruments plc, Abingdon, UK) that includes the XPP corrections method (matrix correction algorithm to convert k-ratios to element concentrations), which is based on the Rhi-Rho-Z approach. At least 10 analyses for each phase or area of the ingot were performed. The hardness of Nb
5Si
3 in the alloys was measured using a Mitutoyo micro-hardness testing machine (Mitutoyo America, Aurora, IL, USA). The load used was 0.1 kg and was applied for 20 s. At least 10 measurements were taken for each phase. The hardness measurements were taken from silicides in bulk microstructures free of contamination by interstitials and with similar grain sizes. The data for the compressive creep of Nb
5Si
3 was from the references [
33,
49], where the experimental details for the creep experiments were given. No new experimental data were created during the course of this study.
The parameter VEC was calculated using [VEC]
intermetallic = ∑
inCi(
VEC)
i, where
Ci and (
VEC)
i respectively are the concentration (at.%) and VEC of element
i in the silicide. For the Nb
5Si
3 silicide [Δχ]
silicide = ∑
imci(χ
<Nb>i) − ∑
iz < κi(
χ<Si>i), where
ci and
χ<Nb>i respectively are the concentration (at.%) and Pauling electronegativity of Nb and element
i substituting Nb in the silicide and
κi and
χ<Si>i respectively are the concentration (at.%) and Pauling electronegativity of Si and element
i substituting Si in the silicide. Data for electronegativity and VEC was from the same sources as in [
22].
The unalloyed (binary) tetragonal αNb
5Si
3 and the B containing tetragonal Nb
5Si
3 are also known as the T1 and T2 silicides respectively and both have the Cr
5B
3 as their prototype. In Nb-silicide based alloys, the Nb in Nb
5Si
3 can be substituted by other transition and/or refractory metals, e.g., Cr, Hf, Mo, Ta, Ti, W and the Si by other simple metals and metalloids, e.g., Al, B, Ge and Sn [
4,
5,
6,
30,
41,
42,
43,
44,
45,
46,
47,
48]. The solubilities of most of these elements depend on other alloying additions, in particular Ti. An objective of this work was to find out if solvent and solute concentrations in the Nb
5Si
3 are related and whether the concentrations of solute additions in the silicide depend on the parameters VEC and Δχ. The alloying of Nb
5Si
3 can stabilise the high temperature tetragonal βNb
5Si
3 and/or the low temperature tetragonal αNb
5Si
3 and/or the hexagonal γNb
5Si
3 in the microstructure of Nb-silicide based alloys and can change the mechanical properties and oxidation of these silicides. Another objective of this work was to find out how properties of tetragonal Nb
5Si
3 are associated with changes of the parameters VEC and Δχ.
In Ti containing Nb-silicide based alloys, Ti rich Nb
5Si
3 can form in the cast microstructure owing to the partitioning behaviour of Ti [
4,
22]. The Ti rich Nb
5Si
3 tends to persist in the microstructure after heat treatment. In the SEM and EPMA the Ti rich Nb
5Si
3 is recognised by its different contrast in the microstructure under back scatter electron imaging conditions [
4].
First it will be shown that the available microanalysis data can be used to find out how the solubilities of elements that substitute Nb and Si in Nb
5Si
3 are related. The actual chemical composition data for Nb
5Si
3 in Nb-silicide based alloys [
4,
5,
23,
24,
25,
26,
27,
28,
29,
30,
41,
42,
43,
44,
45,
46,
47,
48] showed that the solubilities of the elements that substitute Nb in tetragonal Nb
5Si
3 are Cr ≤ 2.9 at.%, Hf ≤ 10.6 at.%, Mo ≤ 1.9 at.%, Ta ≤ 6.3 at.%, Ti ≤ 32.8 at.% and W ≤ 1.9 at.% and that the solubility range of Ti in Nb
5Si
3 depends on the concentration of Ti in the alloy. For KZ5 type alloys [
4], i.e., for alloys where other transition metals and simple metals and metalloid elements are added to the nominal composition Nb-24Ti-18Si-5Al-5Cr (at.%), which is the composition of the alloy KZ5, the Ti solubility range is 17.1 < Ti < 24.2 at.% and 22.2 < Ti < 32.8 at.% for normal and Ti rich Nb
5Si
3 respectively.
The actual chemical composition data for Nb
5Si
3 in Nb-silicide based alloys [
4,
5,
23,
24,
25,
26,
27,
28,
29,
30,
41,
42,
43,
44,
45,
46,
47,
48] showed that the solubilities of the elements that substitute Si in Nb
5Si
3 in Nb-silicide based alloys are Al ≤ 5.2 at.%, B ≤ 10.4 at.%, Ge ≤ 8.4 at.% and Sn ≤ 3.8 at.% with 33.6 < X < 41.6 at.% (X = Al + B + Ge + Si + Sn), compared with 37.5 < Si < 40.5 at.% for unalloyed (binary) Nb
5Si
3 [
2]. The value of X does not differ significantly between the normal silicide and Ti rich Nb
5Si
3 and is 33.9 < X < 40.9 at.% and 33.6 < X < 41.6 at.% respectively. However, the range of X values would suggest that the solubility range of tetragonal Nb
5Si
3 opens up, or closes down and/or shifts upon alloying, primarily towards the Nb side. These changes are accompanied by changes in the values of the parameters VEC and Δχ.
The values of the parameters VEC and Δχ of the Nb
5Si
3 silicide that were calculated as described above are in the ranges 4.11 < VEC < 4.45 and 0.103 < Δχ < 0.415 respectively. The silicide parameter VEC falls outside the range of VEC values of the Nb
ss [
22]. The range of the values of the silicide parameter Δχ is wider that those of the Nb
ss [
22] and there is a gap in silicide Δχ values in the range 0.13 < Δχ < 0.15, which falls within the 0.13 < Δχ < 0.18 gap for the Δχ of the Nb
ss [
22]. However, in the case of the tetragonal Nb
5Si
3 silicide, the aforementioned gap is observed only for B containing Nb
5Si
3 (i.e., for alloyed T2).
Table 2 shows the solubility range of Si for binary Nb
5Si
3 [
2] and the solubility range of X in (Nb,TM)
5X
3 silicides in KZ5 type alloys, where X = Al + B + Ge + Si + Sn and transition and refractory metals are represented by TM. Data for chemical compositions of alloys in
Table 2 can be found in the references [
4,
6,
41,
50,
51]. For each alloy in
Table 2, the corresponding values of the parameters VEC and Δχ of Nb
5Si
3 were calculated as described above and are given for the cast and heat treated conditions. In the binary (unalloyed) Nb
5Si
3, the Si concentration varies from 37.5 at.%, for which Δχ = 0.288 and VEC = 4.63, to 40.5 at.% (Δχ = 0.183, VEC = 4.6). In the alloy KZ5 [
4] the (Nb,Ti,Cr)
5(Si,Al)
3 has Al + Si in the range 35.3 < X < 36.4 at.% (X = Al + Si) and the values of the parameters change from Δχ = 0.363, VEC = 4.41 (cast) to Δχ = 0.322 and VEC = 4.42 (heat treated). With the addition of 6Ta in the alloy KZ6 [
6], the (Nb,Ti,Cr,Ta)
5(Si,Al)
3 has Al + Si in the range 36.7 < X < 38.7 at.% and the values of the parameters change from Δχ = 0.309, VEC = 4.38 (cast) to Δχ = 0.236 and VEC = 4.41 (heat treated). With the addition of 5B the (Nb,Ti,Cr)
5(Si,Al,B)
3 has X = Al + B + Si in the range 36.7 < X < 39 at.% and the values of the parameters change from Δχ = 0.303, VEC = 4.27 (cast) to Δχ = 0.224 and VEC = 4.31 (heat treated).
The data in
Table 2 shows that individually the transition metals Hf, Mo and Ta and the elements B, Ge and Sn (when added to the alloy KZ5) shift the solubility range X of the (Nb,TM)
5X
3 silicide towards Nb, with Hf and Ge having the strongest effect. Boron in synergy with Hf or Mo or Sn opens up the solubility range beyond 40.5 at.%. It should be noted that for each alloy a shift towards higher X concentrations is accompanied by a decrease in the value of Δχ. However, the change of the parameter VEC (meaning increase or decrease) depends on the alloying addition(s), for example when 5 at.% Hf is added to the alloy KZ5 the parameter VEC decreases but when 6 at.% Ta is added the parameter VEC increases.
The availability of data about the actual chemical composition of alloyed Nb
5Si
3 makes it possible to study the alloying of tetragonal Nb
5Si
3. The Si concentration in the silicide decreases with increasing Ti concentration in the silicide and the Cr and Al concentrations increase with increasing Ti concentration.
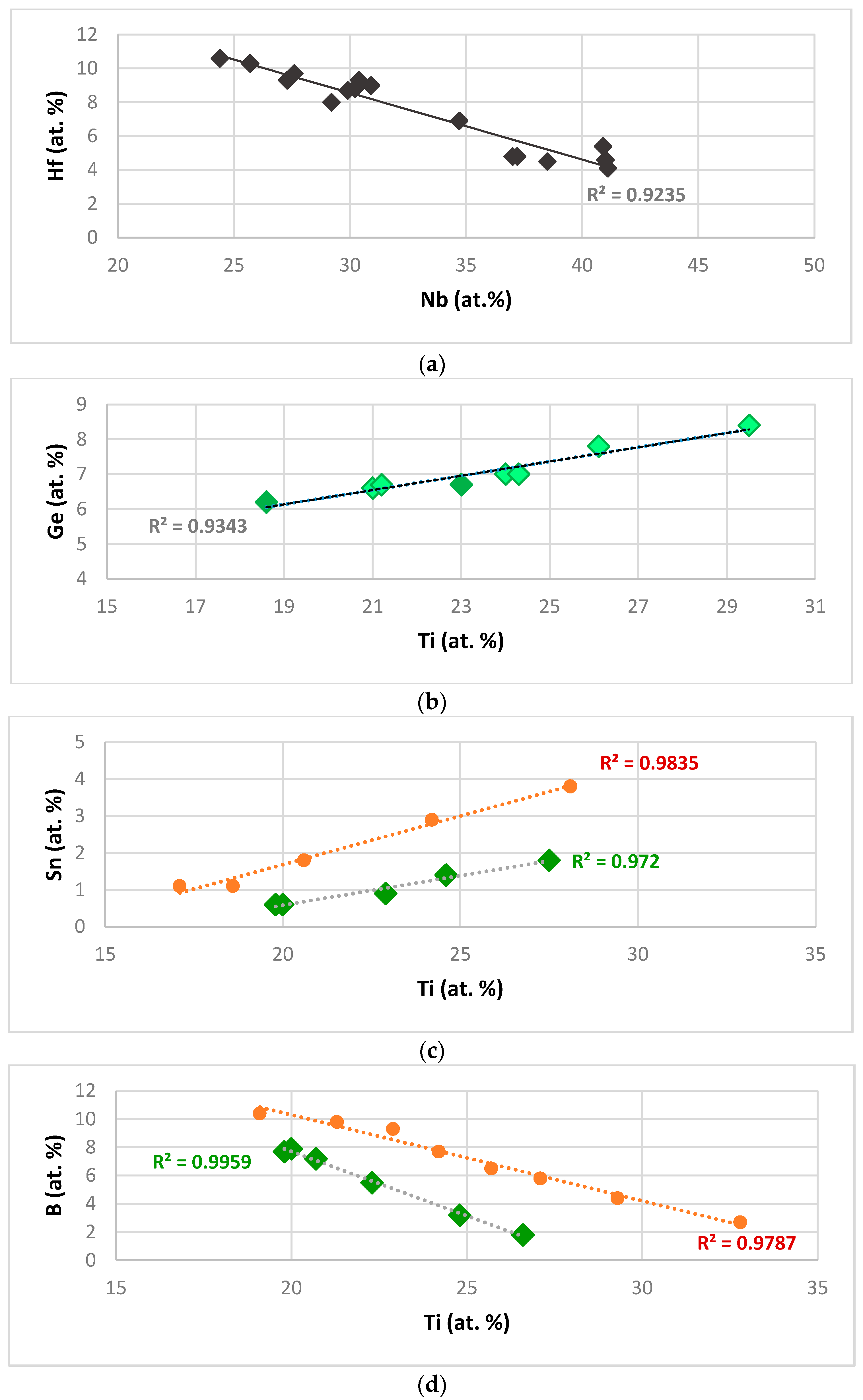
Figure 1a shows that the Hf concentration in Nb
5Si
3 decreases linearly with increasing Nb concentration in the silicide. The linear fit of the data is better in the Hf versus Nb plot (R
2 = 0.9235) compared with the Ti versus Nb plot (not shown, R
2 = 0.8095) that indicates that the Hf concentration in the Nb
5Si
3 increases with its Ti concentration. This would suggest that the Hf concentration in the Nb
5Si
3 depends on both Nb and Ti in the Nb
5Si
3.
Figure 1b shows that the concentration of Ge in Nb
5Si
3 increases with that of Ti. Note that in
Figure 1b the data for Ge and Sn containing Nb
5Si
3 (darker diamonds) falls on the same line as that for Nb
5Si
3 in Ge containing alloys with no B and Sn. The dependence of the concentration of Sn and B on that of Ti in Nb
5Si
3 is shown in
Figure 1c,d respectively. The different sets of data in each part are not for normal Nb
5Si
3 and Ti rich Nb
5Si
3 but for different solutes in the silicide as indicated in the figure caption. The
Figure 1c,d would suggest that the solubilities of B and Sn in Nb
5Si
3 depend strongly on the other elements that are present in the alloy. In all parts of
Figure 1 the linear fit of data, shown by the R
2 values, is very good.
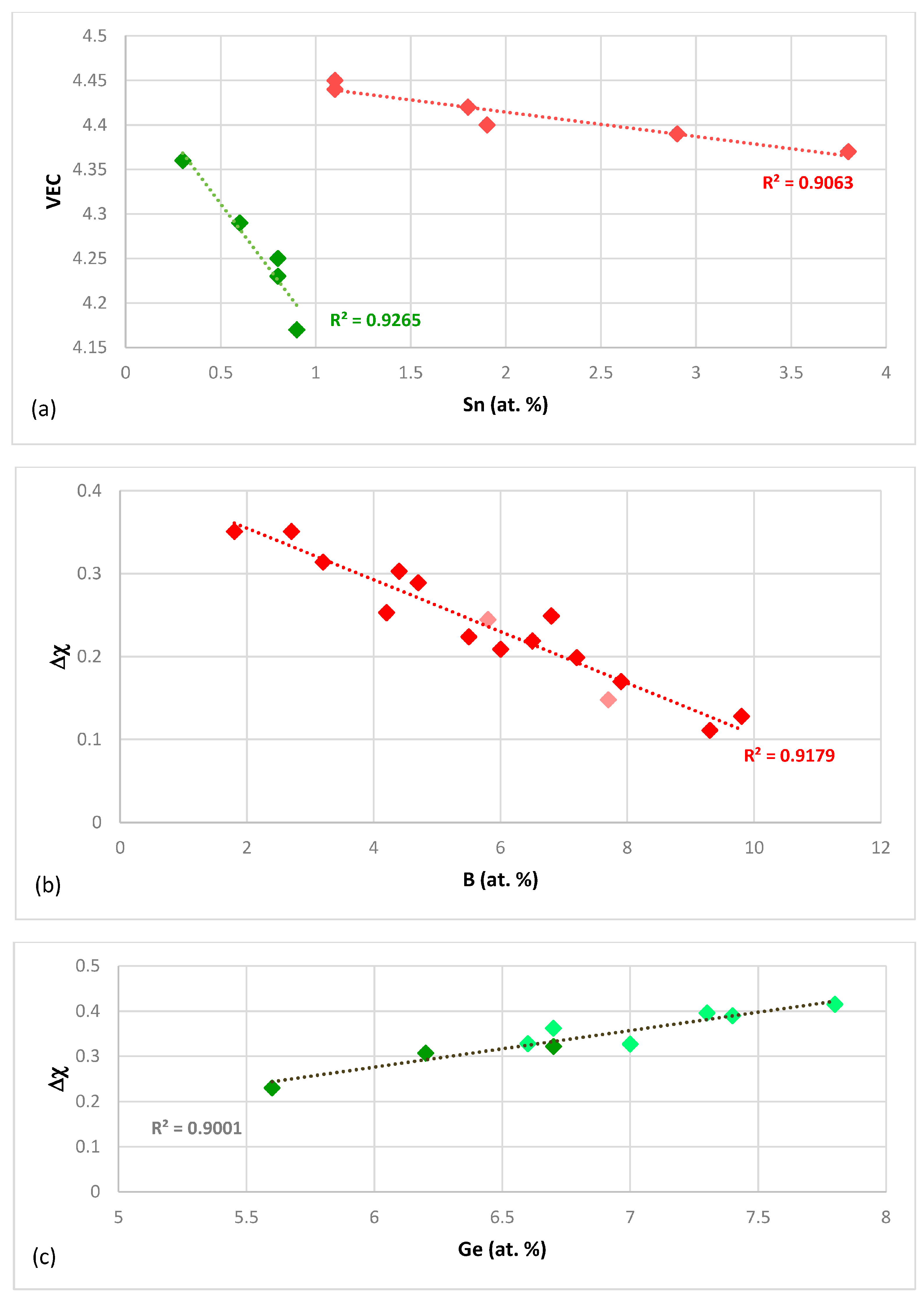
What can be learned about the alloying of Nb
5Si
3 from the silicide parameters VEC and Δχ? The silicide parameter VEC can separate the alloying behaviour of Hf in the normal and Ti rich Nb
5Si
3. The value of the silicide parameter VEC decreases with increasing Hf concentration in Nb
5Si
3 but there is no strong relationship (the R
2 value is low). However, the silicide parameter VEC can better describe the alloying behaviour of Sn in Nb
5Si
3 (
Figure 2a), which is shown to depend strongly on the elements that are present in the alloy (as was the case in
Figure 1c), with B having a strong effect on the change of VEC with Sn concentration in the silicide, compared with that of Ge.
The silicide parameter Δχ also can separate the alloying behaviour of Hf in the normal Nb
5Si
3 and Ti rich Nb
5Si
3 but the data falls in two distinct parts with no strong relationship (the R
2 value is low). When the data for Sn is considered, the silicide parameter Δχ can separate the data into two groups for B and Sn and Ge and Sn containing alloys but there is no good linear fit of the data compared with the silicide parameter VEC (
Figure 2a). The silicide parameter Δχ also can describe the alloying behaviour of B in Nb
5Si
3 (
Figure 2b) well but cannot separate the effect of transition metal addition in the alloy, which was demonstrated in
Figure 1d. The parameter Δχ decreases with increasing B concentration in Nb
5Si
3 (
Figure 2b). The alloying of Nb
5Si
3 with Ge also can be described well by Δχ, which increases with Ge concentration in the alloy (
Figure 2c). Note that the trends in
Figure 2b,c regarding the changes of the silicide parameter Δχ with B and Ge concentration in Nb
5Si
3 are the same with the trends in the change of the concentrations of these elements with Ti in Nb
5Si
3, shown in
Figure 1c,d respectively. This is not the case for the silicide parameter VEC and the concentration of Sn in Nb
5Si
3 (
Figure 1c and
Figure 2a).
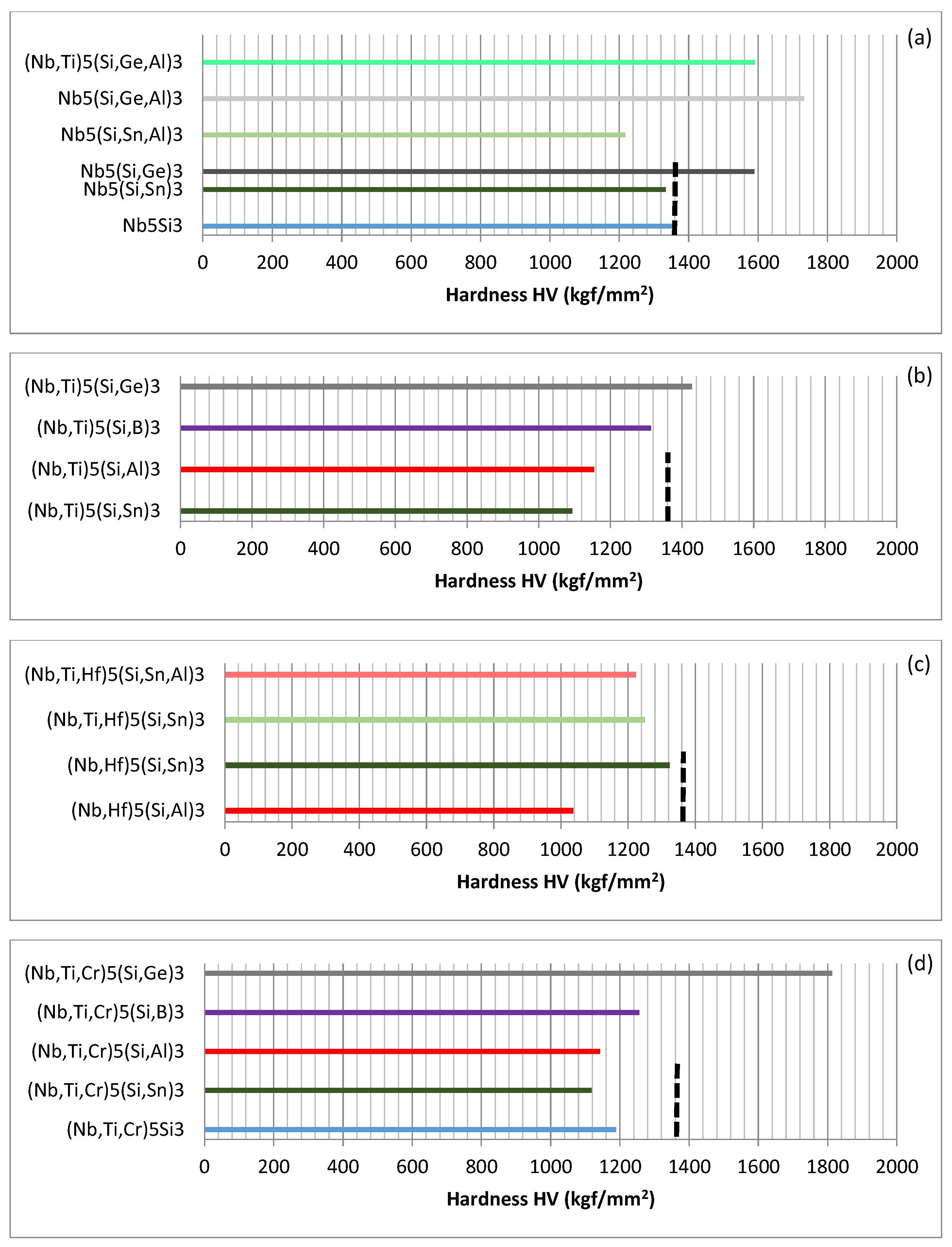
Links between alloying and properties will now be considered. The effects of alloying on the hardness of tetragonal Nb
5Si
3 are shown in
Figure 3, which shows the data for the average Vickers hardness (HV) of tetragonal Nb
5Si
3 silicide, where Nb and Si are substituted by different elements. The data in
Figure 3 shows that Ge increases significantly the hardness of Nb
5Si
3 compared with Sn, which hardly changes the hardness (see
Figure 3a). The effect of Al on the hardness of Nb
5Si
3 depends on the other element that substitute Si in the silicide. Aluminium has a strong negative and positive effect when it is in synergy with Sn or Ge respectively (see
Figure 3a). Comparison of the data for Nb
5(Si,Ge,Al)
3 with that for (Nb,Ti)
5(Si,Ge,Al)
3 in
Figure 3a suggests that the substitution of Nb by Ti decreases the hardness of the 5-3 silicide. This cannot be confirmed for the ternary silicide, because, to the author’s knowledge, there is no experimental data available for the hardness of (Nb,Ti)
5Si
3.
The effect of alloying with Ti on the Young’s modulus is shown in
Table 3, where data for unalloyed αNb
5Si
3, βNb
5Si
3 and alloyed α(Nb,Ti)
5Si
3 and β(Nb,Ti)
5Si
3 with 12.5 at.% Ti is given together with the Young’s moduli of other TM tetragonal 5-3 silicides. In [
19,
42] it was shown that (i) the βNb
5Si
3 has lower Young’s modulus E, shear modulus G and G/B ratio (B is the bulk modulus) compared with the αNb
5Si
3 and; (ii) that substitution of Nb by Ti increases and decreases the E, G and G/B respectively for the αNb
5Si
3 and βNb
5Si
3.
The effect of Ti on the hardness of (Nb,Ti)
5Si
3 can be deduced using data for E, G, the G/B ratio and Poisson’s ratio ν for unalloyed and Ti alloyed tetragonal Nb
5Si
3 silicides from [
19,
42] (see
Table 4). The calculations showed that the hardness of βNb
5Si
3 is lower than that of αNb
5Si
3 and alloying with Ti respectively decreases and increases the hardness of these silicides. The hardness of βNb
5Si
3 (HV = 1286) that was calculated using data for the calculated G/B ratio is closer to the experimental value for unalloyed Nb
5Si
3 (HV = 1360).
Table 4 also gives data for the calculated hardness of the unalloyed hexagonal Ti
5Si
3, which is higher than the measured hardness of unalloyed Ti
5Si
3 (1154 ± 55 HV [
56]). The calculations indicate that alloying Nb
5Si
3 with Ti decreases the hardness of β(Nb,Ti)
5Si
3 only.
The hardness values of Nb
5Si
3 where Nb is substituted by Ti only and Si by Al or B or Ge or Sn are compared in
Figure 3b. The data provides further support that Ti has a negative effect on the hardness and also shows that the synergy of Si, Sn and Ti has the strongest negative effect while that of Ge, Si and Ti has the weakest negative effect, compared with the data in
Figure 3a, with the hardness gradually increasing as the Si is substituted by Sn, Al, B and Ge. The hardness of (Nb,Ti)
5(Si,Ge)
3 is slightly higher than that of the binary (unalloyed) Nb
5Si
3.
When Hf substitutes Nb and Al or Sn substitutes Si, the synergy of Al and Hf and Hf and Sn respectively has a stronger negative and positive effect on the hardness compared with that of Al and Ti and Sn and Ti (
Figure 3b,c). When both Ti and Hf substitute Nb and Sn substitutes Si the hardness decreases slightly, compared with (Nb,Hf)
5(Si,Sn)
3 and there is a further small decrease in hardness when both Al and Sn substitute Si (
Figure 3c). Notice that all the 5-3 silicides in
Figure 3c have lower hardness than that of the binary (unalloyed) Nb
5Si
3.
The effect of the substitution of Nb by Cr and Ti on the hardness of Nb
5Si
3 is shown in
Figure 3d. When only Nb is substituted in Nb
5Si
3 the hardness decreased (compared with the unalloyed Nb
5Si
3) and there is further decrease when Si is substituted by Al and Sn and the effect of Al or Sn is essentially the same (compared with (Nb,Ti,Cr)
5Si
3). The hardness increases as Si is substituted by Al and B and increases significantly when Si is substituted by Ge (compared with (Nb,Ti,Cr)
5(Si,Sn)
3). In
Figure 3d, only the (Nb,Ti,Cr)
5(Si,Ge)
3 has hardness higher than that of the unalloyed Nb
5Si
3 and its hardness is the highest of all the 5-3 silicides shown in
Figure 3.
In this paper, the data for Nb
5Si
3 alloyed with Ge was chosen in order to demonstrate how alloying changes the hardness of Nb
5Si
3 and how the change of hardness can be understood using the silicide parameter VEC. The data in
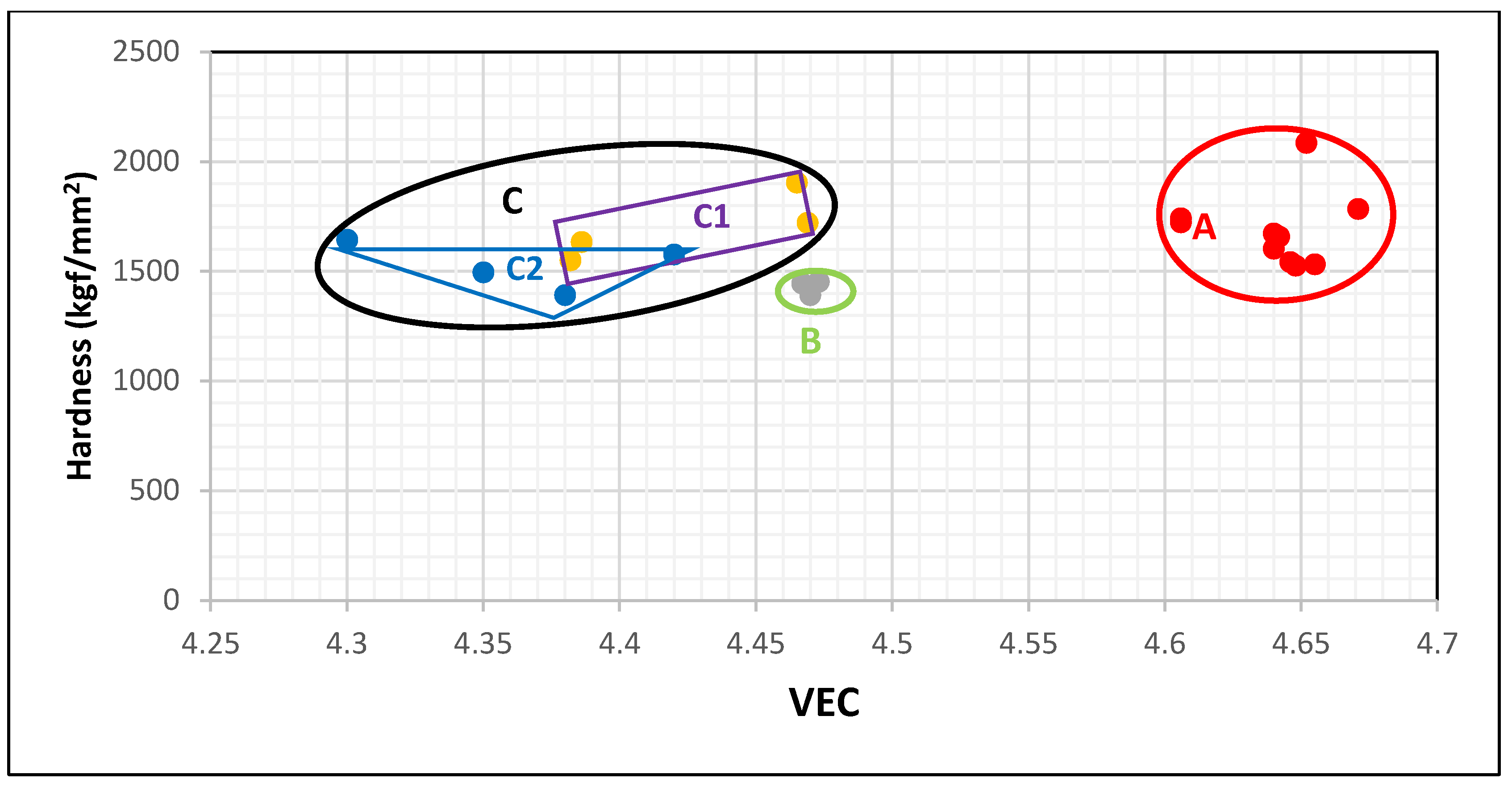
Figure 4 falls in three groups represented by the red, green and black areas that are labelled A, B and C. The data for the silicides Nb
5(SiGe)
3, (Nb,Cr)
5(Si,Ge)
3 and Nb
5(Si,Ge,Al)
3 is in area A. When Nb is substituted only by Ti and Si only by Ge the data shifts towards lower VEC and hardness values to area B, which contains the data for (Nb,Ti)
5(Si,Ge)
3. The individual addition of Al or Cr to the silicide shifts the data upwards (higher hardness but lower VEC) to the rectangular C1 in area C (black ellipse), which has the data for (Nb,Ti,Cr)
5(Si,Ge)
3 and (Nb,Ti)
5(Si,Ge,Al)
3. The substitution of Si by Al shifts VEC and hardness to lower values compared with Cr. The simultaneous presence of Al and Cr in the silicide shifts the data towards lower VEC to the triangle C2 in area C. Thus, the map of silicide hardness versus silicide parameter VEC clearly differentiates the role played by Ti in the hardness of Nb
5Si
3 alloyed with Ge and with no B, Sn, Mo, Ta, or W additions. When no Ti is present in the silicide the hardness exceeds 1500 HV and the silicide parameter VEC is higher than 4.6. The addition of Ti causes VEC to decrease to values below 4.48 and this is accompanied by a shift of the hardness to lower values. There is a gap in VEC values between 4.6 and 4.48 for Nb
5Si
3 alloyed with Ge.
The effects of alloying on properties of Nb
5Si
3 also can be demonstrated using maps of the silicide parameters VEC and Δχ and the available data for the creep of unalloyed and alloyed Nb
5Si
3. Silicide maps are shown in the
Figure 5,
Figure 6,
Figure 7,
Figure 8,
Figure 9,
Figure 10 and
Figure 11. Note that the data in the
Figure 7,
Figure 8,
Figure 9,
Figure 10 and
Figure 11 is for different alloys.
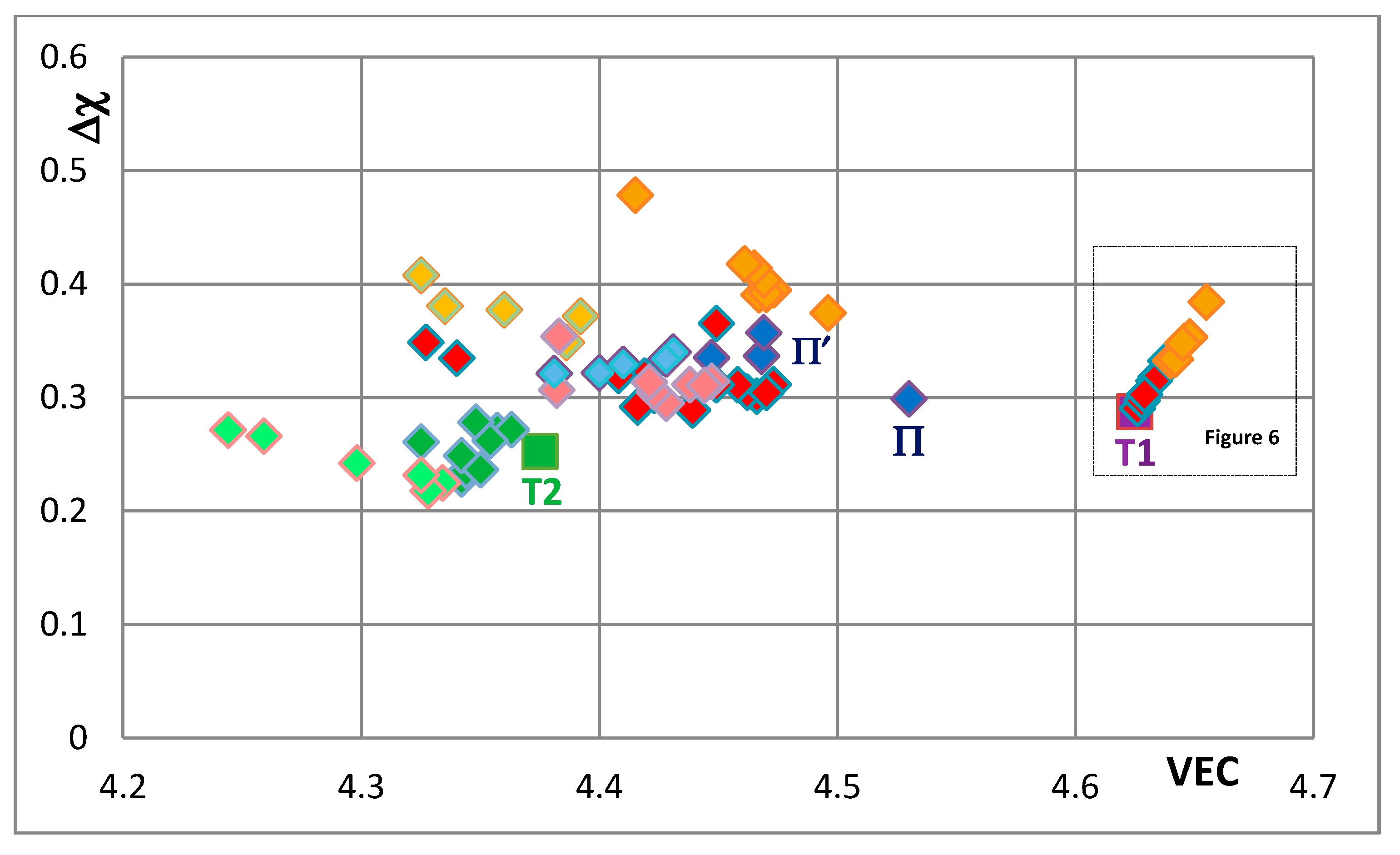
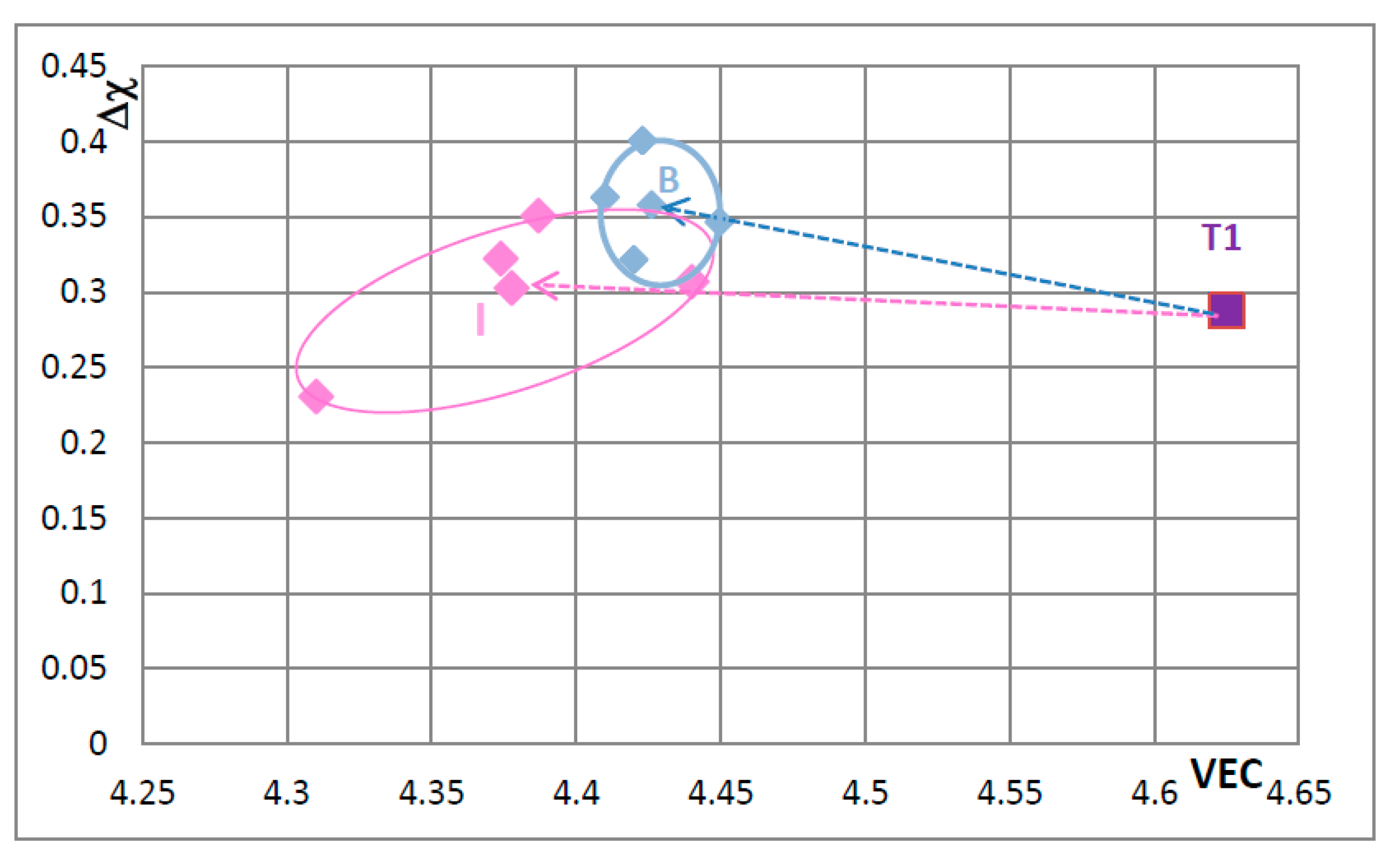
Figure 5 is the map for all the tetragonal Nb
5Si
3 silicides in the studied developmental alloys. When Si is substituted by Ge or Sn, the values of the silicide parameters VEC and Δχ increase but the opposite is the case when B substitutes Si (see
Figure 5 and
Figure 6 and compare the positions of T1 and T2—the composition of the silicide shown by T2 in
Figure 5 is 62.5Nb-12.5Si-25B) or Ti substitutes Nb (see Π in
Figure 5, which corresponds to the silicide 53Nb-10Ti-37Si [
49]) and the concentration of Ti in the silicide is increased (see Π’ in
Figure 5 that corresponds to the silicide 46.8Nb-17.4Ti-35.8Si). When both Nb and Si are substituted the values of the silicide parameter VEC decrease further (all data shifts to the left of Π) and Ge and B have the strongest effect on the silicide parameter Δχ with the former increasing and the latter decreasing Δχ (compared with T1) while the effect of Sn depends on alloying additions. When Si is substituted only by Al the silicide parameter VEC decreases further and there is a slight reduction of the value of the silicide parameter Δχ. When Al is substituting Si, the shift towards lower VEC values is increased only for the silicides where Al is simultaneously present with B or Ge but this is not the case when Al and Sn are simultaneously present in the silicide.
Figure 7,
Figure 8,
Figure 9,
Figure 10 and
Figure 11 show how the position of Nb
5Si
3 changes with alloying additions in the VEC versus Δχ silicide maps. Note differences in the VEC and Δχ axes compared with
Figure 5 and
Figure 6 and differences in the VEC axes between
Figure 7,
Figure 8,
Figure 9,
Figure 10 and
Figure 11. The data used in these maps is for normal and Ti rich Nb
5Si
3 in cast and heat treated alloys. In
Figure 7,
Figure 8,
Figure 9,
Figure 10 and
Figure 11 the unalloyed Nb
5Si
3 is shown as T1. The alloying additions in Nb
5Si
3 are Cr, Hf, Mo, Ta, which substitute Nb and Al, B, Ge and Sn, which substitute Si. Substituting Nb with Ti and Cr and Si with Al shifts the silicide in area B, meaning that the normal Nb
5Si
3 and Ti rich Nb
5Si
3 in the cast and heat treated alloy “moves” in this area as the concentrations of Al, Cr and Ti in the Nb
5Si
3 change. Area B is included in
Figure 7,
Figure 8,
Figure 9,
Figure 10 and
Figure 11 to show how alloying changes the position of the Nb
5Si
3 in the maps.
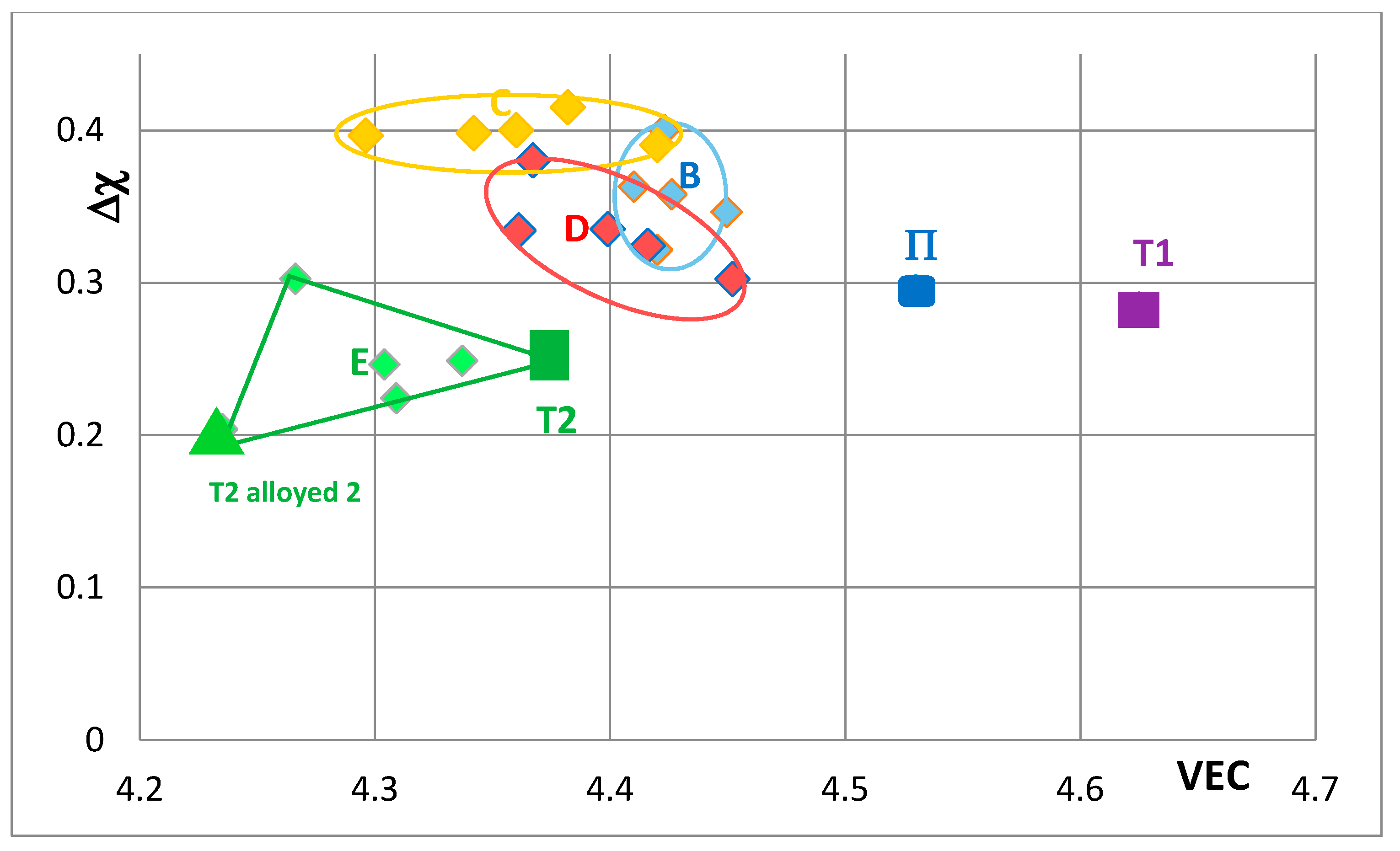
Figure 7 shows changes caused by the substitution of Si with B, Ge and Sn. In this figure, the Nb in the silicide is substituted by Ti and Cr. The Nb
5Si
3 “shifts” from area B to areas C, D and E in
Figure 7, when Ge or Sn or B is present in the alloy. Note that the Nb
5Si
3 alloyed with B occupies the distinctly different area E. In
Figure 8 the effect of substituting Nb with Hf (and Ti and Cr) and Si with B, Ge and Sn in Nb
5Si
3 is shown. The silicide shifts from area B to areas C to F. The substitution of Nb by Cr, Hf and Ti shifts the silicide from area B to area F. When Si in the silicide is substituted by Ge, area C (silicide with Ge and Hf) is entirely within the area F. This however is not the case when Si in the silicide is substituted by Sn in area D, which is for silicides with Hf and Sn. Area D spreads into area E (silicides with B and Hf). Note that with the addition of Hf area E expands towards higher Δχ and lower VEC values. The compositions of the alloyed silicides indicated as T2 alloyed1 and T2 alloyed2 respectively were 38.5Nb-16Ti-6Hf-1Cr-37Si-1Al-0.5B and 41.5Nb-13Ti-3Hf-4Cr-12.5Si-25.5B-0.5Al [
49].
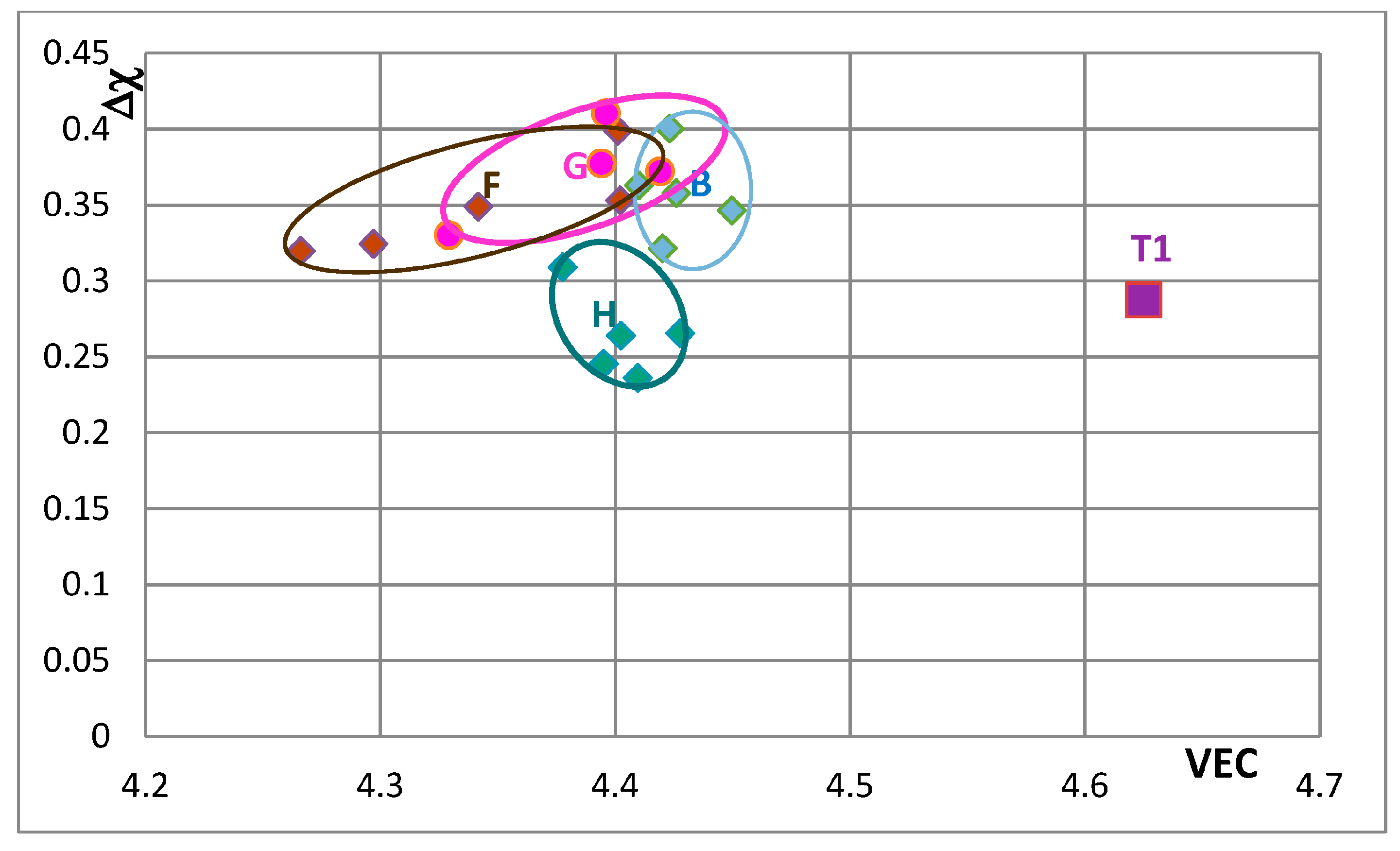
In
Figure 9 the effect of substituting Nb with other transition metals such as Hf and Mo and Ta and Si only with Al is considered in order to show and compare the effects of Mo and Ta in comparison with the effect of Hf. The Nb
5Si
3 alloyed with Ta occupies its own area (H), which is separate from area B. The silicide alloyed with Mo falls almost entirely in area F, which is the same as area F in
Figure 8. In
Figure 10 the substitutions Si with B and Sn and Nb with Hf or Mo or Ta are considered to show the effect of the simultaneous presence of B with each of the other elements. The addition of B causes a significant change in area F (compare
Figure 8,
Figure 9 and
Figure 10), area H expands (compare
Figure 9 and
Figure 10) and area G shifts to lower VEC and Δχ values (compare
Figure 9 and
Figure 10). In
Figure 10 the silicides that contain B occupy the area of the map defined by Δχ and VEC with values less than about 0.35 and 4.362 respectively. The higher value of Δχ should also be noted in
Figure 8. The simultaneous presence of B and Sn has the strongest effect (compare positions of areas B and J in the map).
Figure 11 shows that when Ge and Sn are simultaneously present in the alloy area B shifts to area I, which in the map occupies a position similar to but larger than area D in the
Figure 8, that parts of areas B and I overlap and that Δχ is below 0.35.
In
Figure 7,
Figure 8,
Figure 9,
Figure 10 and
Figure 11 the “average” positions of the Nb
5Si
3 in the areas B to I are indicated by the data point that is closest to the letter of the area. One could use an arrow to link the T1 with the average in each area to show “the direction of change” in the map with specific alloying addition(s). To avoid crowding the maps with extra lines, the “direction of change” is demonstrated only in
Figure 11, where T1 is connected with the average positions in areas B and I. It is noted that the average Δχ value of the Nb
5Si
3 changed very little compared with that of the unalloyed silicide (T1) when Ge and Sn were simultaneously present in the silicide.
The shift of the position of the Nb
5Si
3 in the VEC versus Δχ maps in
Figure 5,
Figure 6,
Figure 7,
Figure 8,
Figure 9,
Figure 10 and
Figure 11—when Nb and Si of the silicide were substituted by alloying additions in Nb-silicide based alloys and the change of the composition of the silicide as the alloy microstructure evolved following exposure to high temperature—should be accompanied with changes of the properties of the 5-3 silicide. These changes affect creep and oxidation of the alloys. This will be discussed in a separate paper.
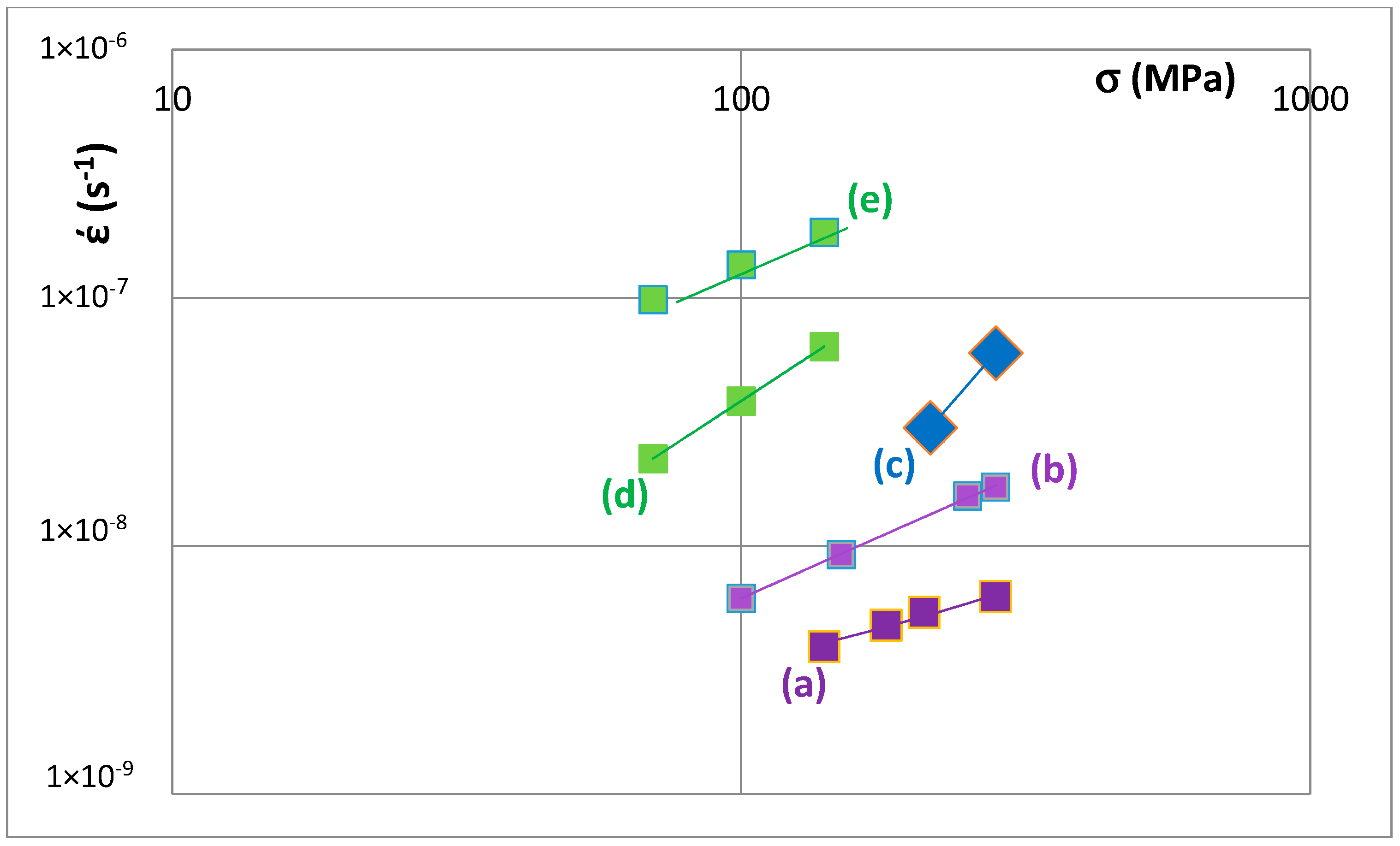
Creep data for unalloyed and alloyed tetragonal Nb
5Si
3 is shown in the Norton
σ
n plots for 1473 K in
Figure 12. The data sets (a) and (b) are for unalloyed αNb
5Si
3 prepared using (a) powder metallurgy processing (PM) and heat treatment (HT) with powders from crashed arc melted material and (b) arc melting + HT. The data shows that the creep rate increases as Nb is substituted by Cr, Hf and Ti and Si by Al and B. The positions of the unalloyed and alloyed silicides in
Figure 12 are indicated in the VEC versus Δχ maps, for example see
Figure 8. Increase in creep rate of Nb
5Si
3 results from alloying with Ti (compare T1 and Π) and with Cr, Hf and Ti and Al and B (compare T1 with T2 alloyed1 and T2 alloyed2) and these increases in creep rate are associated with decrease in the VEC and increase and decrease in the Δχ values (
Figure 8).