Thermal Stress Relaxation and High-Temperature Corrosion of Cr-Mo Steel Processed Using Multifunction Cavitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Material and Processing Conditions
2.2. High-Temperature Corrosion Conditions
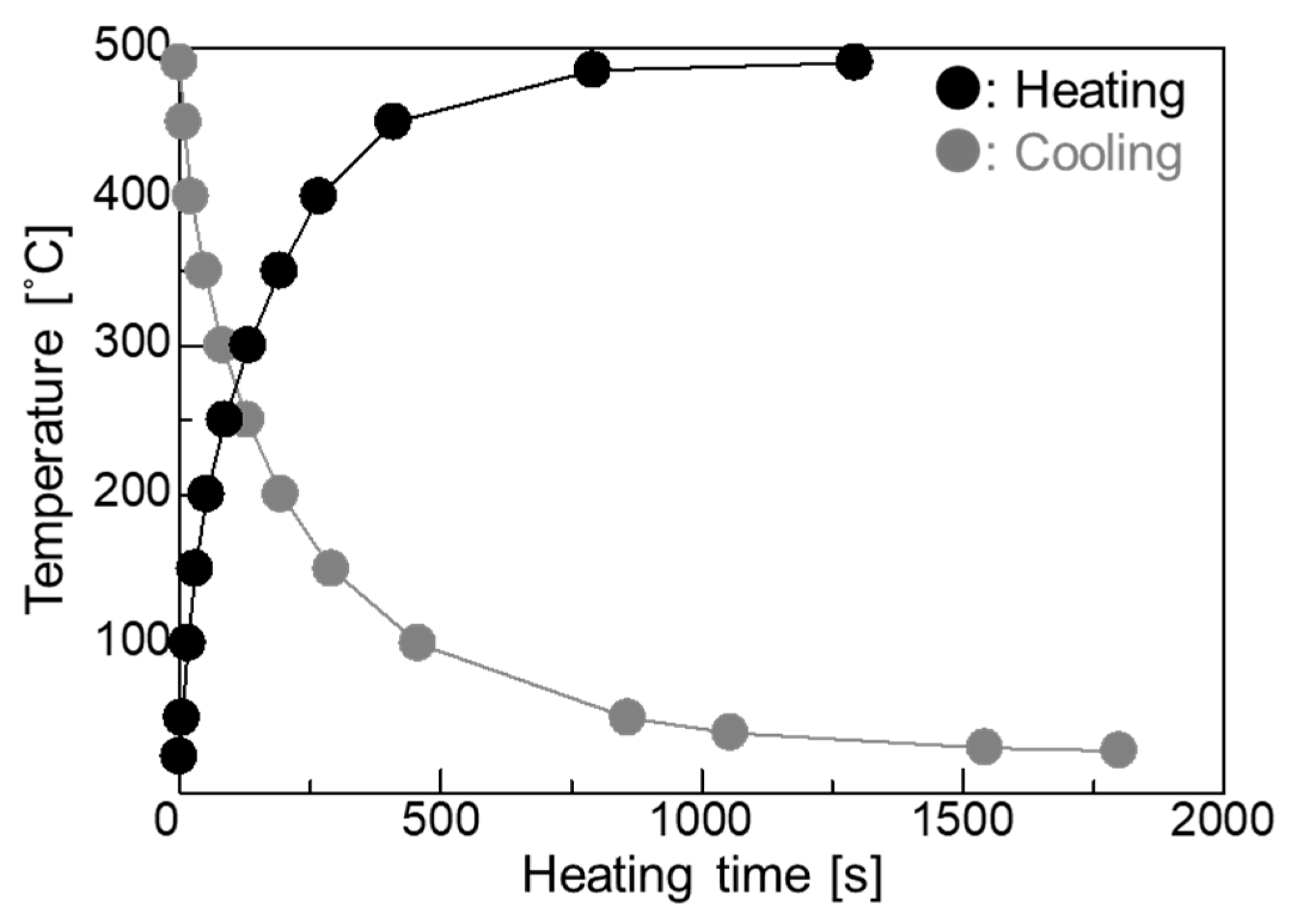
2.3. Heat Cycle Conditions
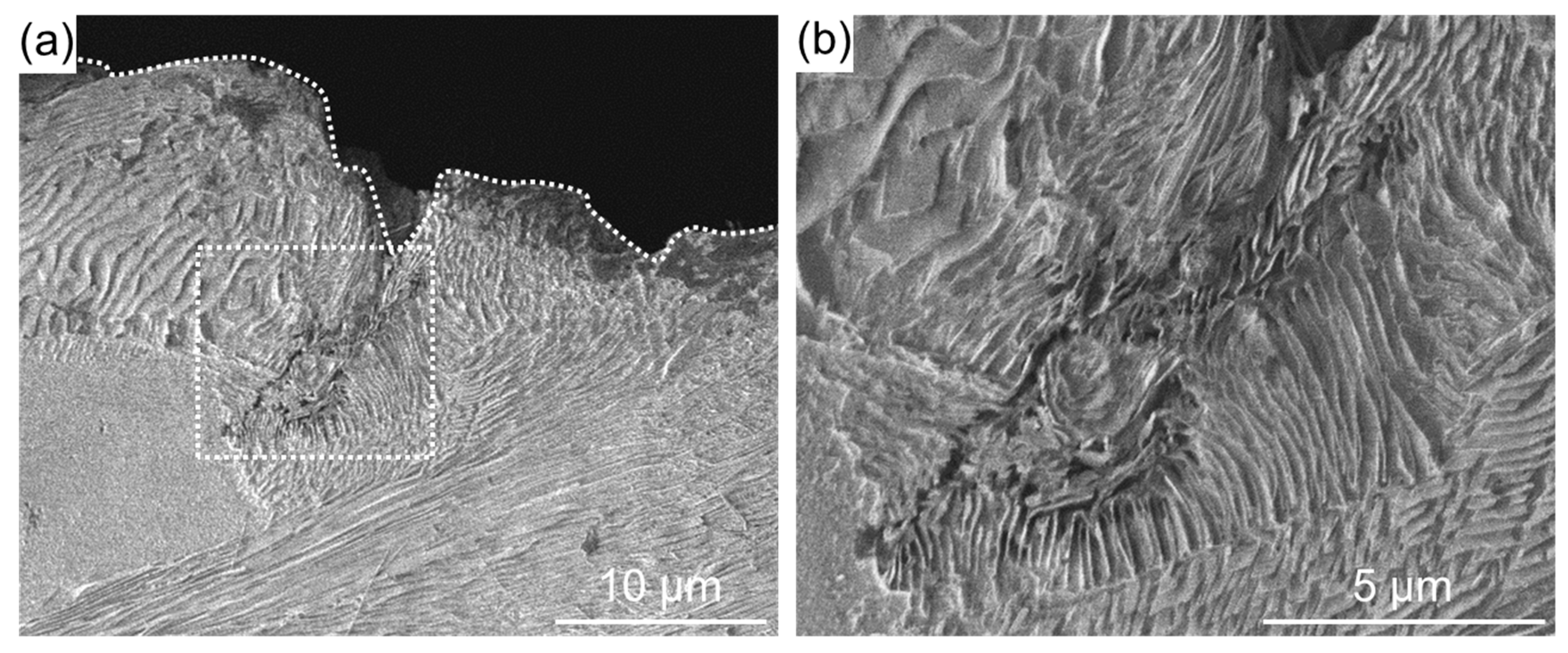
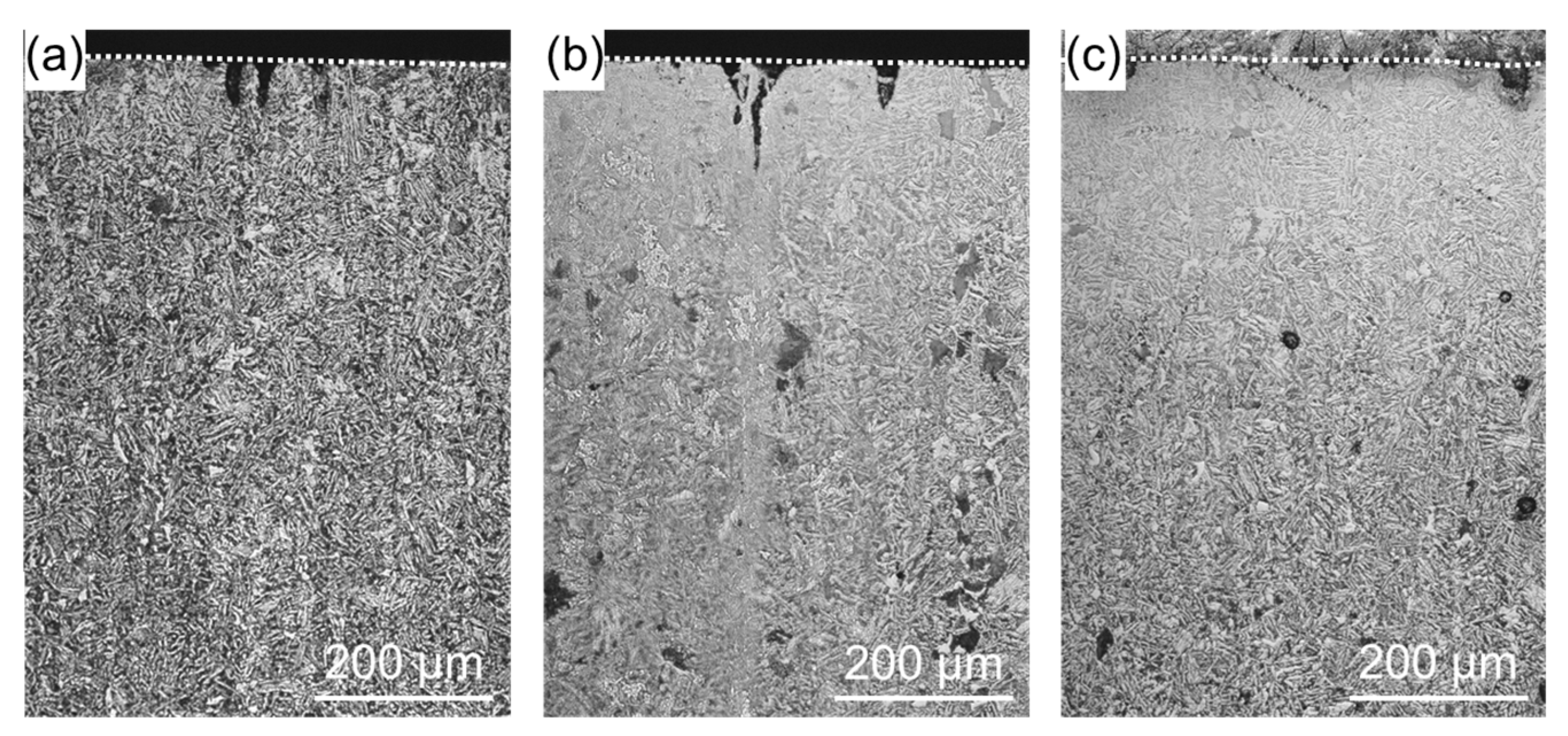
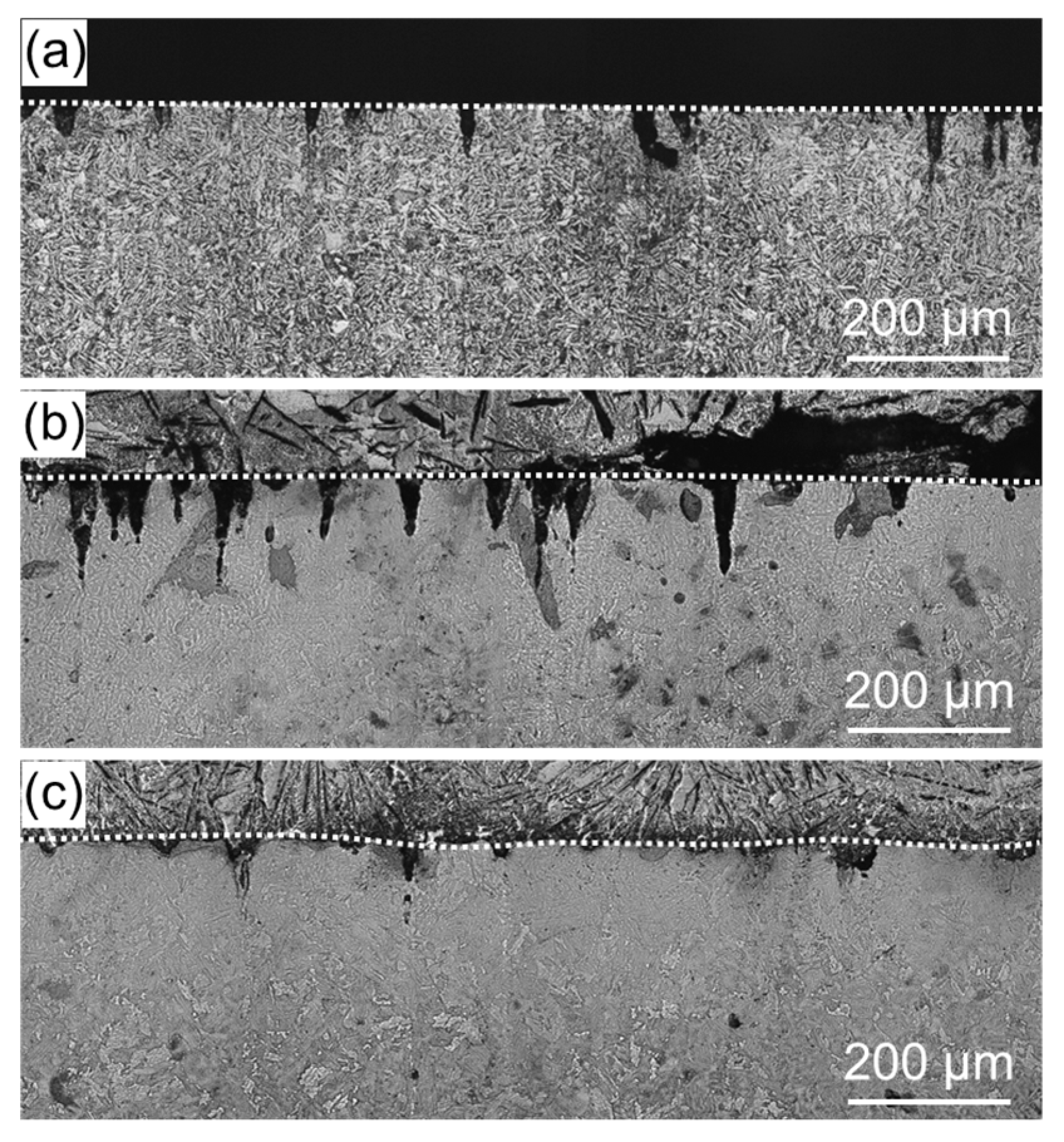
3. Results and Discussion
- The oxide film that forms on the specimen surface reduces the compressive residual stress and decreased the heat transfer from the surface to the interior.
- Since voids and cracks are unlikely to form in the interior, cracks that can be generated by thermal stress caused by high-temperature corrosion are unlikely to occur.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kawahara, Y.; Kira, M. Effect of physical properties of molten deposits on high temperature corrosion of alloys in waste incineration environment. Zairyo-to-Kankyo 1997, 46, 8–15. [Google Scholar] [CrossRef]
- Saitou, N.; Enomoto, K.; Kurosawa, K.; Morinaka, R.; Hayashi, E.; Ishikawa, T.; Yoshimura, T. Development of water jet peening technique for reactor internal components of nuclear power plan. J. Jet Flow Eng. 2003, 20, 4–12. [Google Scholar]
- Hirano, K.; Enomoto, K.; Hayashi, E.; Kurosawa, K. Effects of water jet peening on corrosion resistance and fatigue strength of type 304 stainless steel. J. Soc. Mater. Sci. 1996, 45, 740–745. [Google Scholar] [CrossRef]
- Ijiri, M.; Shimonishi, D.; Nakagawa, D.; Yoshimura, T. Evolution of microstructure from the surface to the interior of Cr-Mo steel by water jet peening. Mater. Sci. Appl. 2017, 8, 708–715. [Google Scholar] [CrossRef]
- Yoshimura, T.; Tanaka, K.; Yoshinaga, N. Development of mechanical-electrochemical cavitation technology. J. Jet Flow Eng. 2016, 32, 10–17. [Google Scholar]
- Yoshimura, T.; Tanaka, K.; Yoshinaga, N. Nano-level material processing by multifunction cavitation. Nanosci. Nanotechnol.-Asia 2018, 8, 41–54. [Google Scholar] [CrossRef]
- Yoshimura, T.; Tanaka, K.; Yoshinaga, N. Material processing by mechanical-electrochemical cavitation. In Proceedings of the BHR Group 2016 Water Jetting, New Orleans, LA, USA, 2–3 November 2016; pp. 223–235. [Google Scholar]
- Ijiri, M.; Shimonishi, D.; Nakagawa, D.; Tanaka, K.; Yoshimura, T. Surface Modification of Ni-Cr-Mo steel by Multifunction cavitation. J. Mater. Sci. Eng. 2017, 7, 290–296. [Google Scholar] [CrossRef]
- Ijiri, M.; Yoshimura, T. Evolution of surface to interior microstructure of SCM435 steel after ultra-high-temperature and ultra-high-pressure cavitation processing. J. Mater. Process. Technol. 2018, 251, 160–167. [Google Scholar] [CrossRef]
- Ijiri, M.; Yoshimura, T. Improvement of corrosion resistance of low-alloy steels by resurfacing using multifunction cavitation in water. IOP Conf. Ser. Mater. Sci. Eng. 2018, 307, 012040. [Google Scholar] [CrossRef] [Green Version]
- Ijiri, M.; Shimonishi, D.; Nakagawa, D.; Yoshimura, T. Effect of water jet peening using ultrasonic waves on pure Al and Al–Cu alloy surfaces. Int. J. Lightweight Mater. Manuf. 2018, in press. [Google Scholar] [CrossRef]
- Ijiri, M.; Yoshimura, T. Effect of ultrasonic irradiation conditions on metal surface during multifunction cavitation. Mater. Sci. Appl. 2018, 9, 698–704. [Google Scholar] [CrossRef]
- Ijiri, M.; Yoshimura, T. Sustainability of compressive residual stress on the processing time of water jet peening using ultrasonic power. Heliyon 2018, 4, e00747. [Google Scholar] [CrossRef] [PubMed]
- Ijiri, M.; Shimonishi, D.; Nakagawa, D.; Yoshimura, T. New water jet cavitation technology to increase number and size of cavitation bubbles and its effect on pure Al surface. Int. J. Lightweight Mater. Manuf. 2018, 1, 12–20. [Google Scholar] [CrossRef]
- Cook, L.P.; McMurdie, H.F. Phase Diagram for Ceramists, Vol. VII; National Institute of Standards and Technology (National Bureau of Standards); Figure 7018; The American Ceramic Soc.: Columbus, OH, USA, 1989; p. 51. [Google Scholar]
- Shimotori, K.; Kawaguchi, K.; Miyauchi, M.; Tomita, T. Experiments on V2O5-Na2SO4 synthetic ash-corrosion test at 1100 °C on various high Cr, Ni-base superalloys. J. Jpn. Inst. Met. 1973, 37, 715–724. [Google Scholar] [CrossRef]






| C | Si | Mn | P | Ni | Cr | Mo | Cu | Fe |
|---|---|---|---|---|---|---|---|---|
| 0.37 | 0.32 | 0.81 | 0.014 | 0.012 | 0.95 | 0.15 | 0.14 | Bal. |
| After Machining (MPa) | After Thermal Cycling (MPa) | |
|---|---|---|
| As received | +147.33 | +43.14 |
| WJP | −366.78 | −79.83 |
| MFC | −409.58 | −272.90 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ijiri, M.; Okada, N.; Kanetou, S.; Yamamoto, M.; Nakagawa, D.; Tanaka, K.; Yoshimura, T. Thermal Stress Relaxation and High-Temperature Corrosion of Cr-Mo Steel Processed Using Multifunction Cavitation. Materials 2018, 11, 2291. https://doi.org/10.3390/ma11112291
Ijiri M, Okada N, Kanetou S, Yamamoto M, Nakagawa D, Tanaka K, Yoshimura T. Thermal Stress Relaxation and High-Temperature Corrosion of Cr-Mo Steel Processed Using Multifunction Cavitation. Materials. 2018; 11(11):2291. https://doi.org/10.3390/ma11112291
Chicago/Turabian StyleIjiri, Masataka, Norihiro Okada, Syouta Kanetou, Masato Yamamoto, Daisuke Nakagawa, Kumiko Tanaka, and Toshihiko Yoshimura. 2018. "Thermal Stress Relaxation and High-Temperature Corrosion of Cr-Mo Steel Processed Using Multifunction Cavitation" Materials 11, no. 11: 2291. https://doi.org/10.3390/ma11112291





