The Mechanical Properties of Biocompatible Apatite Bone Cement Reinforced with Chemically Activated Carbon Fibers
Abstract
:1. Introduction
2. Results and Discussion
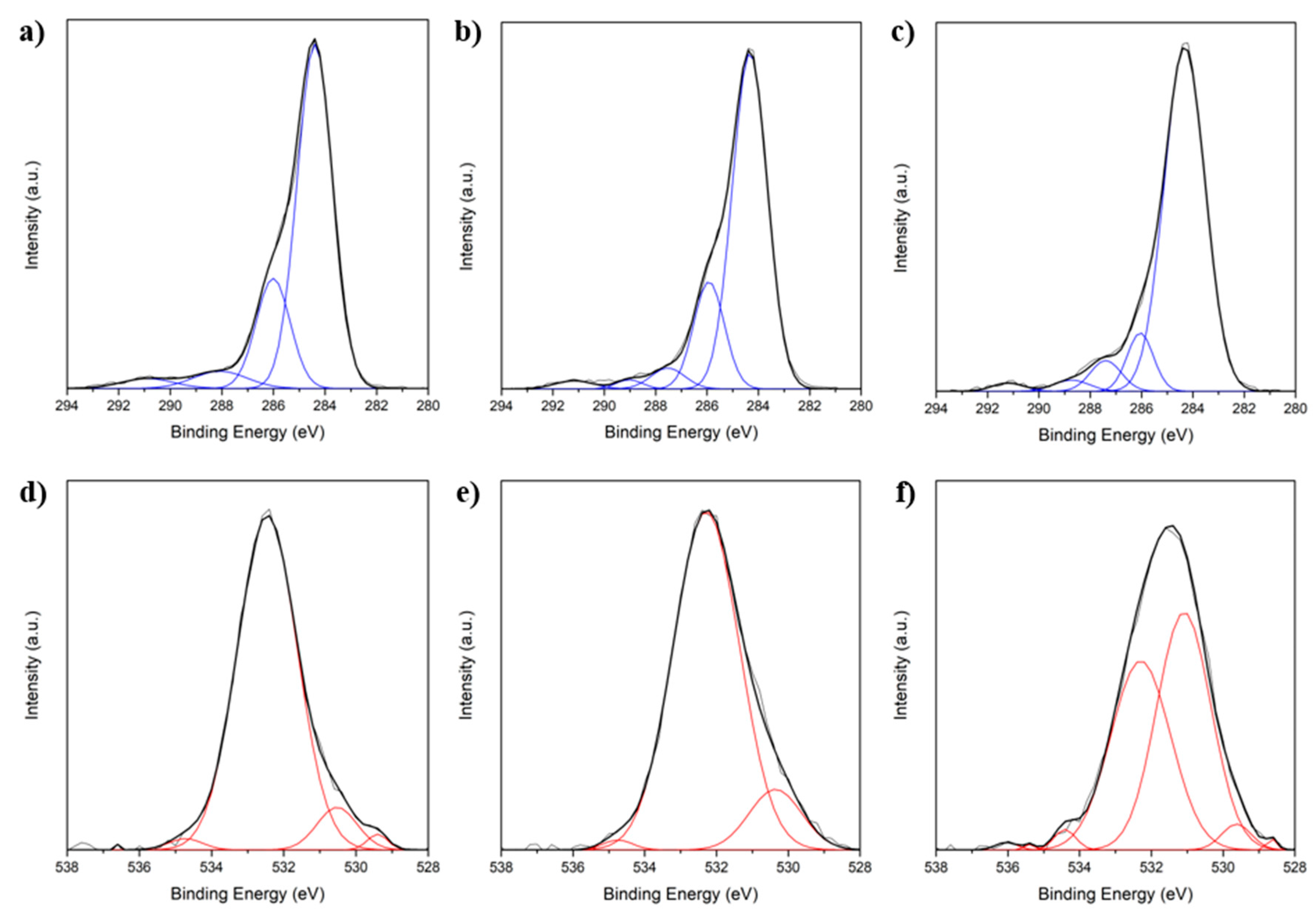
2.1. Fiber Activation
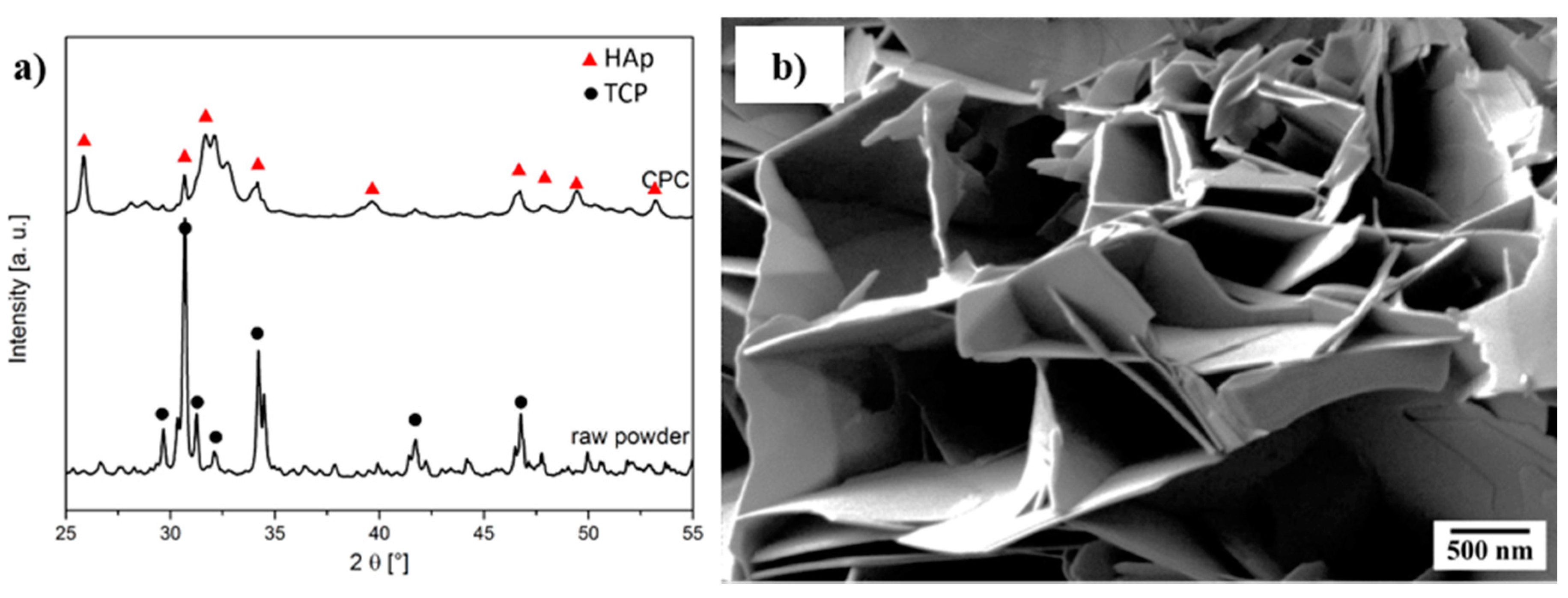
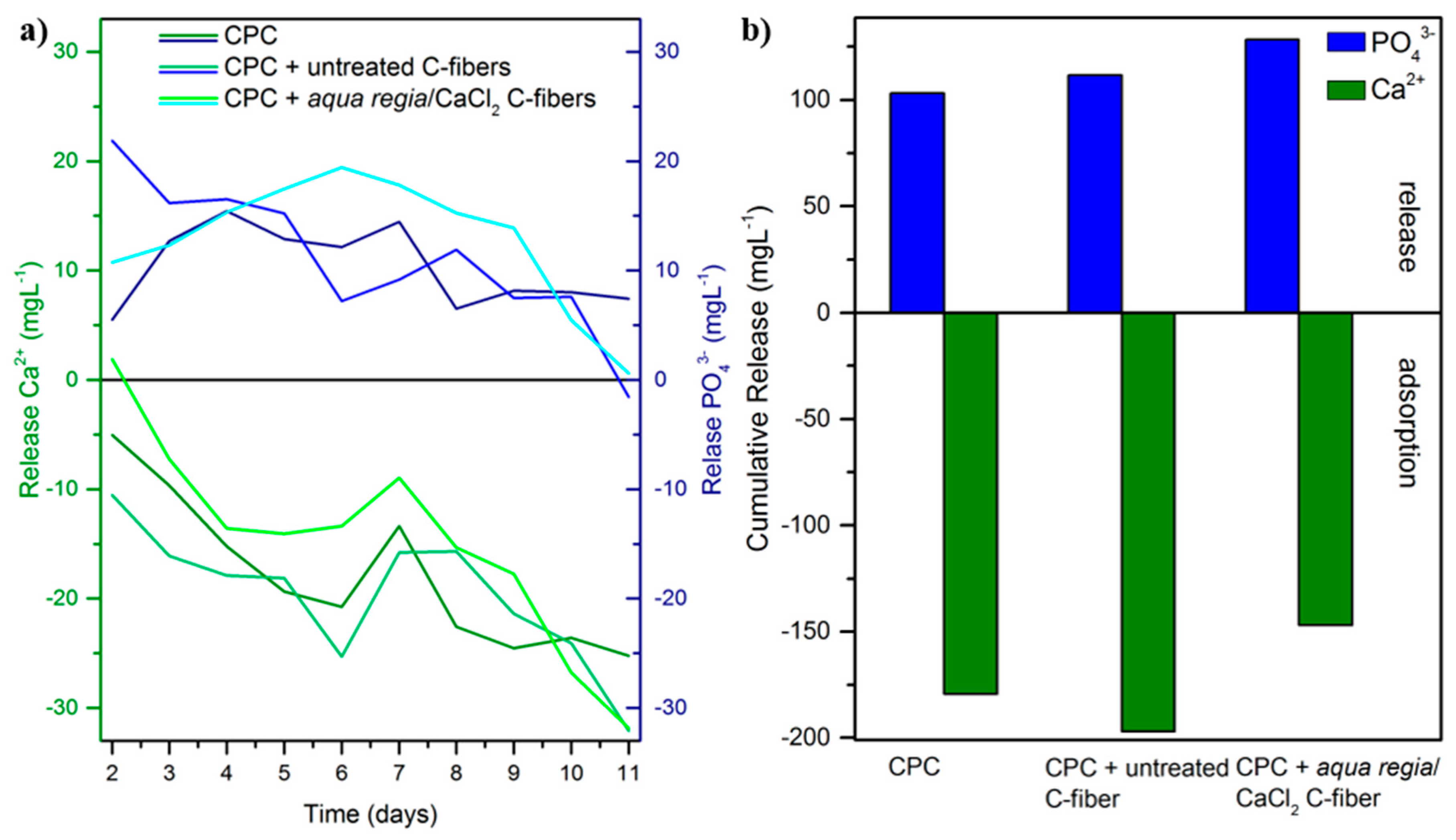
2.2. Cement Setting
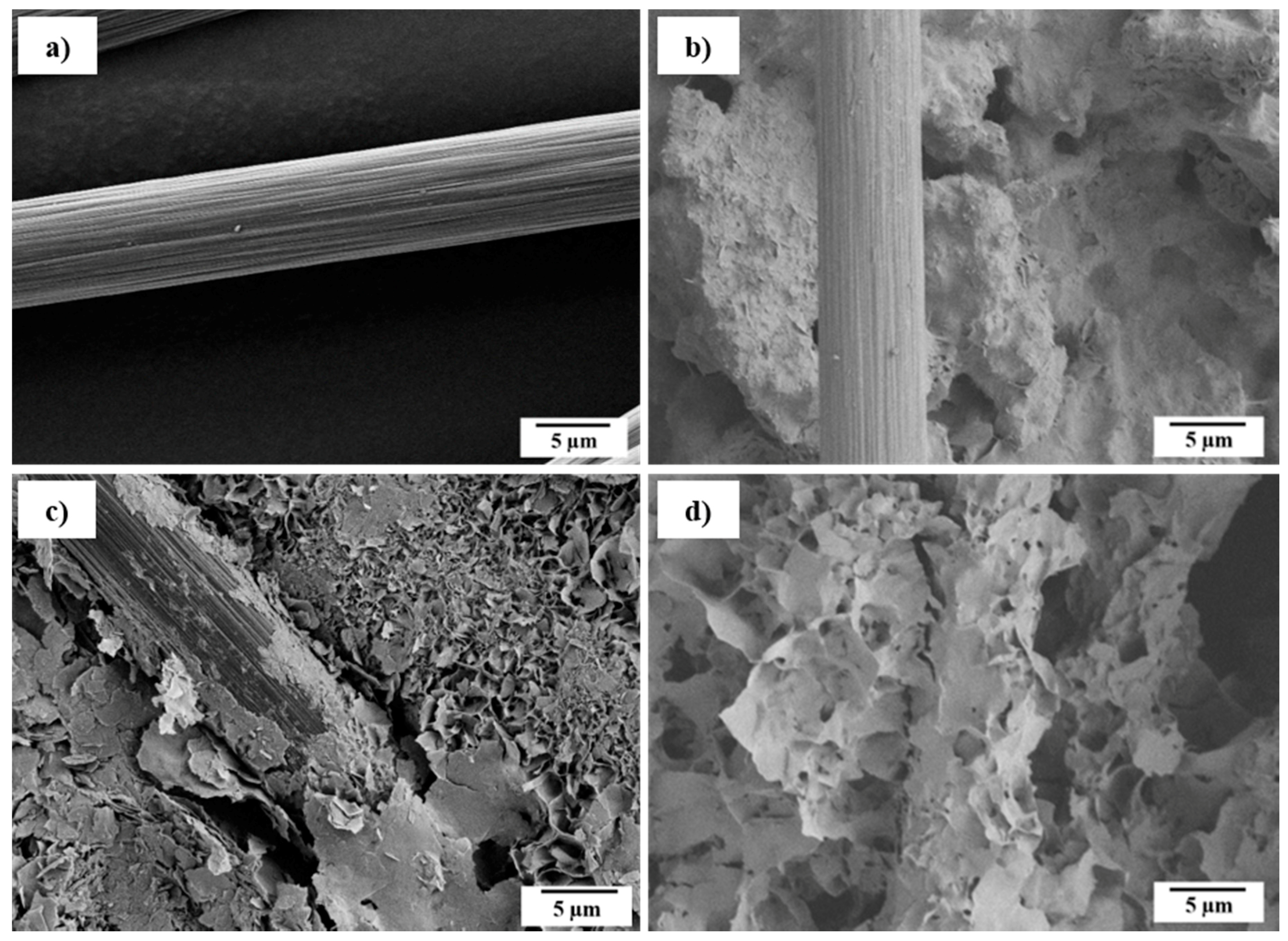
2.3. Mechanical Properties
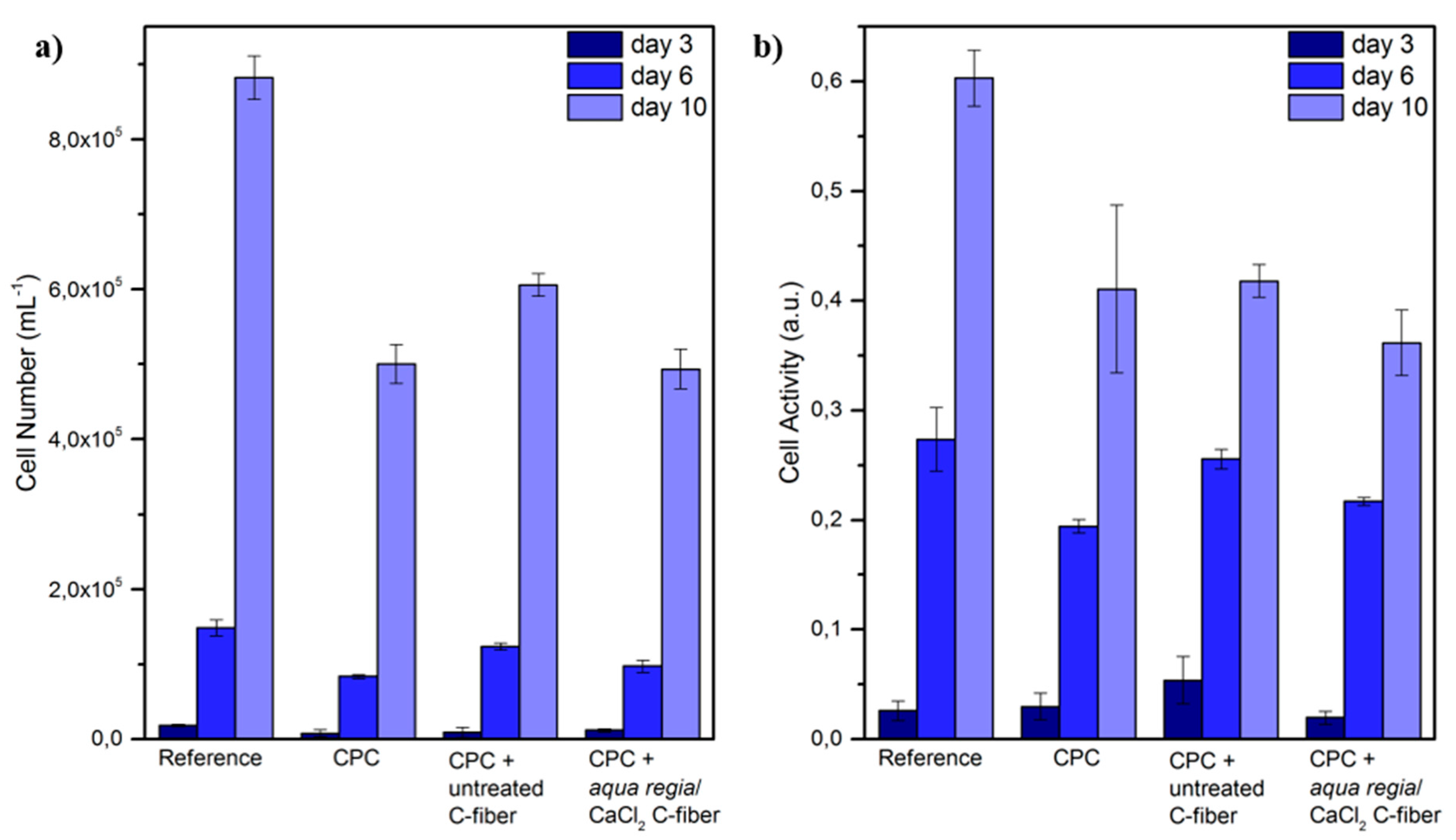
2.4. In Vitro Biocompatibility.
3. Materials and Methods
3.1. Fiber Modification
3.2. Cement Synthesis
3.3. In Vitro Biocompatibility
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dorozhkin, S.V. Calcium orthophosphates: Occurrence, properties, biomineralization, pathological calcification and biomimetic applications. Biomatter 2011, 1, 121–164. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Bioceramics. J. Am. Ceram. Soc. 1998, 81, 1705–1728. [Google Scholar] [CrossRef]
- Osborn, J.F.; Newesely, H. The material science of calcium phosphate ceramics. Biomaterials 1980, 1, 108–111. [Google Scholar] [CrossRef]
- Reznikov, N.; Shahar, R.; Weiner, S. Bone hierarchical structure in three dimensions. Acta Biomater. 2014, 10, 3815–3826. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.E.; Chow, L.C. Dental Resptorative Cement Pastes. United States Patent US-4518430 A, 21 May 1985. [Google Scholar]
- Bohner, M. Reactivity of calcium phosphate cements. J. Mater. Chem. 2007, 17, 3980–3986. [Google Scholar] [CrossRef]
- Bohner, M.; Gbureck, U.; Barralet, J.E. Technological issues for the development of more efficient calcium phosphate bone cements: A critical assessment. Biomaterials 2005, 26, 6423–6429. [Google Scholar] [CrossRef] [PubMed]
- Canal, C.; Ginebra, M.P. Fibre-reinforced calcium phosphate cements: A review. J. Mech. Behav. Biomed. 2011, 4, 1658–1671. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.; Hannink, G. Injectable bone-graft substitutes: Current products, their characteristics and indications, and new developments. Injury 2011, 42 (Suppl. S2), S30–S34. [Google Scholar] [CrossRef] [PubMed]
- Von Gonten, A.S.; Kelly, J.R.; Antonucci, J.M. Load-bearing behavior of a simulated craniofacial structure fabricated from a hydroxyapatite cement and bioresorbable fiber-mesh. J. Mater. Sci. Mater. Med. 2000, 11, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphates in nature, biology and medicine. Materials 2009, 2, 399–498. [Google Scholar] [CrossRef]
- Krüger, R.; Groll, J. Fiber reinforced calcium phosphate cements – on the way to degradable load bearing bone substitutes? Biomaterials 2012, 33, 5887–5900. [Google Scholar] [CrossRef] [PubMed]
- Geffers, M.; Groll, J.; Gbureck, U. Reinforcement strategies for load-bearing calcium phosphate biocements. Materials 2015, 8, 2700. [Google Scholar] [CrossRef]
- Zorn, K.; Vorndran, E.; Gbureck, U.; Müller, F.A. Reinforcement of a magnesium-ammonium-phosphate cement with calcium phosphate whiskers. J. Am. Ceram. Soc. 2015, 98, 4028–4035. [Google Scholar] [CrossRef]
- Nezafati, N.; Moztarzadeh, F.; Hesaraki, S.; Mozafari, M. Synergistically reinforcement of a self-setting calcium phosphate cement with bioactive glass fibers. Ceram. Int. 2011, 37, 927–934. [Google Scholar] [CrossRef]
- Maenz, S.; Hennig, M.; Mühlstädt, M.; Kunisch, E.; Bungartz, M.; Brinkmann, O.; Bossert, J.; Kinne, R.W.; Jandt, K.D. Effects of oxygen plasma treatment on interfacial shear strength and post-peak residual strength of a plga fiber-reinforced brushite cement. J. Mech. Behav. Biomed. 2016, 57, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, L.A.; De Oliveira, L.C.; Da Silva Rigo, E.C.; Carrodéguas, R.G.; Boschi, A.O.; Fonseca de Arruda, A.C. Fiber reinforced calcium phosphate cement. Artif. Organs 2000, 24, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.K.; Eichmiller, F.C.; Barndt, P.R. Effects of fiber length and volume fraction on the reinforcement of calcium phosphate cement. J. Mater. Sci. Mater. Med. 2001, 12, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.K.; Eichmiller, F.C.; Giuseppetti, A.A. Reinforcement of a self-setting calcium phosphate cement with different fibers. J. Biomed. Mater. Res. 2000, 52, 107–114. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology. Nist X-ray Photoelectron Spectroscopy Database, version 4.1. Available online: http://srdata.nist.gov/xps/ (accessed on 24 January 2018).
- Zielke, U.; Hüttinger, K.J.; Hoffman, W.P. Surface-oxidized carbon fibers: I. Surface structure and chemistry. Carbon 1996, 34, 983–998. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Órfão, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Zhuang, Q.L.; Kyotani, T.; Tomita, A. DRIFT and TK/TPD analyses of surface oxygen complexes formed during carbon gasification. Energy Fuels 1994, 8, 714–718. [Google Scholar] [CrossRef]
- Zhou, J.-H.; Sui, Z.-J.; Zhu, J.; Li, P.; Chen, D.; Dai, Y.-C.; Yuan, W.-K. Characterization of surface oxygen complexes on carbon nanofibers by TPD, XPS AND FT-IR. Carbon 2007, 45, 785–796. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L. Ceramics for medical applications: A picture for the next 20 years. J. Eur. Ceram. Soc. 2009, 29, 1245–1255. [Google Scholar] [CrossRef]
- Bohner, M.; Merkle, H.P.; Landuyt, P.V.; Trophardy, G.; Lemaitre, J. Effect of several additives and their admixtures on the physico-chemical properties of a calcium phosphate cement. J. Mater. Sci. Mater. Med. 2000, 11, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Lemaitre, J.; Ring, T.A. Effects of sulfate, pyrophosphate, and citrate ions on the physicochemical properties of cements made of β-tricalcium phosphate-phosphoric acid-water mixtures. J. Am. Ceram. Soc. 1996, 79, 1427–1434. [Google Scholar] [CrossRef]
- Fernandez, E.; Gil, F.J.; Ginebra, M.P.; Driessens, F.C.M.; Planell, J.A.; Best, S.M. Calcium phosphate bone cements for clinical applications. Part II: Precipitate formation during setting reactions. J. Mater. Sci. Mater. Med. 1999, 10, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Marshall, D.B.; Cox, B.N.; Evans, A.G. The mechanics of matrix cracking in brittle-matrix fiber composites. Acta Metall. 1985, 33, 2013–2021. [Google Scholar] [CrossRef]
- Evans, A.G.; Faber, K.T. Crack-growth resistance of microcracking brittle materials. J. Am. Ceram. Soc. 1984, 67, 255–260. [Google Scholar] [CrossRef]
- Faber, K.T.; Evans, A.G. Crack deflection processes—I. Theory. Acta Metall. 1983, 31, 565–576. [Google Scholar] [CrossRef]
- Faber, K.T.; Evans, A.G. Crack deflection processes—II. Experiment. Acta Metall. 1983, 31, 577–584. [Google Scholar] [CrossRef]
- Li Victor, C.; Leung Christopher, K.Y. Steady-state and multiple cracking of short random fiber composites. J. Eng. Mech. 1992, 118, 2246–2264. [Google Scholar]
- Schamel, M.; Barralet, J.E.; Groll, J.; Gbureck, U. In vitro ion adsorption and cytocompatibility of dicalcium phosphate ceramics. Biomater. Res. 2017, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, J.; Ginebra, M.P.; Planell, J.; Engel, E. Osteoblast-like cellular response to dynamic changes in the ionic extracellular environment produced by calcium-deficient hydroxyapatite. J. Mater. Sci. Mater. Med. 2012, 23, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Meleti, Z.; Shapiro, I.M.; Adams, C.S. Inorganic phosphate induces apoptosis of osteoblast-like cells in culture. Bone 2000, 27, 359–366. [Google Scholar] [CrossRef]
- Liu, Y.K.; Lu, Q.Z.; Pei, R.; Ji, H.J.; Zhou, G.S.; Zhao, X.L.; Tang, R.K.; Zhang, M. The effect of extracellular calcium and inorganic phosphate on the growth and osteogenic differentiation of mesenchymal stem cells in vitro: Implication for bone tissue engineering. Biomed. Mater. 2009, 4, 025004. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, S.; Dernell, W.S.; Bandyopadhyay, A.; Bose, S. SrO− and MgO−doped microwave sintered 3D printed tricalcium phosphate scaffolds: Mechanical properties and in vivo osteogenesis in a rabbit model. J. Biomed. Mater. Res. Part B 2015, 103, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Thormann, U.; Ray, S.; Sommer, U.; ElKhassawna, T.; Rehling, T.; Hundgeburth, M.; Henß, A.; Rohnke, M.; Janek, J.; Lips, K.S.; et al. Bone formation induced by strontium modified calcium phosphate cement in critical-size metaphyseal fracture defects in ovariectomized rats. Biomaterials 2013, 34, 8589–8598. [Google Scholar] [CrossRef] [PubMed]
- Ni, G.X.; Chiu, K.Y.; Lu, W.W.; Wang, Y.; Zhang, Y.G.; Hao, L.B.; Li, Z.Y.; Lam, W.M.; Lu, S.B.; Luk, K.D.K. Strontium-containing hydroxyapatite bioactive bone cement in revision hip arthroplasty. Biomaterials 2006, 27, 4348–4355. [Google Scholar] [CrossRef] [PubMed]
- Meininger, S.; Mandal, S.; Kumar, A.; Groll, J.; Basu, B.; Gbureck, U. Strength reliability and in vitro degradation of three-dimensional powder printed strontium-substituted magnesium phosphate scaffolds. Acta Biomater. 2016, 31, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Kanter, B.; Geffers, M.; Ignatius, A.; Gbureck, U. Control of in vivo mineral bone cement degradation. Acta Biomater. 2014, 10, 3279–3287. [Google Scholar] [CrossRef] [PubMed]
- Gbureck, U.; Barralet, J.E.; Spatz, K.; Grover, L.M.; Thull, R. Ionic modification of calcium phosphate cement viscosity. Part i: Hypodermic injection and strength improvement of apatite cement. Biomaterials 2004, 25, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Gbureck, U.; Barralet, J.E.; Radu, L.; Klinger, H.G.; Thull, R. Amorphous α-tricalcium phosphate: Preparation and aqueous setting reaction. J. Am. Ceram. Soc. 2004, 87, 1126–1132. [Google Scholar] [CrossRef]
- Currey, J.D. Physical characteristics affecting the tensile failure properties of compact bone. J. Biomech. 1990, 23, 837–844. [Google Scholar] [CrossRef]


| Chemical Treatment | Contact Angle (°) | Initial Setting Time (min) | Final Setting Time (min) |
|---|---|---|---|
| Pure CPC | - | 25.0 ± 0.5 | 105 ± 5 |
| untreated | 71 ± 4 | 20.0 ± 0.5 | 65 ± 2 |
| H2O2 | 64 ± 6 | 21.0 ± 0.5 | 80 ± 2 |
| aqua regia followed by CaCl2 | 62 ± 4 | 18.5 ± 0.5 | 50 ± 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boehm, A.V.; Meininger, S.; Tesch, A.; Gbureck, U.; Müller, F.A. The Mechanical Properties of Biocompatible Apatite Bone Cement Reinforced with Chemically Activated Carbon Fibers. Materials 2018, 11, 192. https://doi.org/10.3390/ma11020192
Boehm AV, Meininger S, Tesch A, Gbureck U, Müller FA. The Mechanical Properties of Biocompatible Apatite Bone Cement Reinforced with Chemically Activated Carbon Fibers. Materials. 2018; 11(2):192. https://doi.org/10.3390/ma11020192
Chicago/Turabian StyleBoehm, Anne V., Susanne Meininger, Annemarie Tesch, Uwe Gbureck, and Frank A. Müller. 2018. "The Mechanical Properties of Biocompatible Apatite Bone Cement Reinforced with Chemically Activated Carbon Fibers" Materials 11, no. 2: 192. https://doi.org/10.3390/ma11020192





