Influence of PEG Stoichiometry on Structure-Tuned Formation of Self-Assembled Submicron Nickel Particles
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Synthesis of Nickel Submicron Particles
2.3. Characterization
3. Results and Discussion
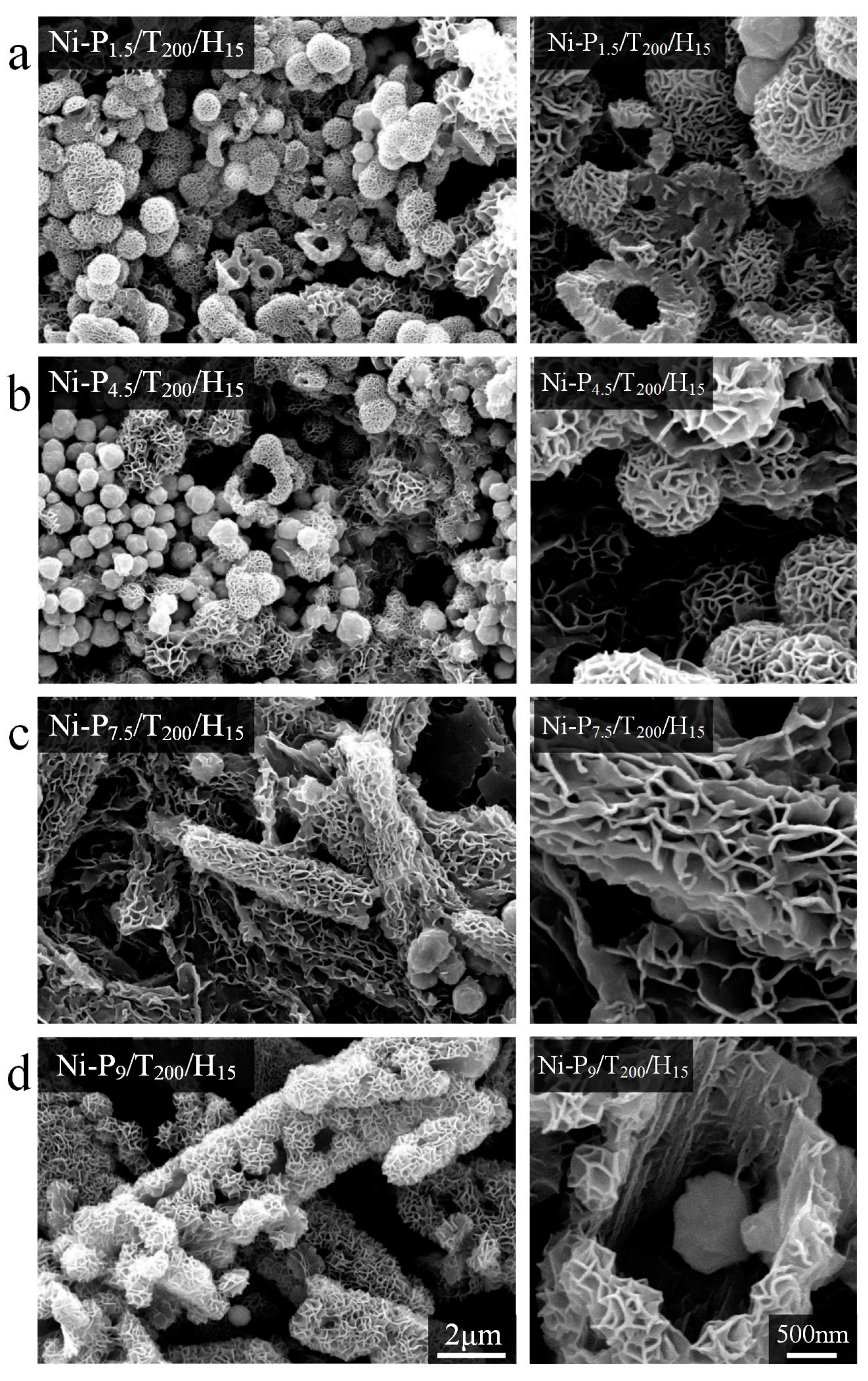
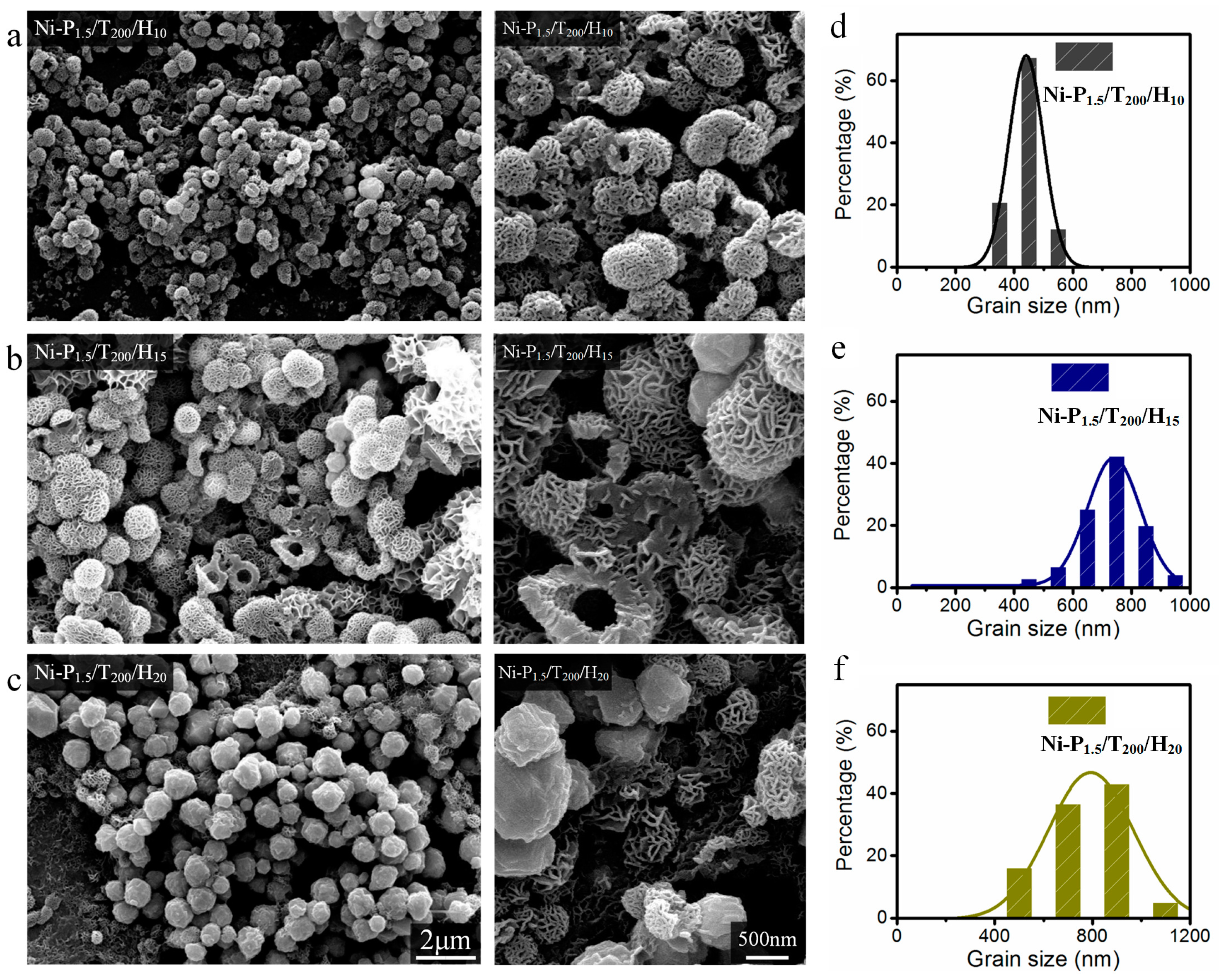
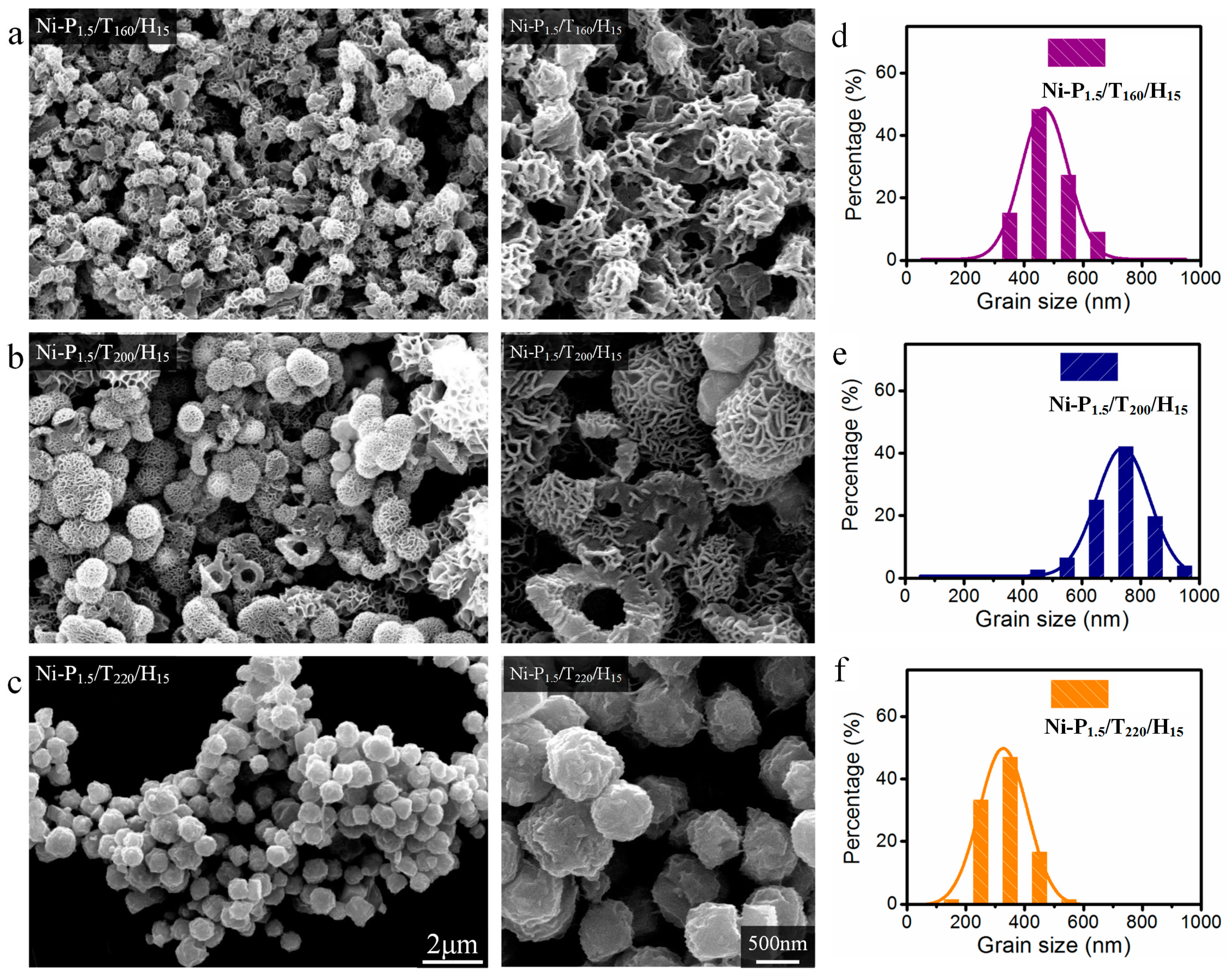
3.1. Morphological Properties
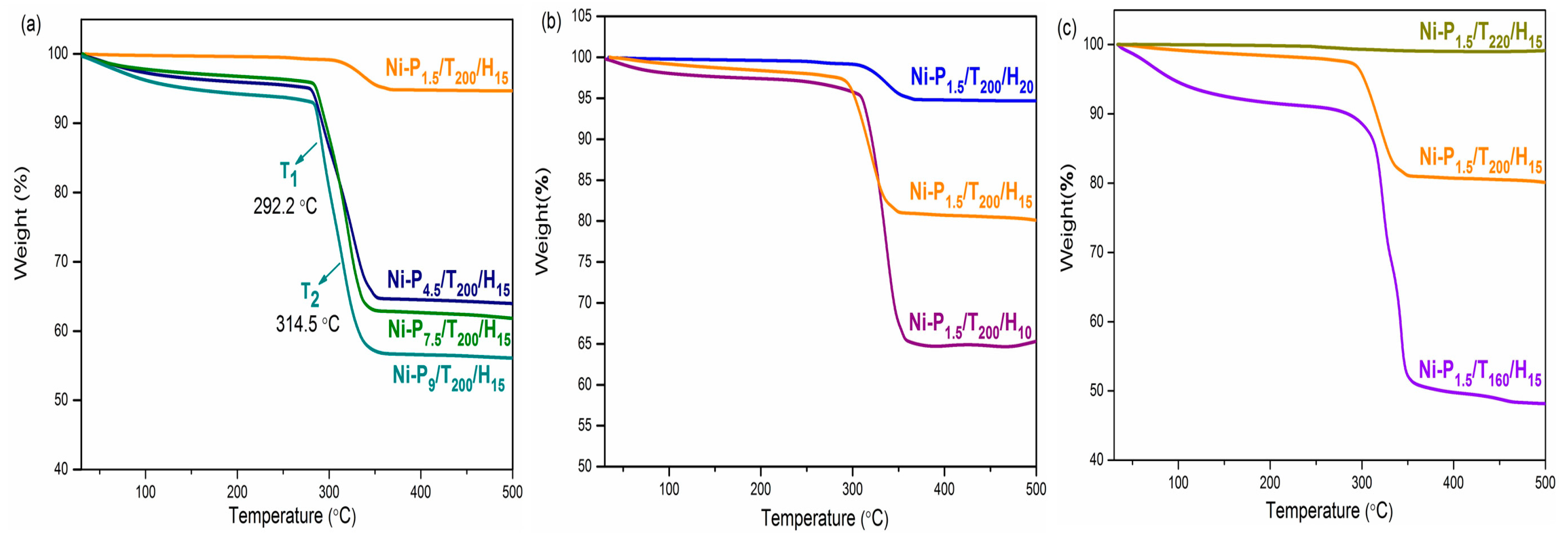
3.2. Thermal Properties
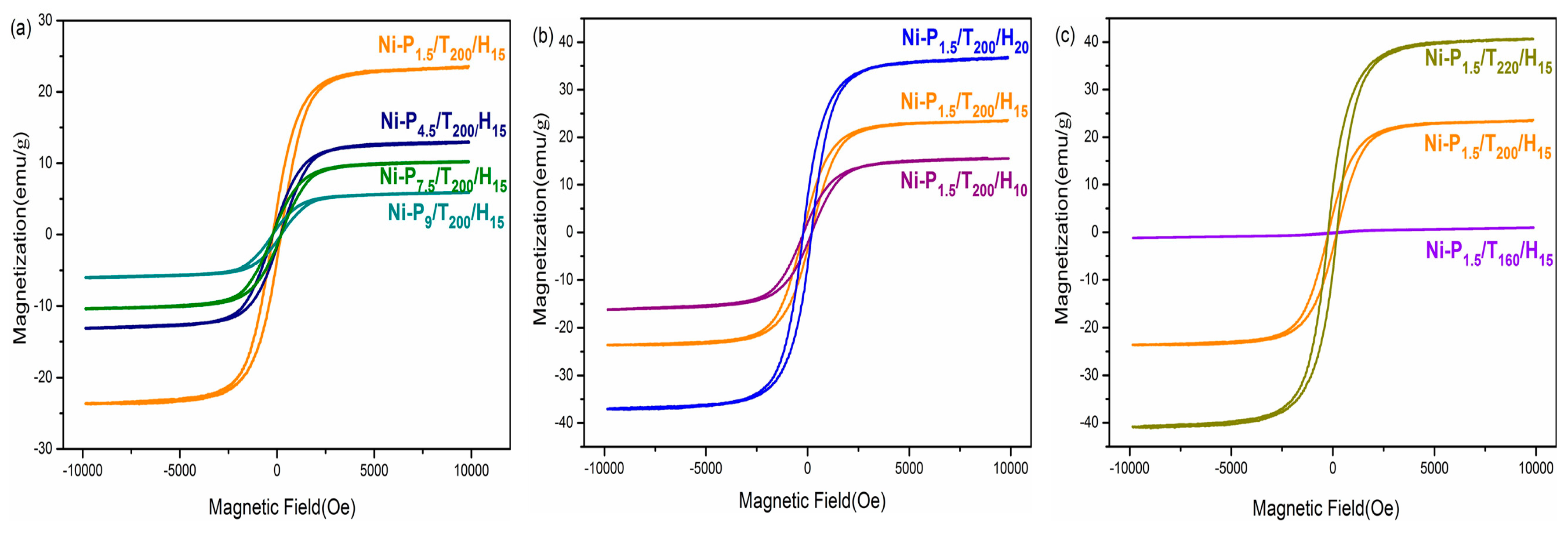
3.3. Magnetic Properties
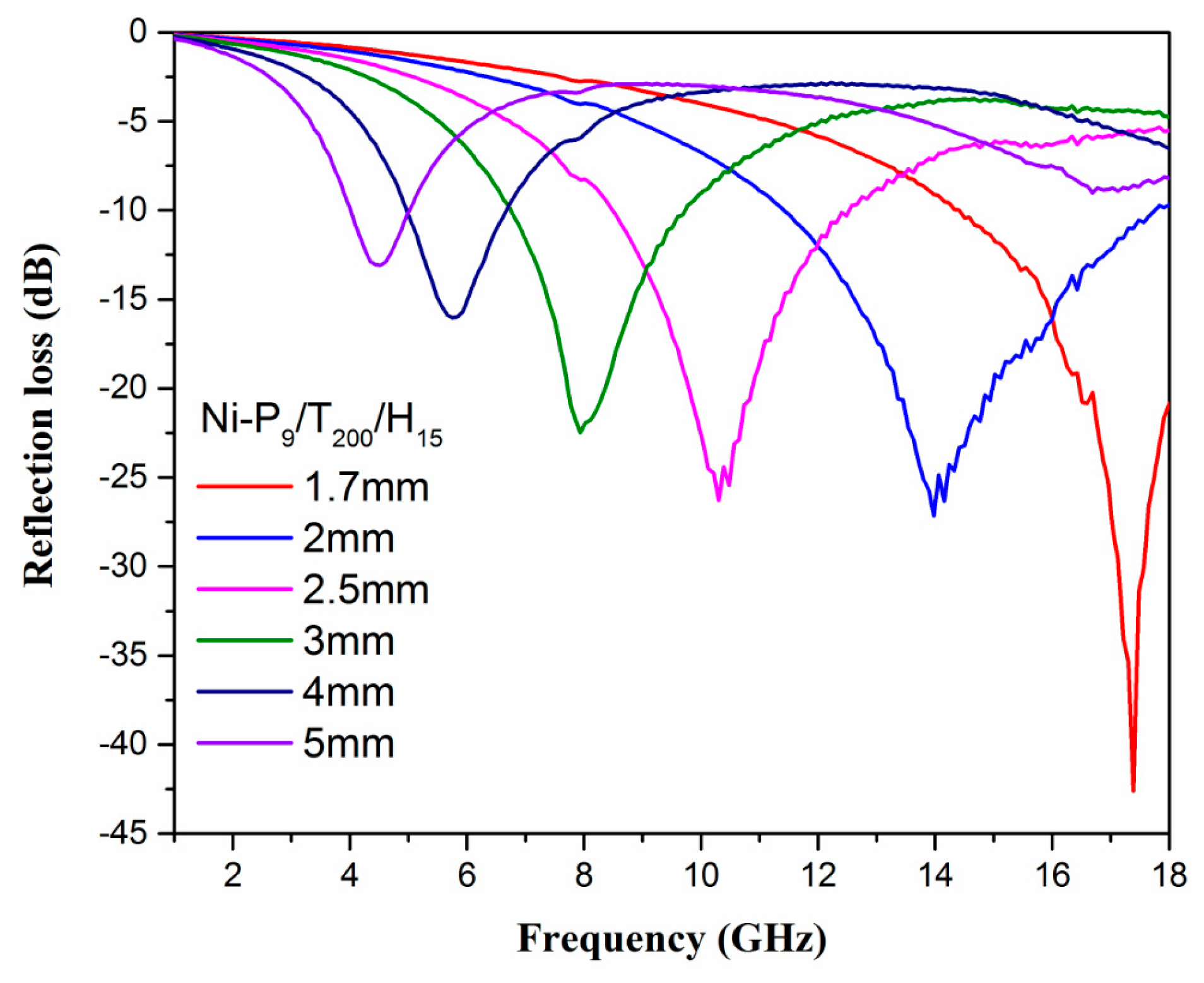
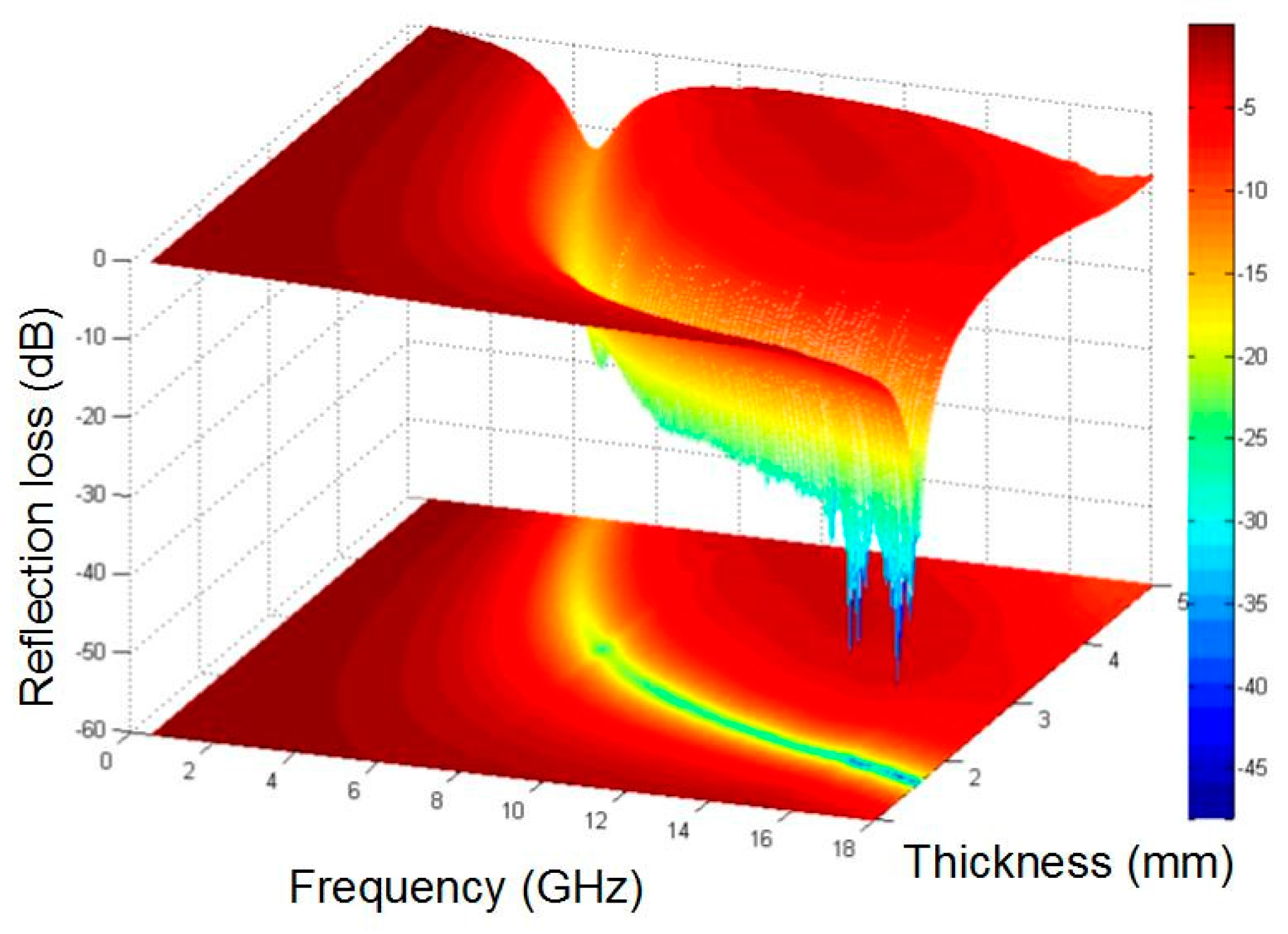
3.4. Microwave Absorption Properties
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Murray, C.B.; Sun, S.; Doyle, H.; Betley, T. Monodisperse 3D transition-metal (Co,Ni,Fe) nanoparticles and their assembly into nanoparticle superlattices. MRS Bull. 2001, 26, 985–991. [Google Scholar] [CrossRef]
- Miao, F.; Tao, B.; Sun, L.; Liu, T.; You, J.; Wang, L.; Chu, P.K. Preparation and characterization of novel nickel–palladium electrodes supported by silicon microchannel plates for direct methanol fuel cells. J. Power Sources 2010, 195, 146–150. [Google Scholar] [CrossRef]
- Yan, Y.; Cheng, G.; Wang, P.; He, D.; Chen, R. Facile hydrothermal selective fabrication of Ni(OH)2 and Ni(HCO3)2 nanoparticulates and their electrochemical performances. RSC Adv. 2014, 4, 49303–49307. [Google Scholar] [CrossRef]
- Maringa, A.; Mugadza, T.; Antunes, E.; Nyokong, T. Characterization and electrocatalytic behaviour of glassy carbon electrode modified with nickel nanoparticles towards amitrole detection. J. Electroanal. Chem. 2013, 700, 86–92. [Google Scholar] [CrossRef]
- Bao, J.; Tie, C.; Xu, Z.; Zhou, Q.; Shen, D.; Ma, Q. Template synthesis of an array of nickel nanotubules and its magnetic behavior. Adv. Mater. 2001, 13, 1631–1633. [Google Scholar] [CrossRef]
- Toneguzzo, P.; Viau, G.; Acher, O.; Fiévet-Vincent, F.; Fiévet, F. Monodisperse ferromagnetic particles for microwave applications. Adv. Mater. 1998, 98, 1032–1035. [Google Scholar] [CrossRef]
- Liu, J.; Itoh, M.; Terada, M.; Horikawa, T.; Machida, K. Enhanced electromagnetic wave absorption properties of Fe nanowires in gigaherz range. Appl. Phys. Lett. 2007, 91, 093101. [Google Scholar] [CrossRef]
- Li, N.; Cao, M.; Hu, C. A simple approach to spherical nickel-carbon monoliths as light-weight microwave absorbers. J. Mater. Chem. 2012, 22, 18426–18432. [Google Scholar] [CrossRef]
- Tao, M.; Xin, Z.; Meng, X.; Lv, Y.; Bian, Z. Impact of double-solvent impregnation on the Ni dispersion of Ni/SBA-15 catalysts and catalytic performance for the syngas methanation reaction. RSC Adv. 2016, 6, 35875–35883. [Google Scholar] [CrossRef]
- Roca, A.G.; Costo, R.; Rebolledo, A.F.; Veintemillas-Verdaguer, S.; Tartaj, P.; Gonzalez-Carreno, T.; Morales, M.P.; Serna, C.J. Progress in the preparation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2009, 42, 224002–224012. [Google Scholar] [CrossRef]
- Cao, L.; Ni, Y.; Wang, M.; Ma, X. Magnetic Ni/α-Ni(OH)2 porous superstructures: Synthesis, influencing factors and applications in the removal of heavy metals. RSC Adv. 2013, 3, 3585–3591. [Google Scholar] [CrossRef]
- Hu, H.; Sugawara, K. Selective synthesis of metallic nickel particles with control of shape via wet chemical process. Mater. Lett. 2008, 62, 4339–4342. [Google Scholar] [CrossRef]
- Qiao, L.; Han, X.; Gao, B.; Wang, J.; Wen, F.; Li, F. Microwave absorption properties of the hierarchically branched Ni nanowire composites. J. Appl. Phys. 2009, 105, 053911. [Google Scholar] [CrossRef]
- Xu, S.; Fei, G.; Ouyang, H.; Zhang, Y.; Huo, P.; Zhang, L. Controllable fabrication of nickel nanoparticle chains based on electrochemical corrosion. J. Mater. Chem. C 2015, 3, 2072–2079. [Google Scholar] [CrossRef]
- Brosseau, C.; Talbot, P. Effective magnetic permeability of Ni and Co micro- and nanoparticles embedded in a ZnO matrix. J. Appl. Phys. 2005, 97, 104325. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Shape-controlled synthesis of gold and silver nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhao, L. Study on the morphologies of nickel crystals and their magnetic properties. Mater. Lett. 2012, 79, 142–144. [Google Scholar] [CrossRef]
- Tokarev, A.; Yatvin, J.; Trotsenko, O.; Locklin, J.; Minko, S. Nanostructured soft matter with magnetic nanoparticles. Adv. Funct. Mater. 2016, 26, 3761. [Google Scholar] [CrossRef]
- Guo, H.; Pu, B.; Chen, H.; Yang, J.; Zhou, Y.; Yang, J.; Bismark, B.; Li, H.; Niu, X. Surfactant-assisted solvothermal synthesis of pure nickel submicron spheres with microwave-absorbing properties. Nanoscale Res. Lett. 2016, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, G.; Liu, Y. Low-temperature solution synthesis of CuO nanorods with thin diameter. Mater. Lett. 2013, 93, 60–63. [Google Scholar] [CrossRef]
- Cao, J.; Lü, X.; Zhao, L.; Qu, S.; Gao, W. Influence of initial growth conditions and Mg-surfactant on the quality of GaN film grown by MOVPE. J. Semicond. 2015, 36, 023005. [Google Scholar] [CrossRef]
- Wang, F.; Richards, V.N.; Shields, S.P.; Buhro, W.E. Kinetics and mechanisms of aggregative nanocrystal growth. Chem. Mater. 2014, 26, 5–21. [Google Scholar] [CrossRef]
- Zheng, H.; Smith, R.K.; Jun, Y.; Kisielowski, C.; Dahmen, U.; Alivisatos, A.P. Observation of single colloidal platinum nanocrystal growth trajectories. Science 2009, 324, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Deng, G.; Guo, X.; Liu, H.; Jiang, W.; Li, F. Preparation of nickel and Ni3Sn nanoparticles via extension of conventional citric acid and ethylene diamine tetraacetic acid mediated solegel method. J. Alloys Compd. 2016, 668, 159–168. [Google Scholar] [CrossRef]
- Song, H.J.; Jia, X.H.; Yang, X.F.; Tang, H.; Li, Y.; Su, Y.T. Controllable synthesis of monodisperse polyhedral nickel nanocrystals. CrystEngComm 2012, 14, 405–410. [Google Scholar] [CrossRef]
- Liu, J.; Hu, R.; Liu, H.; Ma, J. Chips assembled cuboid-like nickel hydroxide/rGO composite material for high performance supercapacitors. J. Alloys Compd. 2017, 718, 349–355. [Google Scholar] [CrossRef]
- Patnaik, P.; Biswal, A.; Tripathy, B.; Pradhan, S.; Das, B.; Sakthivel, R.; Subbaiah, T. Synthesis and characterization of fibrous nickel hydroxide obtained from spent nickel catalyst. Trans. Nonferrous Met. Soc. China 2013, 23, 2977–2983. [Google Scholar] [CrossRef]
- Koltypin, Y.; Katabi, G.; Cao, X.; Prozorov, R.; Gedanken, A. Sonochemical preparation of amorphous nickel. J. Non-Cryst. Solids 1996, 201, 159–162. [Google Scholar] [CrossRef]
- Yoon, S.; Dornseiffer, J.; Schneller, T.; Hennings, D.; Iwaya, S.; Pithan, C.; Waser, R. Percolative BaTiO3–Ni composite nanopowders from alkoxide-mediated synthesis. J. Eur. Ceram. Soc. 2010, 30, 561–567. [Google Scholar] [CrossRef]
- Liu, Z.P.; Li, S.; Yang, Y.; Peng, S.; Hu, Z.K.; Qian, Y.T. Complex-surfactant-assisted hydrothermal route to ferromagnetic nickel nanobelts. Adv. Mater. 2003, 15, 1946–1948. [Google Scholar] [CrossRef]
- Ni, X.; Zhao, Q.; Zhang, D.; Zhang, X.; Zheng, H. Novel Hierarchical nanostructures of nickel: Self-assembly of hexagonal nanoplatelets. J. Phys. Chem. C 2007, 111, 601–605. [Google Scholar] [CrossRef]
- Mathew, A.; Munichandraiah, N.; Rao, G.M. Synthesis and magnetic studies of flower-like nickel nanocones. Mater. Sci. Eng. B 2009, 158, 7–12. [Google Scholar] [CrossRef]
- Feng, Y.; Qiu, T. Preparation, characterization and microwave absorbing properties of FeNi alloy prepared by gas atomization method. J. Alloys Compd. 2012, 513, 455–459. [Google Scholar] [CrossRef]
- Feng, Y.B.; Qiu, T.; Shen, C.Y. Absorbing properties and structural design of microwave absorbers based on carbonyl iron and barium ferrite. J. Magn. Magn. Mater. 2007, 318, 8–13. [Google Scholar] [CrossRef]
- Zhang, C.; Yao, Y.; Zhan, J.; Wu, J.; Li, C. Template-free synthesis of Ni microfibres and their electromagnetic wave absorbing properties. J. Phys. D Appl. Phys. 2013, 46, 495308. [Google Scholar] [CrossRef]
- Liu, J.; Feng, Y.; Qiu, T. Synthesis, characterization, and microwave absorption properties of Fe–40 wt %Ni alloy prepared by mechanical alloying and annealing. J. Magn. Magn. Mater. 2011, 323, 3071–3076. [Google Scholar] [CrossRef]
- Naito, Y.; Suetake, K. Application of ferrite to electromagnetic wave absorber and its characteristics. IEEE Trans. Microw. Theory Tech. 1971, 19, 65–72. [Google Scholar] [CrossRef]
- Michielssen, E.; Sajer, J.; Ranjithan, S.; Mittra, R. Design of lightweight, broad-band microwave absorbers using genetic algorithms. IEEE Trans. Microw. Theory Tech. 1993, 41, 0018–9480. [Google Scholar] [CrossRef]
- Dong, S.; Xu, M.; Wei, J.; Yang, X.; Liu, X. The preparation and wide frequency microwave absorbing properties of tri-substituted-bisphthalonitrile/Fe3O4 magnetic hybrid microspheres. J. Magn. Magn. Mater. 2014, 349, 15–20. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, R.; Liu, X. Only Ku-band microwave absorption by Fe3O4/ferrocenyl-CuPc hybrid nanospheres. J. Magn. Magn. Mater. 2012, 324, 3323–3327. [Google Scholar] [CrossRef]
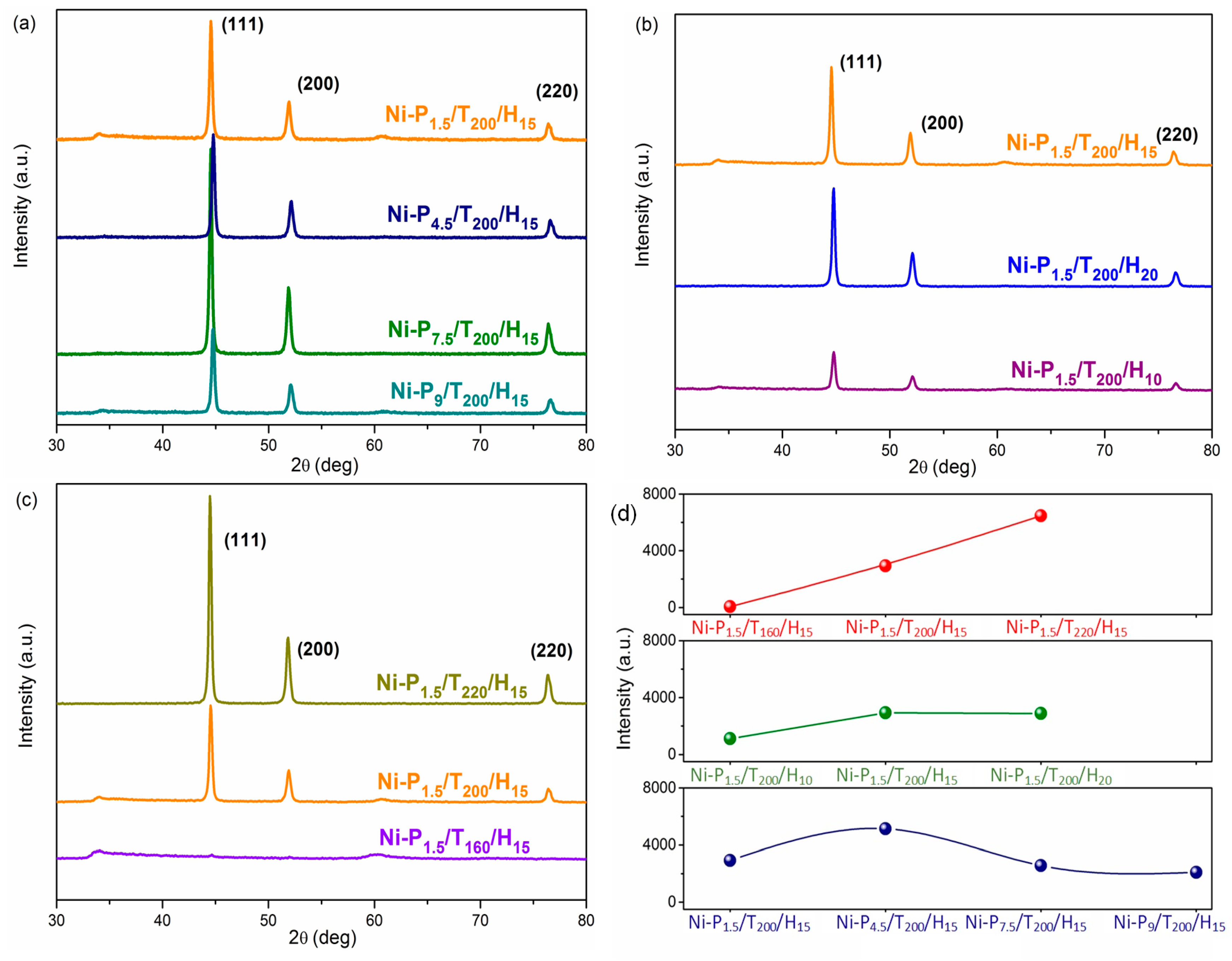

| Samples | Mass of PEG (g) | Temperature (°C) | Time (h) | Crystal Size (nm) |
|---|---|---|---|---|
| Ni-P1.5/T200/H15 | 1.5 | 200 | 15 | 730 |
| Ni-P4.5/T200/H15 | 4.5 | 200 | 15 | 640 |
| Ni-P7.5/T200/H15 | 7.5 | 200 | 15 | - |
| Ni-P9/T200/H15 | 9.0 | 200 | 15 | 670 |
| Ni-P1.5/T200/H10 | 1.5 | 200 | 10 | 440 |
| Ni-P1.5/T200/H20 | 1.5 | 200 | 20 | 770 |
| Ni-P1.5/T160/H15 | 1.5 | 160 | 15 | 470 |
| Ni-P1.5/T220/H15 | 1.5 | 220 | 15 | 660 |
| Samples | Ms (eum/g) | Mr (eum/g) | Hc (Oe) | T1 (°C) | T2 (°C) |
|---|---|---|---|---|---|
| Ni-P1.5/T200/H15 | 23.5 | 3.7 | 205.7 | 319.24 | - |
| Ni-P4.5/T200/H15 | 13.0 | 1.8 | 200.8 | 291.4 | 326.5 |
| Ni-P7.5/T200/H15 | 10.2 | 1.6 | 218.0 | 292.2 | 320.1 |
| Ni-P9/T200/H15 | 5.9 | 0.87 | 222.2 | 292.2 | 314.5 |
| Ni-P1.5/T200/H10 | 15.9 | 2.1 | 197.8 | 336.0 | - |
| Ni-P1.5/T200/H20 | 36.8 | 7.4 | 206.8 | 337.6 | - |
| Ni-P1.5/T160/H15 | 1.1 | 0.08 | 185.6 | 252.5 | 280.3 |
| Ni-P1.5/T220/H15 | 40.7 | 8.5 | 216.6 | 322.5 | 341.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, B.; Wang, L.; Guo, H.; Yang, J.; Chen, H.; Zhou, Y.; Yang, J.; Zhao, B.; Niu, X. Influence of PEG Stoichiometry on Structure-Tuned Formation of Self-Assembled Submicron Nickel Particles. Materials 2018, 11, 222. https://doi.org/10.3390/ma11020222
Pu B, Wang L, Guo H, Yang J, Chen H, Zhou Y, Yang J, Zhao B, Niu X. Influence of PEG Stoichiometry on Structure-Tuned Formation of Self-Assembled Submicron Nickel Particles. Materials. 2018; 11(2):222. https://doi.org/10.3390/ma11020222
Chicago/Turabian StylePu, Bingxue, Liping Wang, Heng Guo, Jian Yang, Haiyuan Chen, Yajun Zhou, Jin Yang, Bin Zhao, and Xiaobin Niu. 2018. "Influence of PEG Stoichiometry on Structure-Tuned Formation of Self-Assembled Submicron Nickel Particles" Materials 11, no. 2: 222. https://doi.org/10.3390/ma11020222




