Effect of Fluoride on the Morphology and Electrochemical Property of Co3O4 Nanostructures for Hydrazine Detection
Abstract
:1. Introduction
2. Results and Discussion
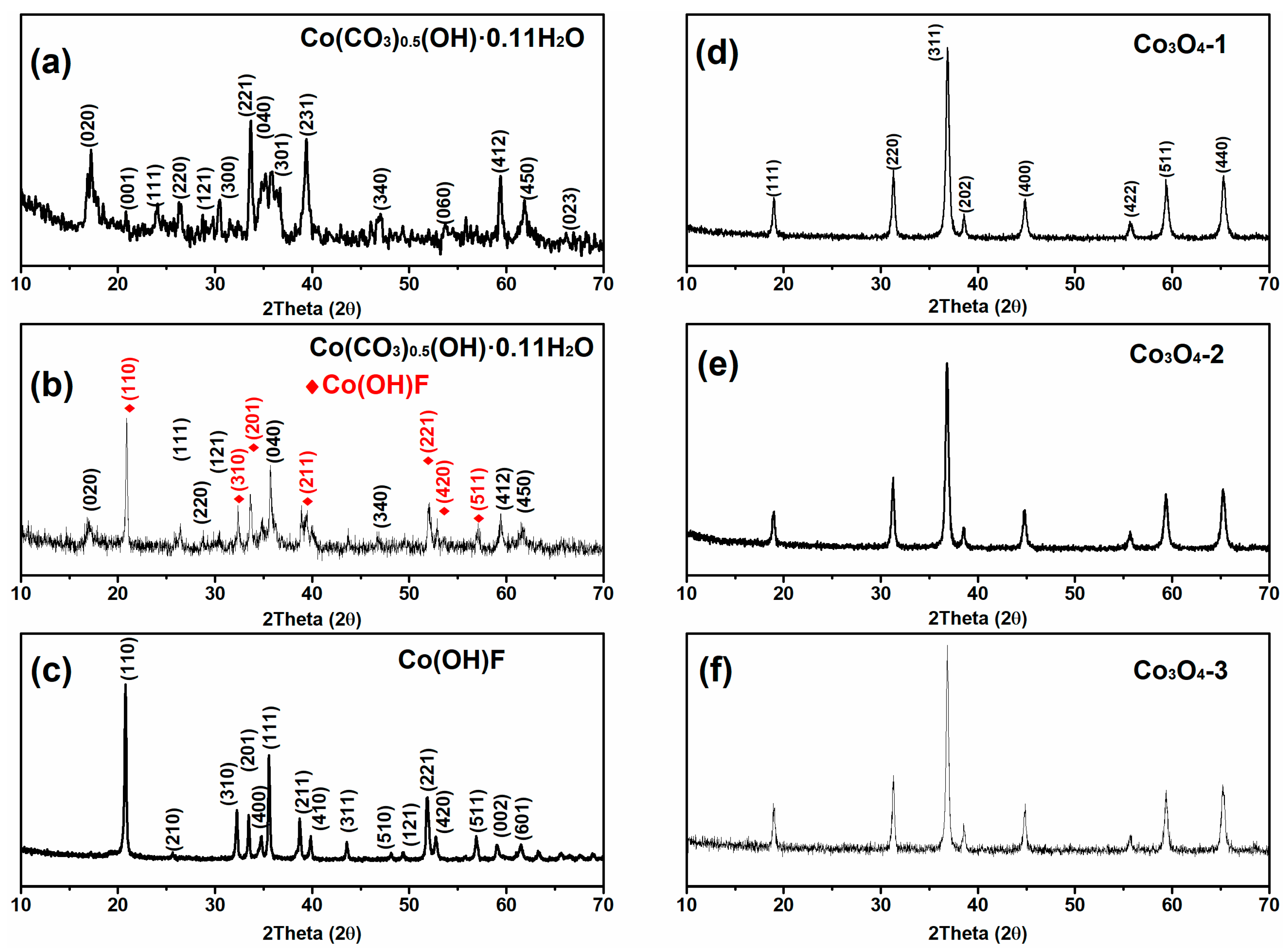
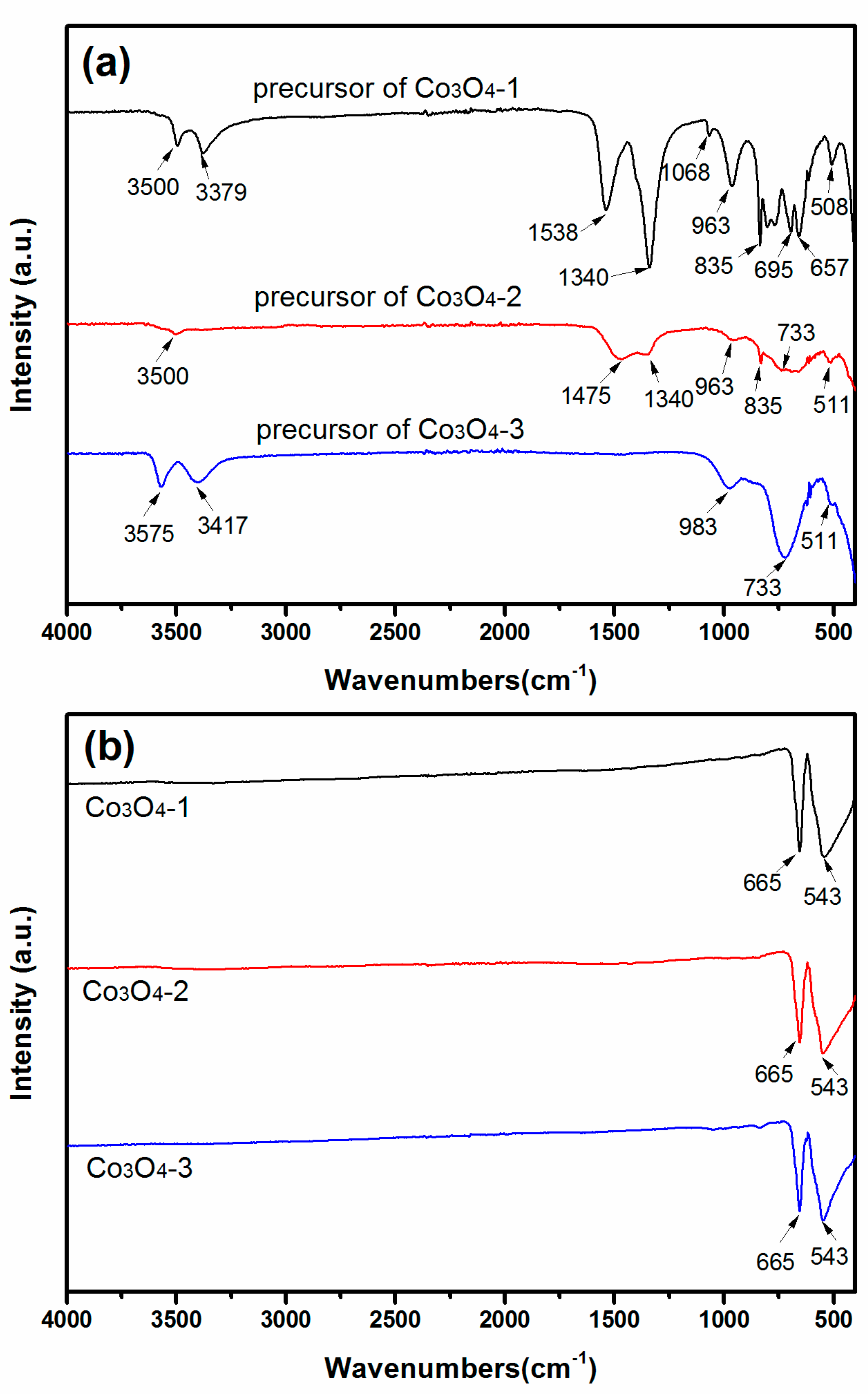
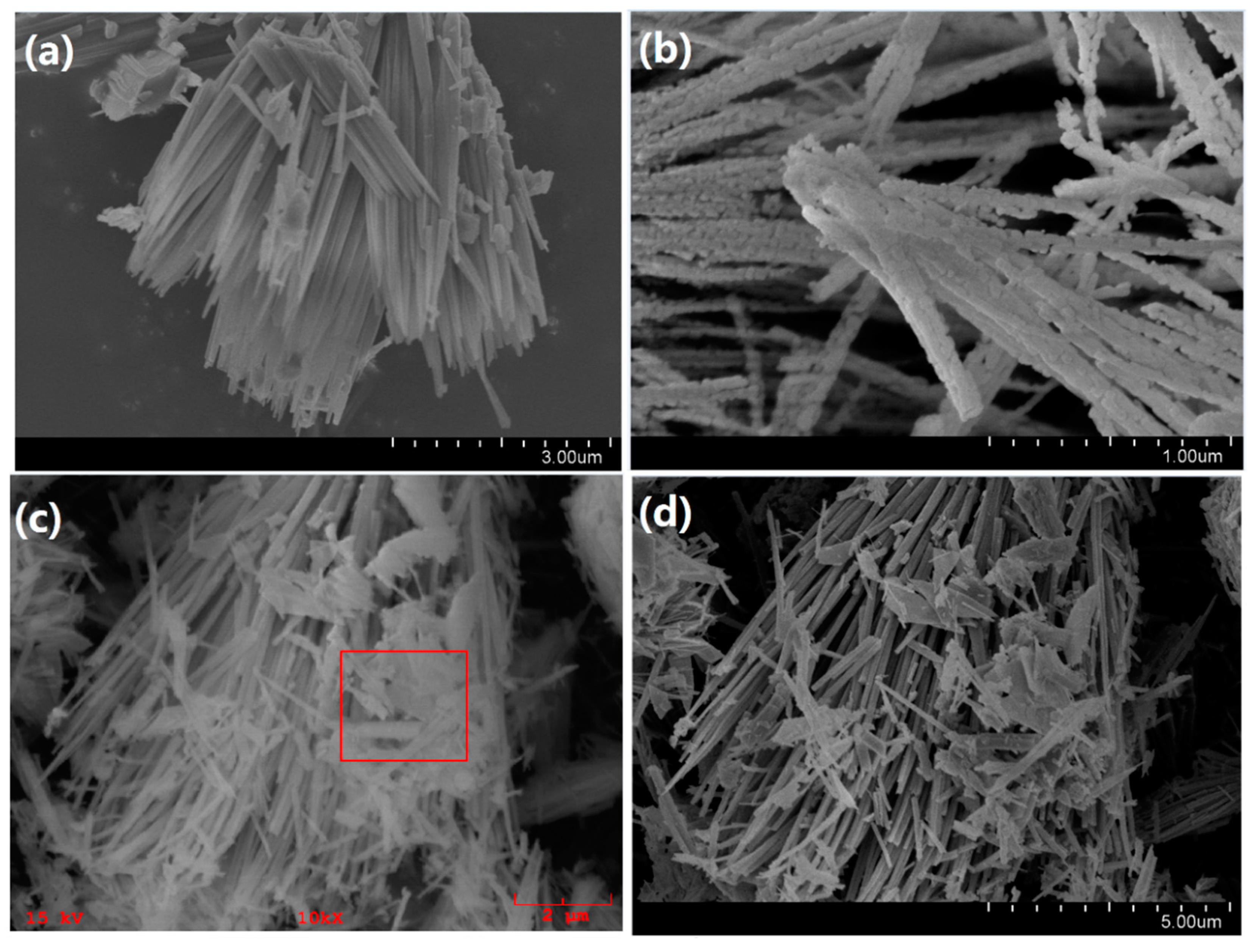
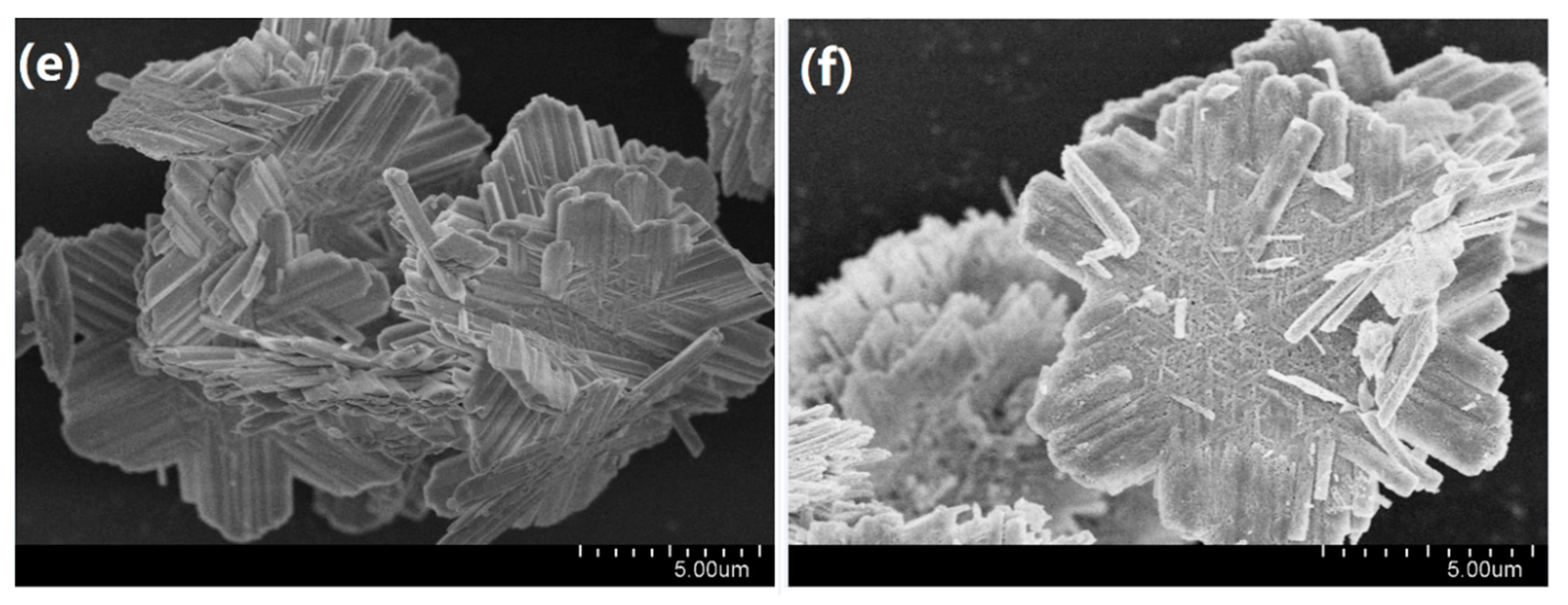
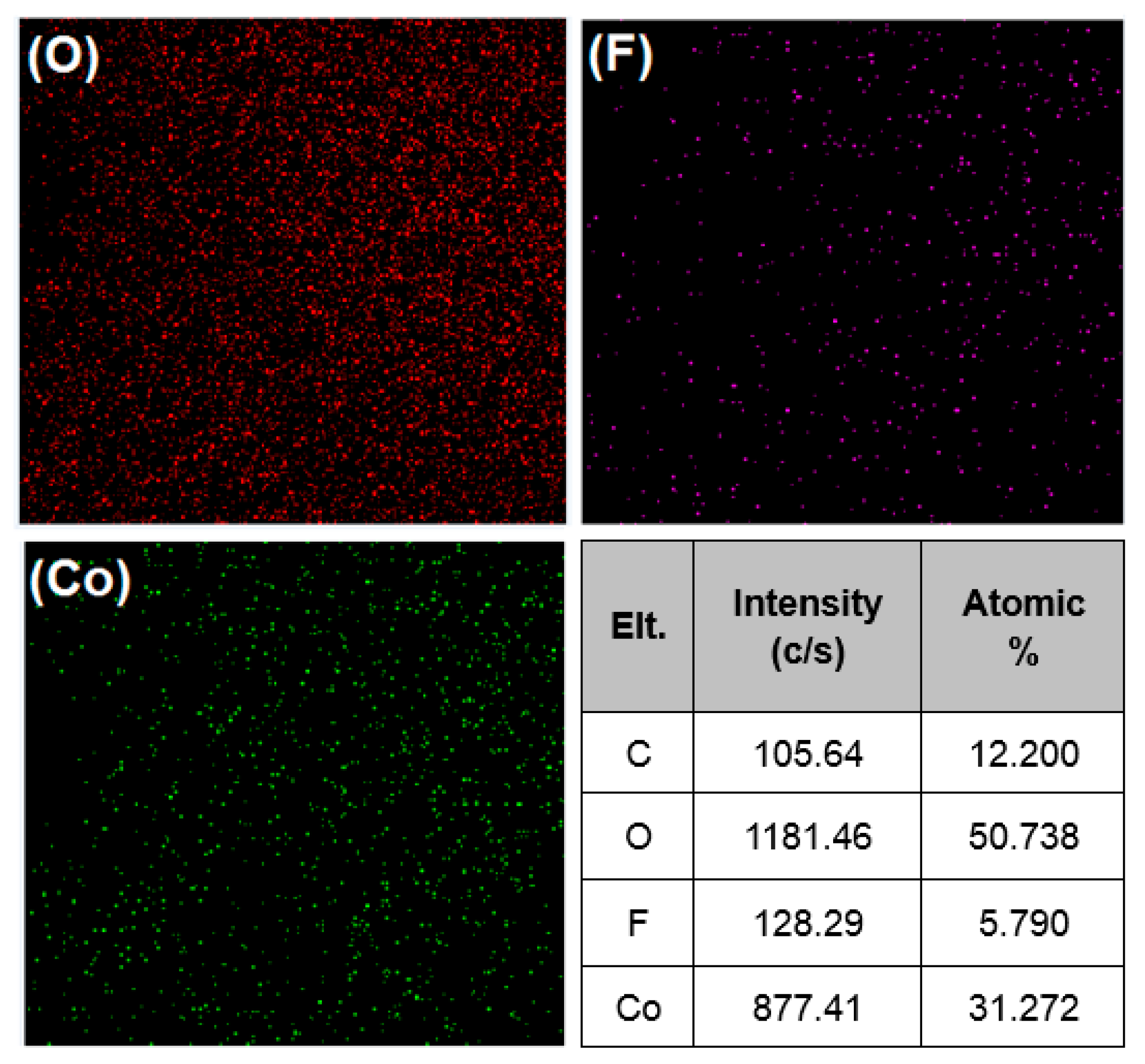
2.1. Structural and Morphological Studies
2.2. Hydrazine Chemical Sensor Studies of Co3O4 Modified Electrodes
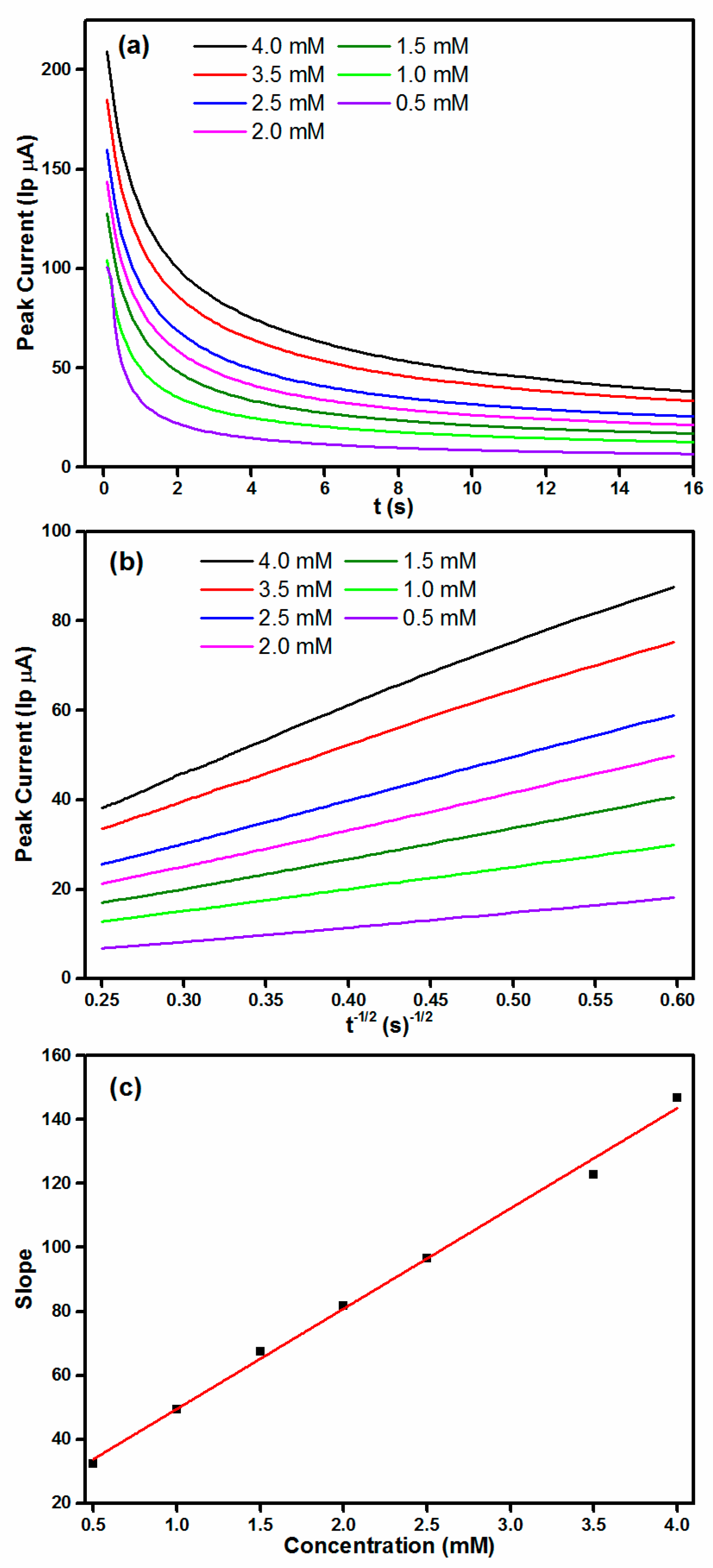
2.3. Amperometric Detection of Hydrazine Using Co3O4 Modified Electrodes
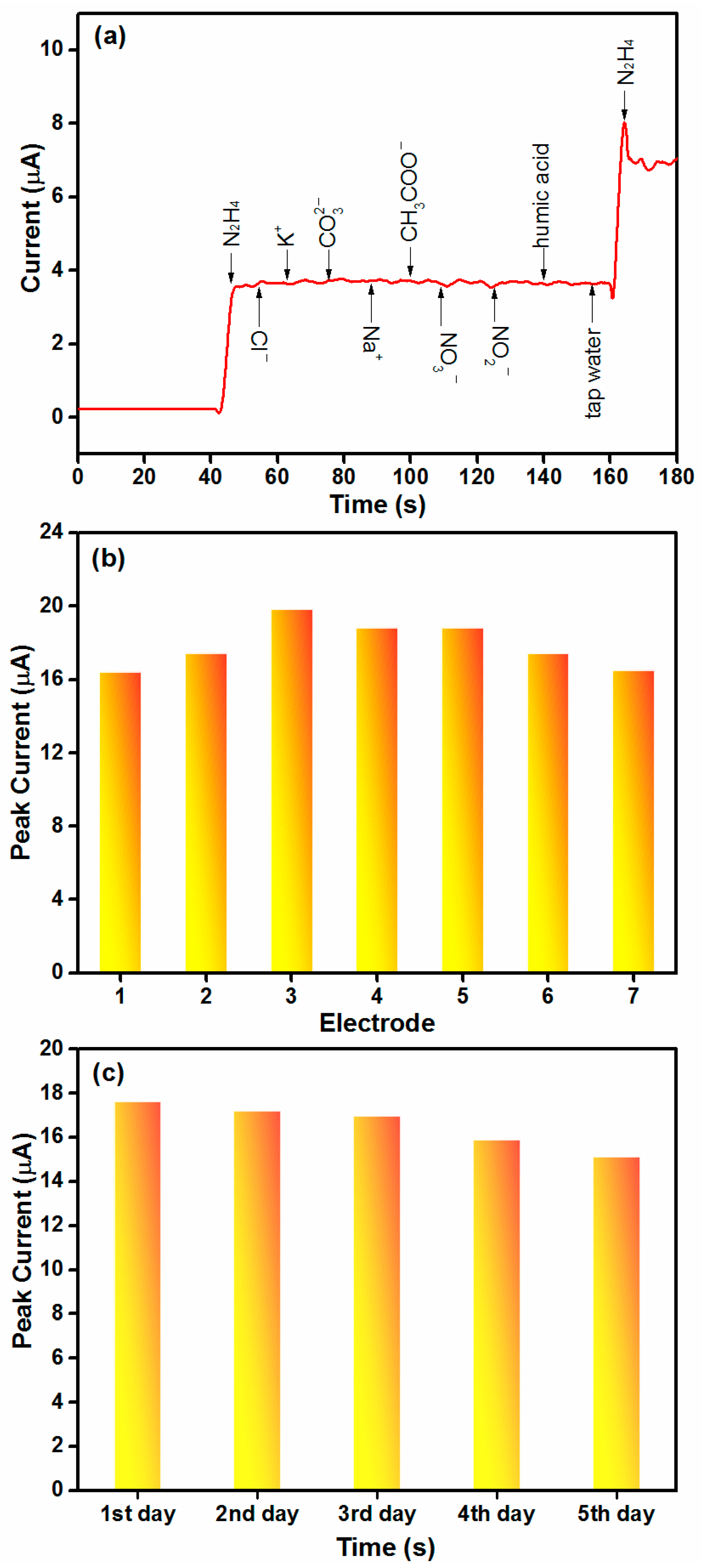
2.4. Selectivity, Reproducibility and Stability Tests
2.5. Real Sample Test
3. Materials and Methods
3.1. Synthesis of Co3O4 Nanostructures
3.2. Electrode Modification
3.3. Characterization of Samples
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ma, X.; Zhang, J.; Jiang, Q.; Liu, X.; Xu, W.; Zheng, M.; Hou, B. Highly Dispersed Ag/Graphene Composites with Enhanced Electro-Oxidation of Hydrazine. Sci. Adv. Mater. 2016, 8, 1305–1308. [Google Scholar] [CrossRef]
- Patel, N.B.; Khan, I.H.; Rajani, S.D. Pharmacological evaluation and characterizations of newly synthesized 1, 2, 4-triazoles. Eur. J. Med. Chem. 2010, 45, 4293–4299. [Google Scholar] [CrossRef] [PubMed]
- Ragnarsson, U. Synthetic methodology for alkyl substituted hydrazines. Chem. Soc. Rev. 2001, 30, 205–213. [Google Scholar] [CrossRef]
- Al-Hadeethi, Y.; Al-Heniti, S.; Umar, A. Highly Sensitive Hydroquinone Chemical Sensor Based on Cd0.5Mg0.4Ca0.1Fe2O4 Nanoparticles. Sci. Adv. Mater. 2017, 9, 2196–2201. [Google Scholar]
- Tafazoli, S.; Mashregi, M.; O’Brien, P.J. Role of hydrazine in isoniazid-induced hepatotoxicity in a hepatocyte inflammation model. Toxicol. Appl. Pharmacol. 2008, 229, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Khatoon, Z.; Parveen, N.; Fouad, H.; Kulkarni, A.; Umar, A.; Ansari, Z.A.; Ansari, S.G. Polyaniline-Functionalized TiO2 Nanoparticles as a Suitable Matrix for Hydroquinone Sensor. Sci. Adv. Mater. 2017, 9, 2032–2038. [Google Scholar]
- Zhou, Q.; Hong, C.; Yao, Y.; Ibrahim, A.M.; Xu, L.; Kumar, R.; Talballa, S.M.; Kim, S.H.; Umar, A. Fabrication and characterization of highly sensitive acetone chemical sensor based on ZnO nanoballs. Materials 2017, 10, 799. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimiasl, S.; Seifi, R.; Nahli, R.E.; Zakaria, A. Ppy/Nanographene Modified Pencil Graphite Electrode Nanosensor for Detection and Determination of Herbicides in Agricultural Water. Sci. Adv. Mater. 2017, 9, 2045–2053. [Google Scholar]
- Ahmad, R.; Tripathy, N.; Ahn, M.S.; Hahn, Y.B. Highly stable hydrazine chemical sensor based on vertically-aligned ZnO nanorods grown on electrode. J. Colloid Interf. Sci. 2017, 494, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhou, T.; Wang, Q.; Umar, A. Morphology and chemical composition dependent synthesis and electrochemical properties of MnO2-based nanostructures for efficient hydrazine detection. Sens. Actuators B Chem. 2016, 224, 878–884. [Google Scholar] [CrossRef]
- Gulino, A.; Fiorito, G.; Fragalá, I. Deposition of thin films of cobalt oxides by MOCVD. J. Mater. Chem. 2003, 13, 861–865. [Google Scholar] [CrossRef]
- Patil, D.; Patil, P.; Subramanian, V.; Joy, P.A.; Potdar, H.S. Highly sensitive and fast responding CO sensor based on Co3O4 nanorods. Talanta 2010, 81, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Zhu, L.; Li, Y.; Zhang, Z.; Ye, Z. Mesoporous Co3O4 nanoneedle arrays for high-performance gas sensor. Sens. Actuators B Chem. 2014, 203, 873–879. [Google Scholar] [CrossRef]
- Vetter, S.; Haffer, S.; Wagner, T.; Tiemann, M. Nanostructured Co3O4 as a CO gas sensor: Temperature-dependent behavior. Sens. Actuators B Chem. 2015, 206, 133–138. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Y.; Yang, F.; Liu, Z.H.; Li, G. Preparation of SnO2-Co3O4/C biochar catalyst as a Lewis acid for corncob hydrolysis into furfural in water medium. J. Ind. Eng. Chem. 2015, 26, 46–54. [Google Scholar] [CrossRef]
- Zeng, T.; Zhang, X.; Wang, S.; Niu, H.; Cai, Y. Spatial confinement of a Co3O4 catalyst in hollow metal-organic frameworks as a nanoreactor for improved degradation of organic pollutants. Environ. Sci. Technol. 2015, 49, 2350–2357. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, K.H.; Bark, C.W. Two-Dimensional Nanomaterials: Their Structures, Synthesis, and Applications. Sci. Adv. Mater. 2017, 9, 1441–1457. [Google Scholar] [CrossRef]
- Benitez, M.J.; Petracic, O.; Salabas, E.L.; Radu, F.; Tüysüz, H.; Schüth, F.; Zabel, H. Evidence for core-shell magnetic behavior in antiferromagnetic Co3O4 nanowires. Phys. Rev. Lett. 2008, 101, 7206–7210. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Kadono, K.; Kamada, K.; Ohta, K. Third-order nonlinear optical responses of nanoparticulate Co3O4 films. Thin Solid Films 2004, 446, 271–276. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, S.; Li, F.; Zhang, A.; Zhao, J.; Fang, S.; Jia, D. 3D hierarchical Co3O4 twin-spheres with an urchin-like structure: Large-scale synthesis, multistep-splitting growth, and electrochemical pseudocapacitors. Adv. Funct. Mater. 2012, 22, 4052–4059. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Yi, H.; Wei, H.; Guo, Z.; Wang, X. One-step preparation of single-crystalline Fe2O3 particles/graphene composite hydrogels as high performance anode materials for supercapacitors. Nano Energy 2014, 7, 86–96. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, J.; He, Y.; Zhong, C.; Hu, W.; Liu, L.; Deng, Y. Sub-3 nm Co3O4 nanofilms with enhanced supercapacitor properties. ACS nano 2015, 9, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Park, Y.K.; An, K.H.; Lee, H.; Jung, S.C. Impregnation of Cobalt on Graphene Sheet Using Liquid Phase Plasma Method for Lithium-Ion Batteries Application. Sci. Adv. Mater. 2016, 8, 1769–1773. [Google Scholar] [CrossRef]
- Tahira, A.; Nafady, A.; Baloach, Q.; Sherazi, S.T.H.; Shaikh, T.; Arain, M.; Magnus, M.; Ibupoto, Z.H. Ascorbic acid assisted synthesis of cobalt oxide nanostructures, their electrochemical sensing application for the sensitive determination of hydrazine. J. Electron. Mater. 2016, 45, 3695–3701. [Google Scholar] [CrossRef]
- Mei, W.; Huang, J.; Zhu, L.; Ye, Z.; Mai, Y.; Tu, J. Synthesis of porous rhombus-shaped Co3O4 nanorod arrays grown directly on a nickel substrate with high electrochemical performance. J. Mater. Chem. 2012, 22, 9315–9321. [Google Scholar] [CrossRef]
- Zhu, L.; Wen, Z.; Mei, W.; Li, Y.; Ye, Z. Porous CoO nanostructure arrays converted from rhombic Co(OH)F and needle-like Co(CO3)0.5(OH)·0.11H2O and their electrochemical properties. J. Phys. Chem. C 2013, 117, 20465–20473. [Google Scholar] [CrossRef]
- Fang, Z.; Yu, H.; Dang, Y.; Gao, N.; Ma, N.; Peng, J.; Xie, K.; Li, L. Electrochemical and Printable Properties of Polydopamine Decorated Carbon Nanotube Ink. Sci. Adv. Mater. 2017, 9, 2039–2044. [Google Scholar]
- Ngai, K.S.; Tan, W.T.; Zainal, Z.; Zawawi, R.M.; Juan, J.C. Electrochemical Sensor Based on Single-Walled Carbon Nanotube/ZnO Photocatalyst Nanocomposite Modified Electrode for the Determination of Paracetamol. Sci. Adv. Mater. 2016, 8, 788–796. [Google Scholar] [CrossRef]
- Herrero, M.; Benito, P.; Labajos, F.M.; Nanosize, V.R. Cobalt oxide-containing catalysts obtained through microwave-assisted methods. Catal. Today 2007, 128, 129–137. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Davar, F.; Mazaheri, M.; Shaterian, M. Preparation of cobalt nanoparticles from [bis(salicylidene)cobalt(II)]-oleylamine complex by thermal decomposition. J. Magn. Magn. Mater. 2008, 320, 575–578. [Google Scholar] [CrossRef]
- Tyuliev, G.; Angelov, S. The nature of excess oxygen in Co3O4+ϵ. Appl. Surf. Sci. 1988, 32, 381–391. [Google Scholar] [CrossRef]
- He, T.; Chen, D.; Jiao, X.; Wang, Y.; Duan, Y. Solubility-controlled synthesis of high-quality Co3O4 nanocrystals. Chem. Mater. 2005, 17, 4023–4030. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, D.; Shi, L.; Xu, J.; Zhang, L.; Huang, L.; Li, H.; Zhang, J. Porous Ni-Mn oxide nanosheets in situ formed on nickel foam as 3D hierarchical monolith de-NOx catalysts. Nanoscale 2014, 6, 7346–7353. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Linsebigler, A.; Yates Jr, J.T. Ti3+ defect sites on TiO2 (110): Production and chemical detection of active sites. J. Phys. Chem. 1994, 98, 11733–11738. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, W.; Dou, M.; Wang, F.; Liu, J.; Li, Z.; Ji, J. Nanorod-constructed porous Co3O4 nanowires: Highly sensitive sensors for the detection of hydrazine. Analyst 2015, 140, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Wang, B.; Lin, Z.; Sun, B.; Pan, L.; Wu, J.; Shi, Y. Enhanced Nonenzymatic Sensing of Hydrogen Peroxide Released from Living Cells Based on Graphene Aerogel/Platinum Nanoparticle. Sci. Adv. Mater. 2016, 8, 1165–1171. [Google Scholar] [CrossRef]
- Yagati, A.K.; Park, I.; Cho, S.; Min, J. Impedimetric Horseradish Peroxidase Sensor Based on Polyaniline-Nanofiber-Modified Microdisk Electrode Arrays. Sci. Adv. Mater. 2017, 9, 1595–1602. [Google Scholar] [CrossRef]
- Li, J.; Lin, X. Electrocatalytic oxidation of hydrazine and hydroxylamine at gold nanoparticle—Polypyrrole nanowire modified glassy carbon electrode. Sens. Actuators B Chem. 2007, 126, 527–535. [Google Scholar] [CrossRef]
- Pinter, J.S.; Brown, K.L.; DeYoung, P.A.; Peaslee, G.F. Amperometric detection of hydrazine by cyclic voltammetry and flow injection analysis using ruthenium modified glassy carbon electrodes. Talanta 2007, 71, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.F.; Yu, W.O. Nanoporous gold particles modified titanium electrode for hydrazine oxidation. J. Electroanal. Chem. 2009, 633, 159–164. [Google Scholar] [CrossRef]
- Perez, E.F.; Neto, G.D.O.; Tanaka, A.A.; Kubota, L.T. Electrochemical sensor for hydrazine based on silica modified with nickel tetrasulfonated phthalocyanine. Electroanalysis 1998, 10, 111–115. [Google Scholar] [CrossRef]
- Salimi, A.; Miranzadeh, L.; Hallaj, R. Amperometric and voltammetric detection of hydrazine using glassy carbon electrodes modified with carbon nanotubes and catechol derivatives. Talanta 2008, 75, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Huang, X.; Wu, C.; Xu, H.; Jiang, P.; Tanaka, T. Fabrication of two-dimensional hybrid sheets by decorating insulating PANI on reduced graphene oxide for polymer nanocomposites with low dielectric loss and high dielectric constant. J. Mater. Chem. 2012, 22, 23477–23484. [Google Scholar] [CrossRef]



| Raw Materials (mmol) | Precursor | Calcination (400 °C) | Crystallinity (%) | SSA (m2∙g−1) | ||
|---|---|---|---|---|---|---|
| Co(NO3)2 | NH4F | CO(NH2)2 | ||||
| 5 | 0 | 10 | P1 1 | Co3O4-1 | 73.87 | 25.83 |
| 5 | 5 | 10 | P1 and P2 | Co3O4 | 80.34 | 21.20 |
| 5 | 10 | 10 | P1 and P2 | Co3O4-2 | 84.24 | 18.44 |
| 5 | 15 | 10 | P1 and P2 | Co3O4 | 88.05 | 14.55 |
| 5 | 20 | 10 | P1 and P2 | Co3O4 | 90.46 | 12.44 |
| 5 | 20 | HMT | P2 1 | Co3O4-3 | 91.62 | 10.57 |
| Sample | Amount of Oxygen Species | |||
|---|---|---|---|---|
| Oxygen Vacancy (OV) | Lattice Oxygen (LO) | |||
| at. % | BE (eV) | at. % | BE (eV) | |
| Co3O4-1 | 44.9 | 530.12 | 55.1 | 531.47 |
| Co3O4-2 | 43.8 | 530.12 | 56.2 | 531.47 |
| Co3O4-3 | 41.8 | 530.12 | 58.2 | 531.47 |
| Electrode Materials | Sensitivity (μA∙μM−1) | Detection Limit (μM) | Linear Range (mM) | Ref. |
|---|---|---|---|---|
| Ru-complex films | – | 8.5 | 0.010–10 | [40] |
| Nano-Au/Ti | 1.117 | 42 | 0.5–4 | [41] |
| Nickel tetrasulfonated phthalocyanine | 0.0079 | 10 | 0.1–0.6 | [42] |
| MWCNTs/Chlorogenic | 4.1 μA∙mM−1∙cm−2 | 8 | 0.0025–0.5 | [43] |
| GO/CTS/Pt | 104.6 μA∙mM−1∙cm−2 | 3.6 | 0.02–1 | [44] |
| Co3O4-1 | 32.42 | 9.73 | 0.010–2.38 | This work |
| Co3O4-2 | 25.28 | 10.74 | 0.027–0.890 |
| Materials | Current (μA) | Sensitivity (μA∙mM−1) | SSA (m2∙g−1) | Surface Area Normalized Current (μA∙m−2∙g) | Surface Area Normalized Sensitivity (μA∙mM−1∙m−2∙g) |
|---|---|---|---|---|---|
| Co3O4-1 | 46.05 | 32.42 | 25.83 | 1.78 | 1.26 |
| Co3O4-2 | 33.58 | 25.28 | 18.44 | 1.81 | 1.37 |
| Co3O4-3 | - | - | 10.57 | - | - |
| Sample | Hydrazine Added (μM) | Hydrazine Founded (μM) | Recovery |
|---|---|---|---|
| Distilled Water | 10 | 10.28 | 102.79% |
| 20 | 19.98 | 99.88% | |
| 50 | 49.88 | 99.77% | |
| Tap Water | 10 | 10.16 | 101.63% |
| 20 | 19.63 | 98.14% | |
| 50 | 49.16 | 98.33% | |
| River Water | 10 | 9.86 | 98.63% |
| 20 | 19.51 | 97.56% | |
| 50 | 49.65 | 99.30% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, T.; Gao, W.; Wang, Q.; Umar, A. Effect of Fluoride on the Morphology and Electrochemical Property of Co3O4 Nanostructures for Hydrazine Detection. Materials 2018, 11, 207. https://doi.org/10.3390/ma11020207
Zhou T, Gao W, Wang Q, Umar A. Effect of Fluoride on the Morphology and Electrochemical Property of Co3O4 Nanostructures for Hydrazine Detection. Materials. 2018; 11(2):207. https://doi.org/10.3390/ma11020207
Chicago/Turabian StyleZhou, Tuantuan, Wanlin Gao, Qiang Wang, and Ahmad Umar. 2018. "Effect of Fluoride on the Morphology and Electrochemical Property of Co3O4 Nanostructures for Hydrazine Detection" Materials 11, no. 2: 207. https://doi.org/10.3390/ma11020207





