A Self-Switchable Polymer Reactor for Controlled Catalytic Chemistry Processes with a Hyperbranched Structure
Abstract
:1. Introduction
2. Experiment Section
2.1. Characterization
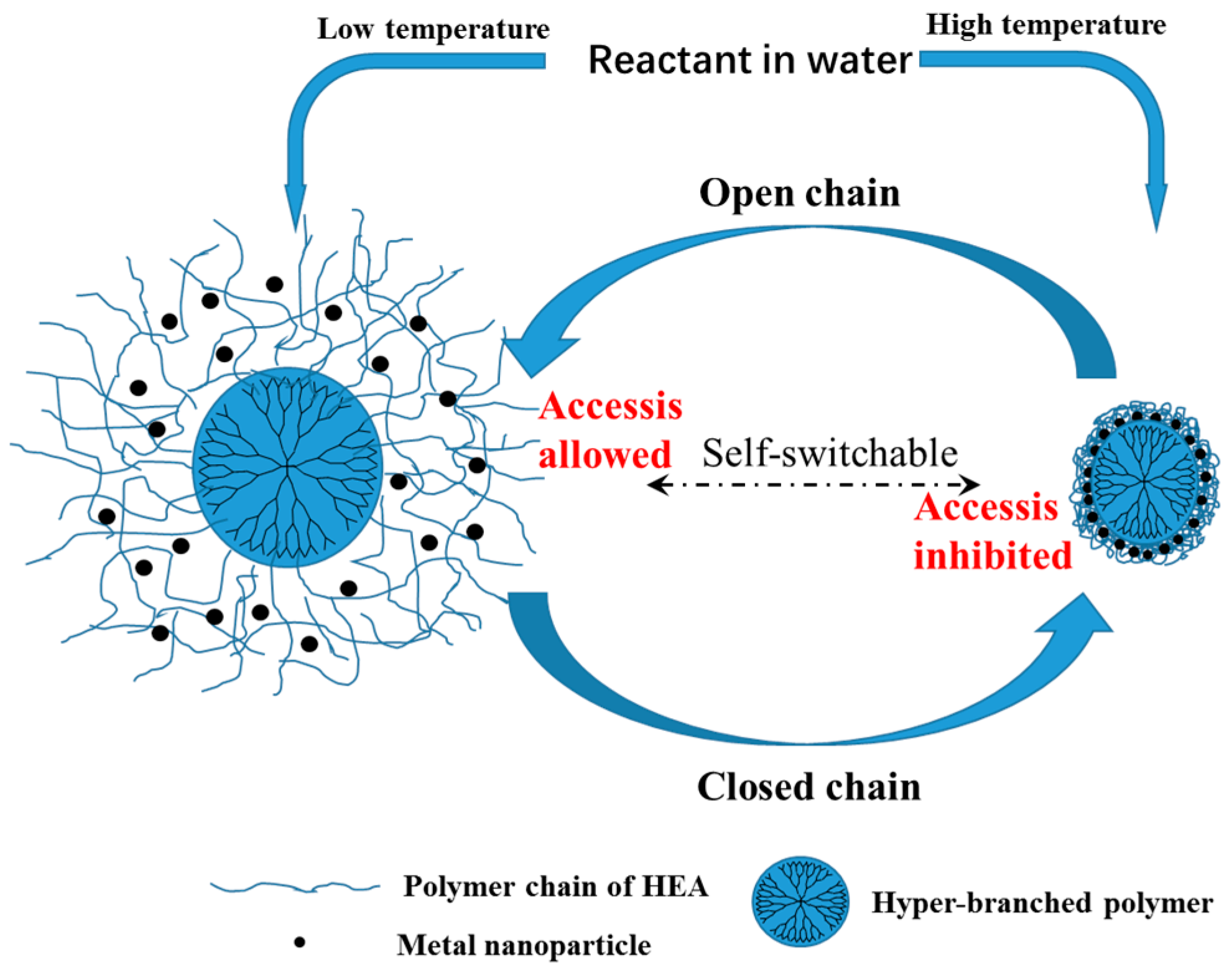
2.2. Self-Switchable Interactions
2.3. Catalysis Test
2.4. Electrochemical Tests
3. Results and Discussion
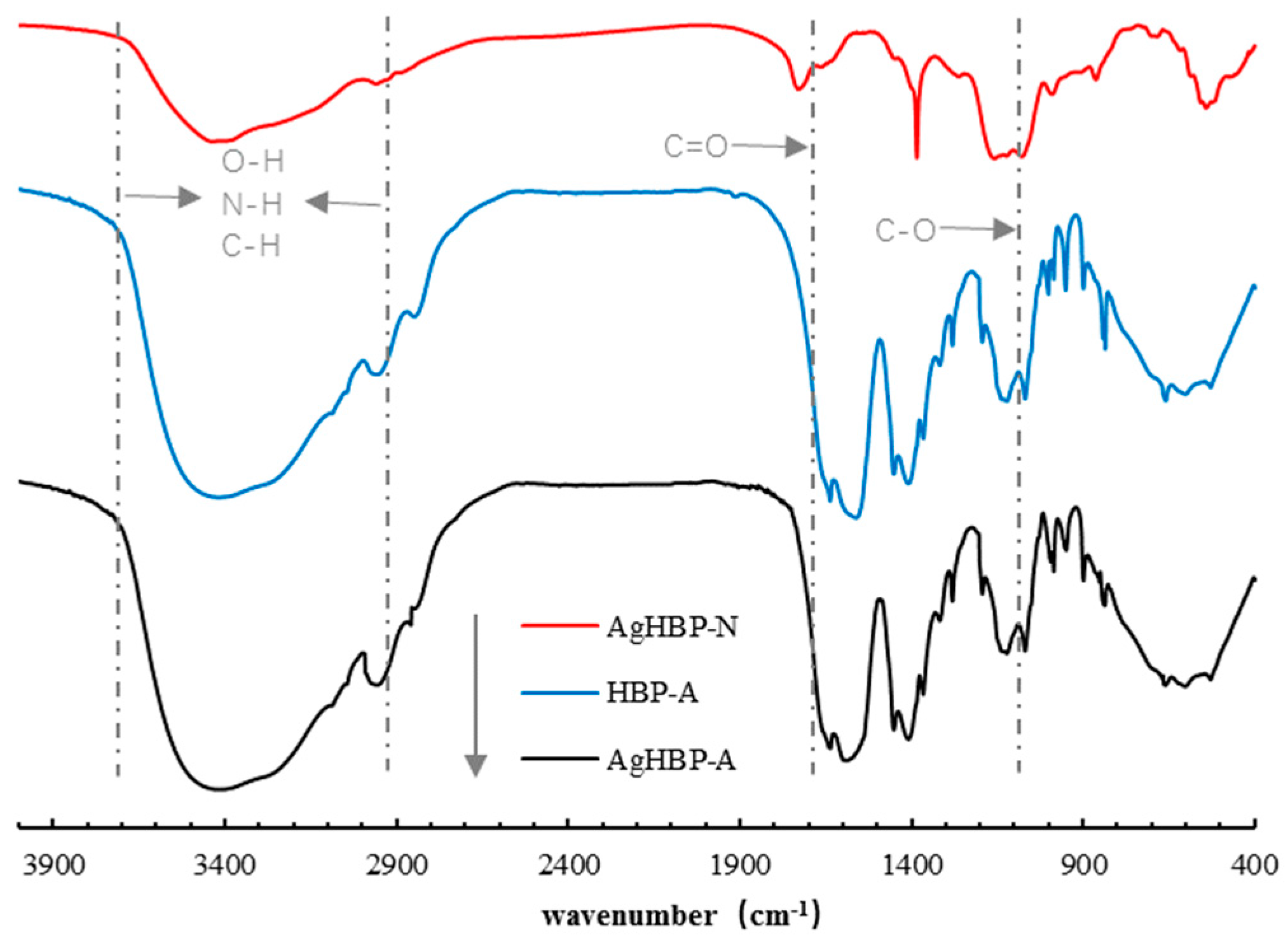
3.1. 1H NMR and FT-IR Analysis
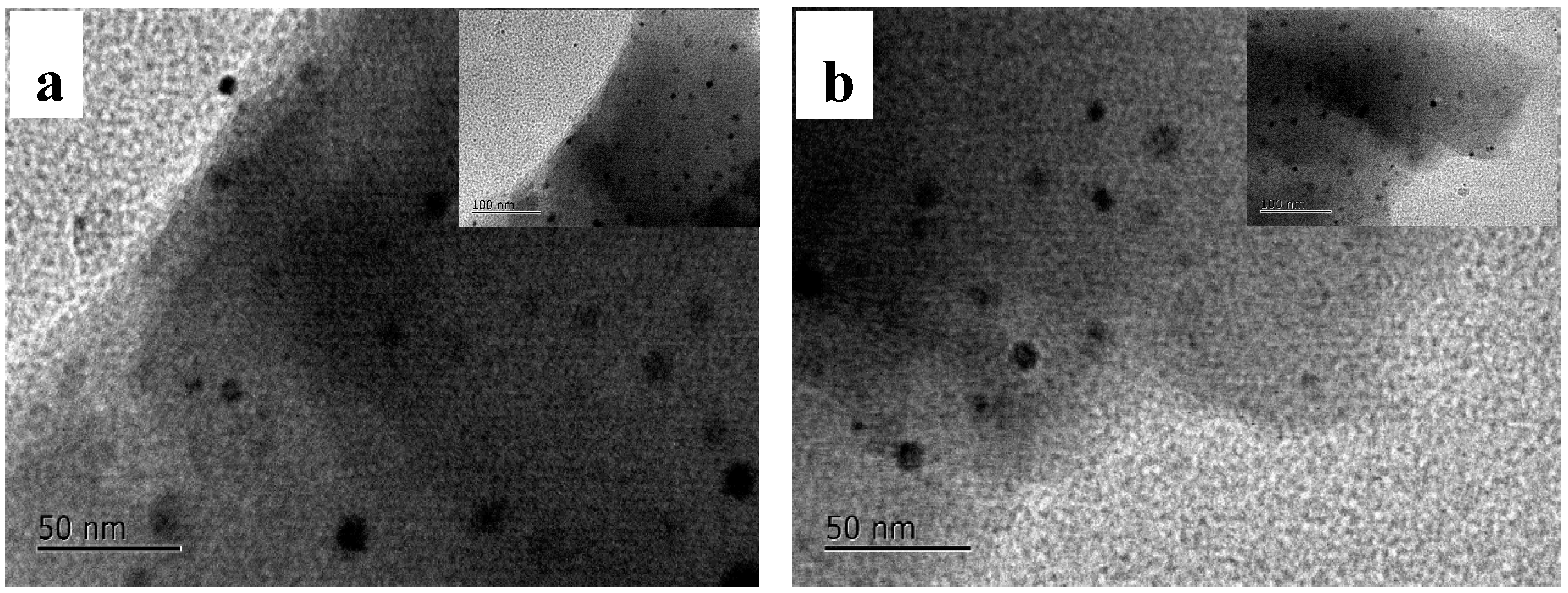
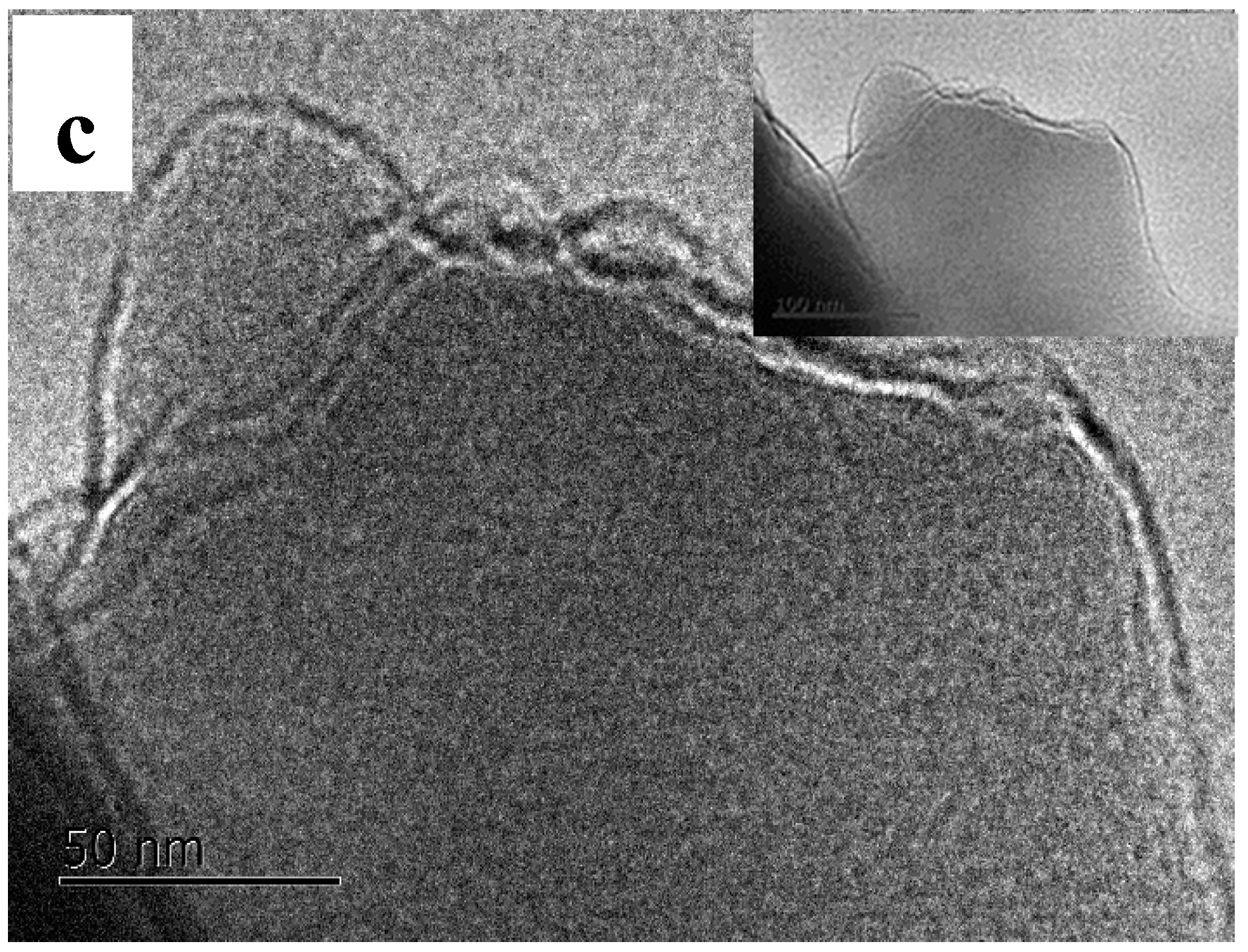
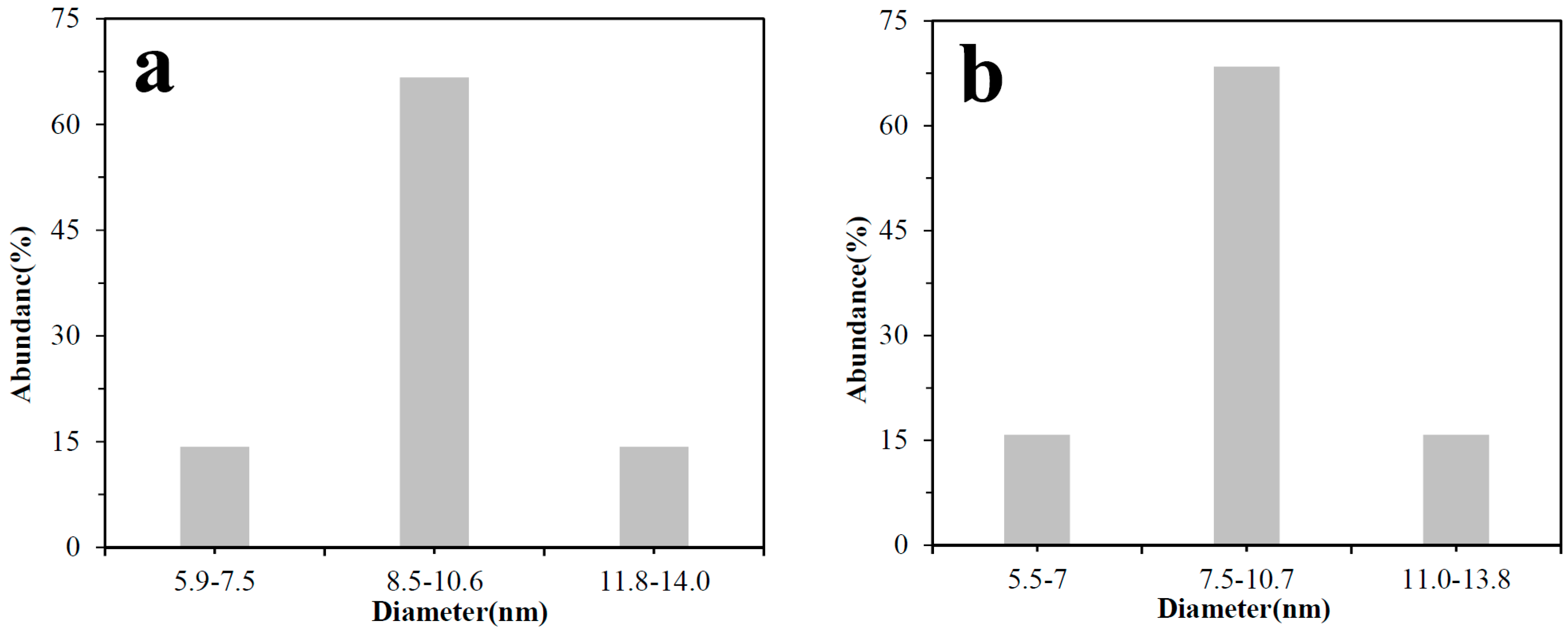
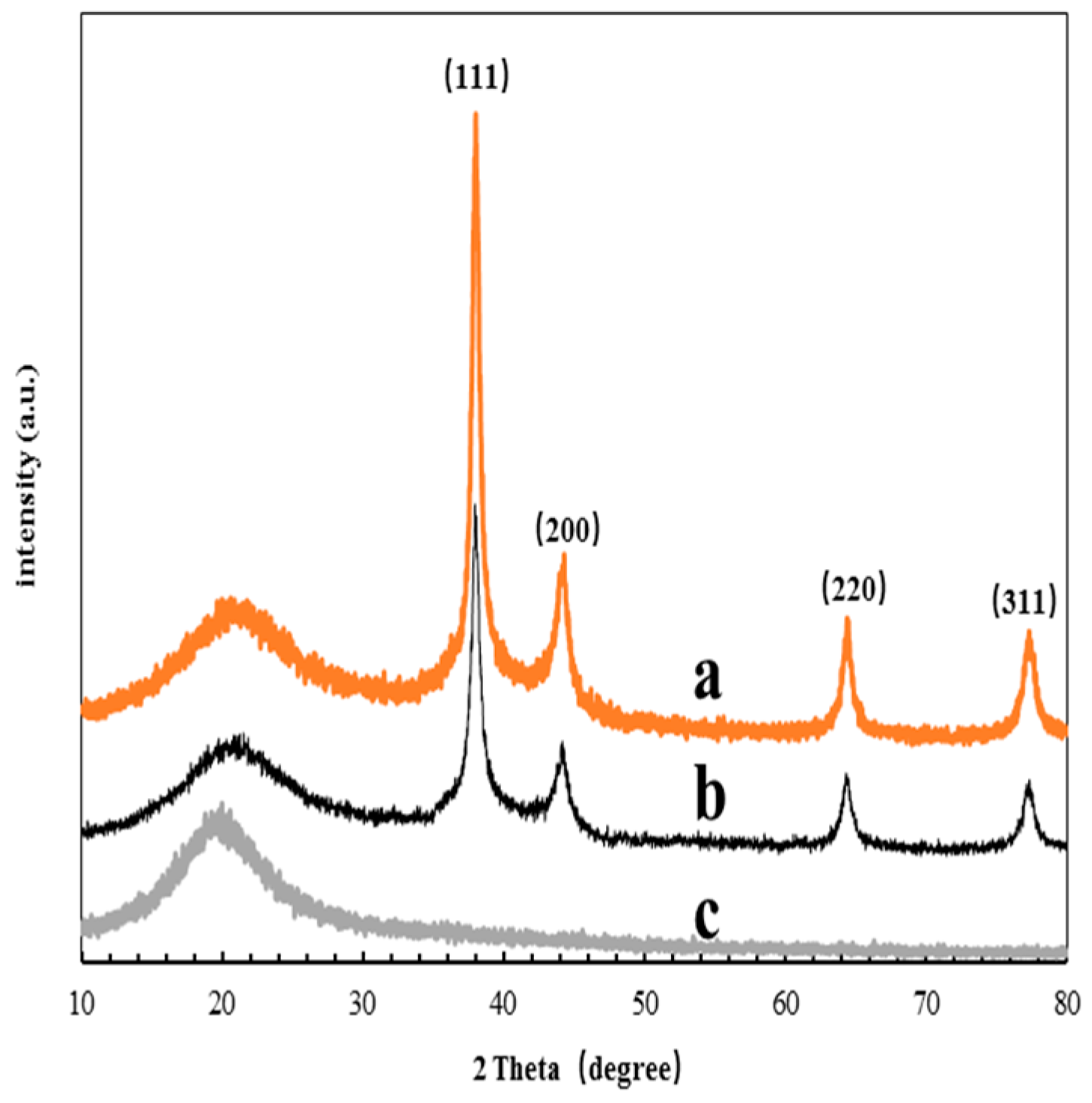
3.2. TEM and XRD Analysis
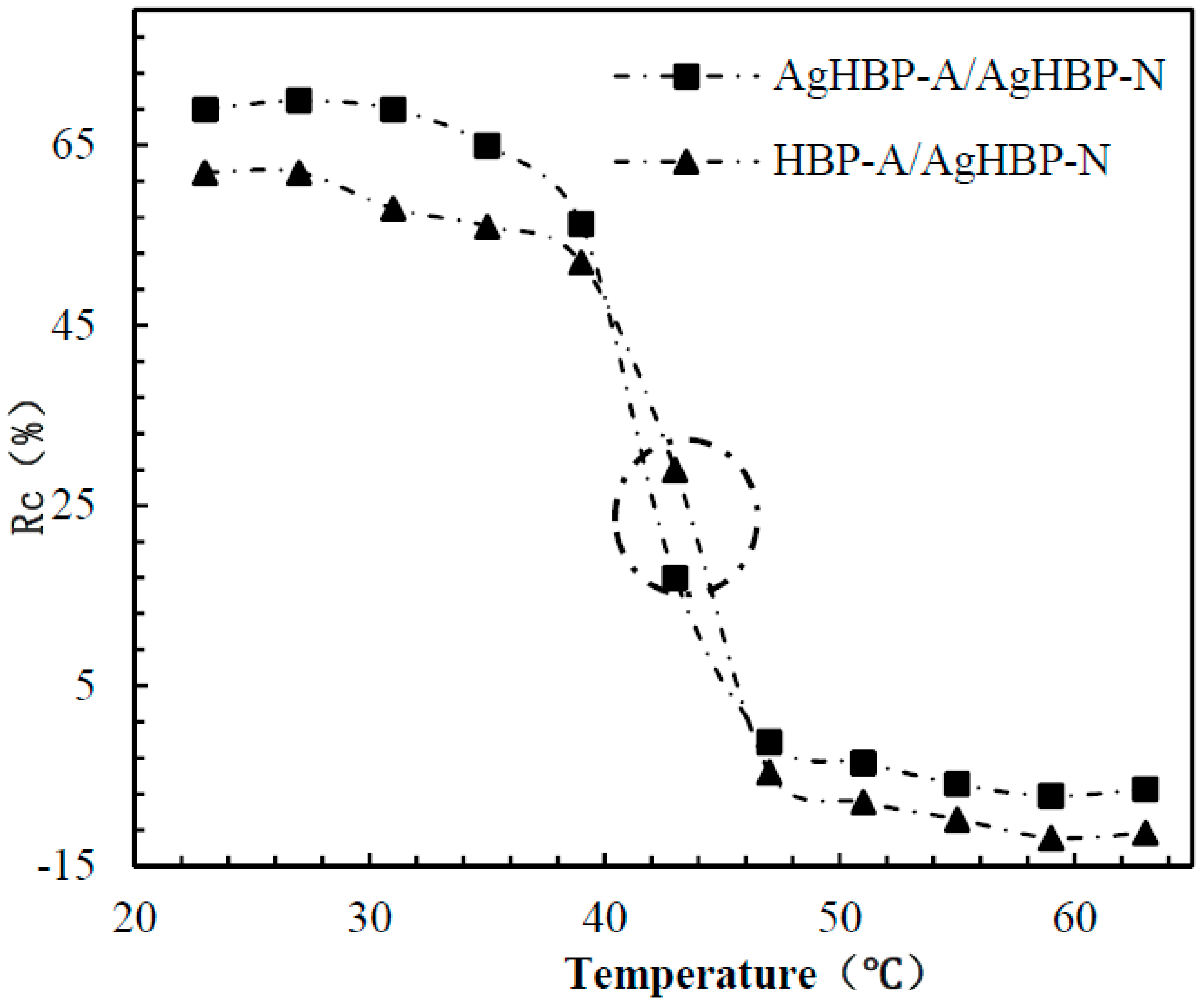
3.3. Evaluation of the Self-Switchable Interaction
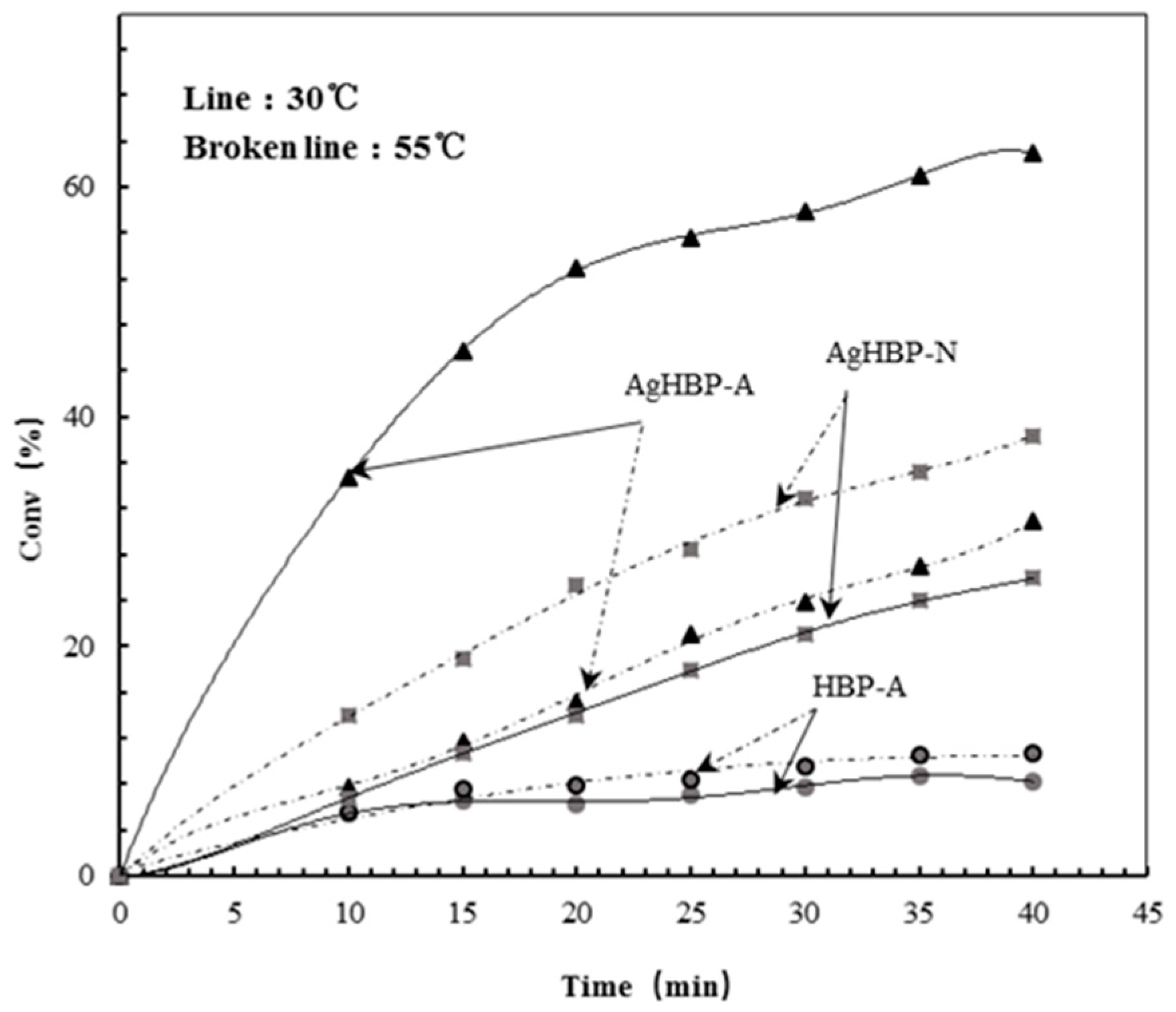
3.4. Switchable Catalysis
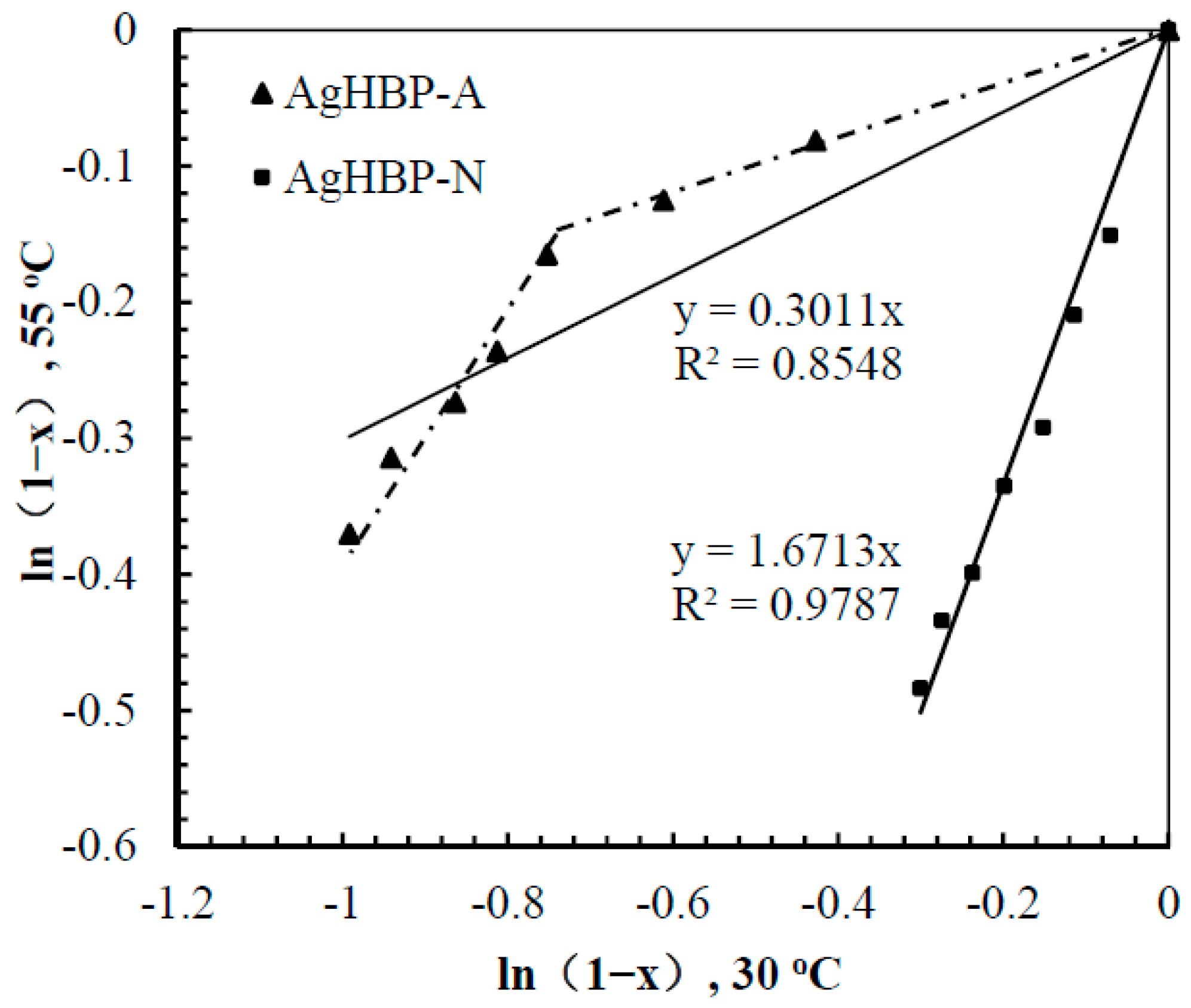
3.5. Kinetic Study of Catalysis
3.6. Dynamic Binding Behavior
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A. Experimental Details
Appendix A.1. Preparation of Polymer Catalysts
Appendix A.2. Synthesis of HBP Intermediate 1
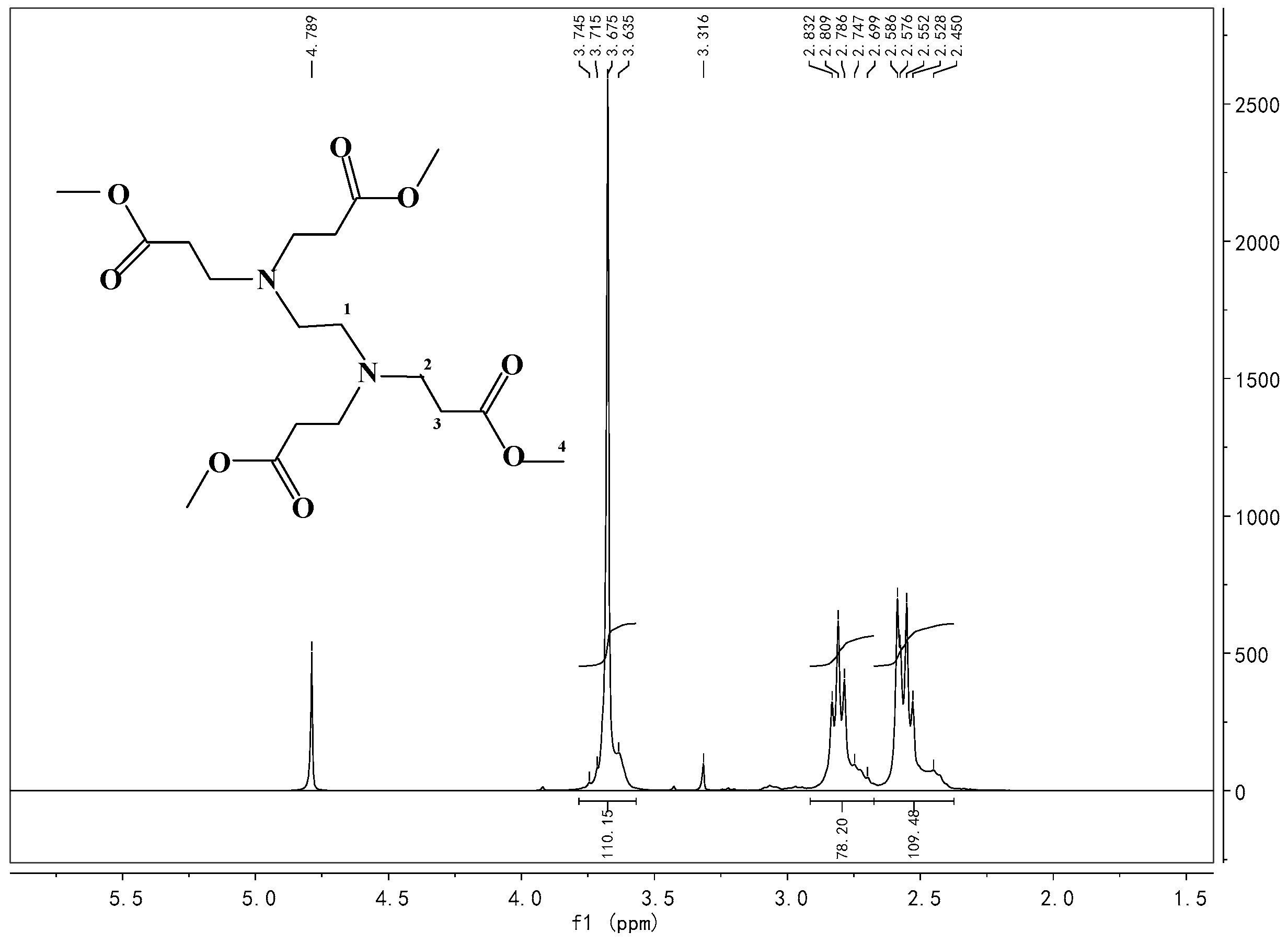
Appendix A.3. Synthesis of HBP Intermediate 2

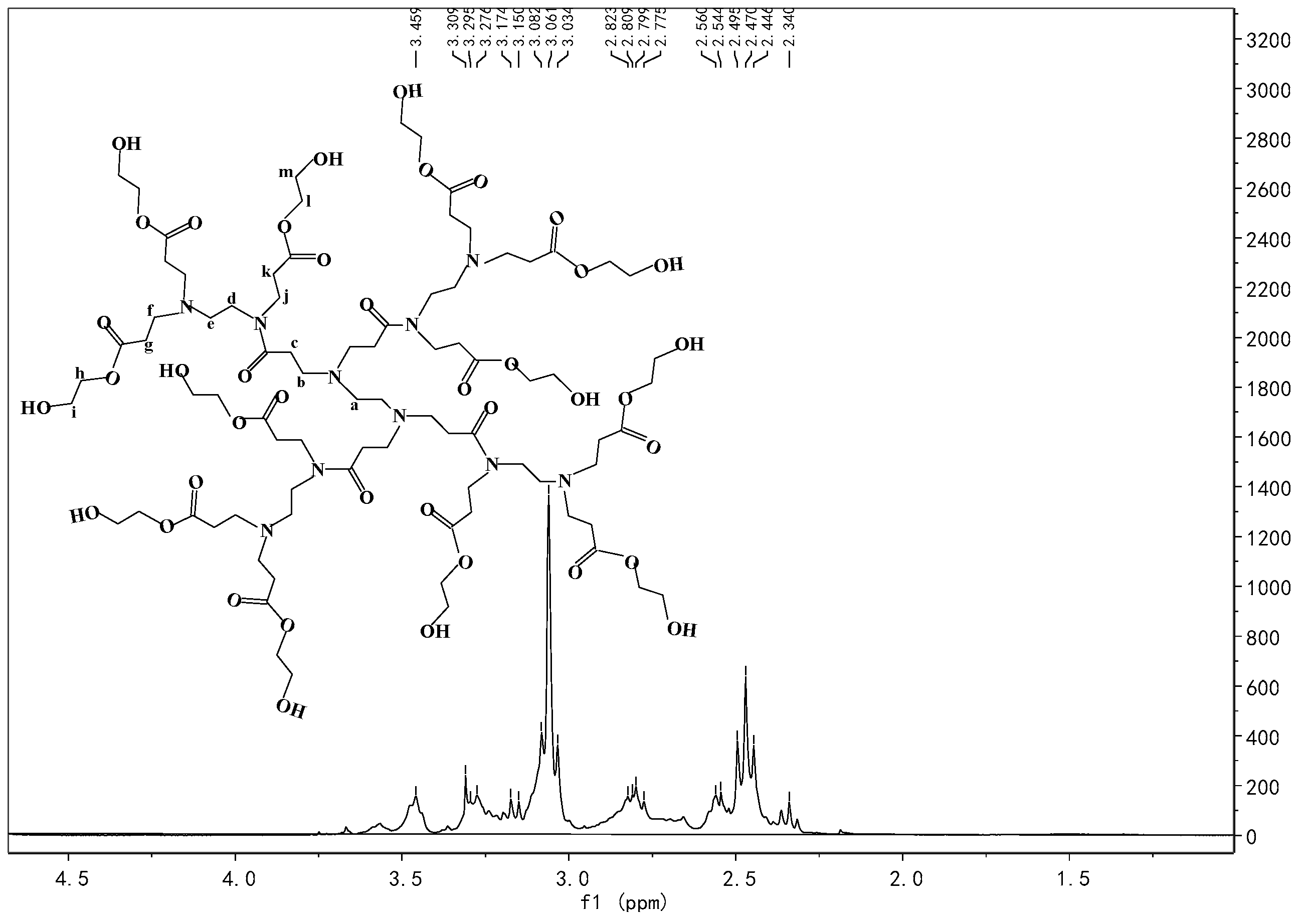
Appendix A.4. Synthesis of Pre-AgHBP-A (HBP-A)

References
- Stavila, V.; Bhakta, R.K.; Alam, T.M.; Majzoub, E.H.; Allendorf, M.D. Reversible hydrogen storage by NaAlH4 confined within a titanium-functionalized MOF-74 (Mg) nanoreactor. ACS Nano 2012, 6, 9807–9817. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Lorenzo, M.; Vaz, B.; Salgueiriño, V.; Correa-Duarte, M.A. Hollow-shelled nanoreactors endowed with high catalytic activity. Chem. Eur. J. 2013, 19, 12196–12211. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Mei, Y.; Drechsler, M.; Ballauff, M. Thermosensitive core-shell particles as carriers for Ag nanoparticles: Modulating the catalytic activity by a phase transition in networks. Angew. Chem. Int. Ed. 2006, 45, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Chen, M.Q.; Serizawa, T.; Akashi, M. In-situ formation of silver nanoparticles on poly (N-isopropylacrylamide)-coated polystyrene microspheres. Adv. Mater. 1998, 10, 1122–1126. [Google Scholar] [CrossRef]
- Nestl, B.M.; Hammer, S.C.; Nebel, B.A.; Hauer, B. New generation of biocatalysts for organic synthesis. Angew. Chem. Int. Ed. 2014, 53, 3070–3095. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Garcia, H. Cascade reactions catalyzed by metal organic frameworks. ChemSusChem 2014, 7, 2392–2410. [Google Scholar] [CrossRef] [PubMed]
- Voit, B. Hyperbranched polymers—All problems solved after 15 years of research? J. Polym. Sci. Part A Polym. Chem. 2005, 43, 2679–2699. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, Y.; Roberts, E.; Duxbury, C.J.; Ding, L.; Irvine, D.J.; Howdle, S.M. Controlling chain growth: A new strategy to hyperbranched materials. Macromolecules 2007, 40, 7184–7194. [Google Scholar] [CrossRef]
- Schulze, A.; Went, M.; Prager, A. Membrane functionalization with hyperbranched polymers. Materials 2016, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Haba, Y.; Harada, A.; Takagishi, T.; Kono, K. Rendering poly (amidoamine) or poly (propylenimine) dendrimers temperature sensitive. J. Am. Chem. Soc. 2004, 126, 12760–12761. [Google Scholar] [CrossRef] [PubMed]
- Aathimanikandan, S.V.; Savariar, E.N.; Thayumanavan, S. Temperature-sensitive dendritic micelles. J. Am. Chem. Soc. 2005, 127, 14922–14929. [Google Scholar] [CrossRef] [PubMed]
- Parrott, M.C.; Marchington, E.B.; Valliant, J.F.; Adronov, A. Synthesis and properties of carborane-functionalized aliphatic polyester dendrimers. J. Am. Chem. Soc. 2005, 127, 12081–12089. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Chen, H.; Zhu, X.; Yan, D. Backbone-thermoresponsive hyperbranched polyethers. J. Am. Chem. Soc. 2006, 128, 8144–8145. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, Q. Facile fabrication of nanoporous Au-Pd bimetallic foams with high catalytic activity for 2-nitrophenol reduction and SERS property. J. Mater. Chem. 2011, 21, 11961–11967. [Google Scholar] [CrossRef]
- Singh, H.P.; Gupta, N.; Sharma, S.K.; Sharma, R.K. Synthesis of bimetallic Pt-Cu nanoparticles and their application in the reduction of rhodamine B. Colloids Surf. A Physicochem. Eng. Asp. 2013, 416, 43–50. [Google Scholar] [CrossRef]
- González Lazo, M.A.; Katrantzis, I.; Dalle Vacche, S.; Karasu, F.; Leterrier, Y. A facile in situ and UV printing process for bioinspired self-cleaning surfaces. Materials 2016, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.R.; Qu, N.; Guo, L.H. Electrochemical displacement method for the investigation of the binding interaction of polycyclic organic compounds with DNA. Anal. Chem. 2008, 80, 3910–3914. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Spyra, P.; Mei, Y.; Ballauff, M.; Pich, A. Composite hydrogels: Robust carriers for catalytic nanoparticles. Macromol. Chem. Phys. 2007, 208, 254–261. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, C.; Meng, D.; Diao, G. In situ synthesis of silver nanostructures on magnetic Fe3O4@C core-shell nanocomposites and their application in catalytic reduction reactions. J. Mater. Chem. A 2013, 1, 2118–2125. [Google Scholar] [CrossRef]
- Li, S.; Ge, Y.; Turner, A.P. A catalytic and positively thermosensitive molecularly imprinted polymer. Adv. Funct. Mater. 2011, 21, 1194–1200. [Google Scholar] [CrossRef]
- Li, S.; Ge, Y.; Tiwari, A.; Cao, S. A Temperature-Responsive Nanoreactor. Small 2010, 6, 2453–2459. [Google Scholar] [CrossRef] [PubMed]
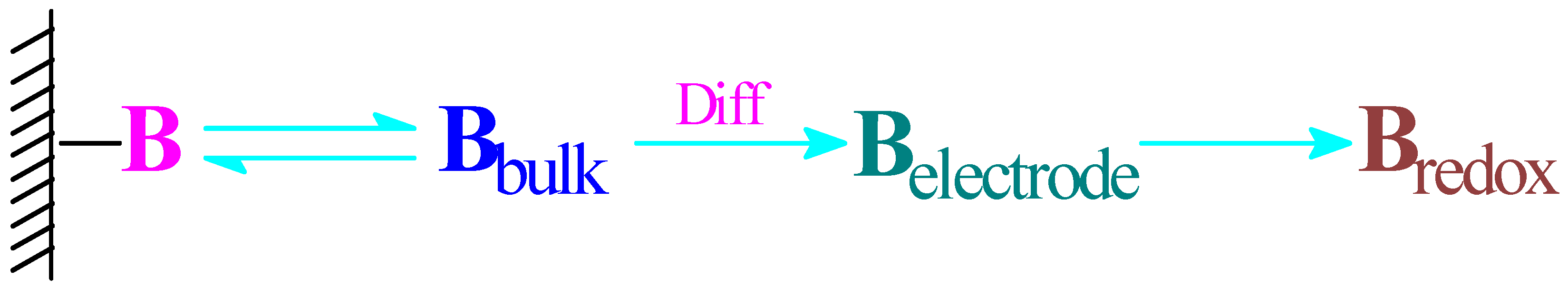
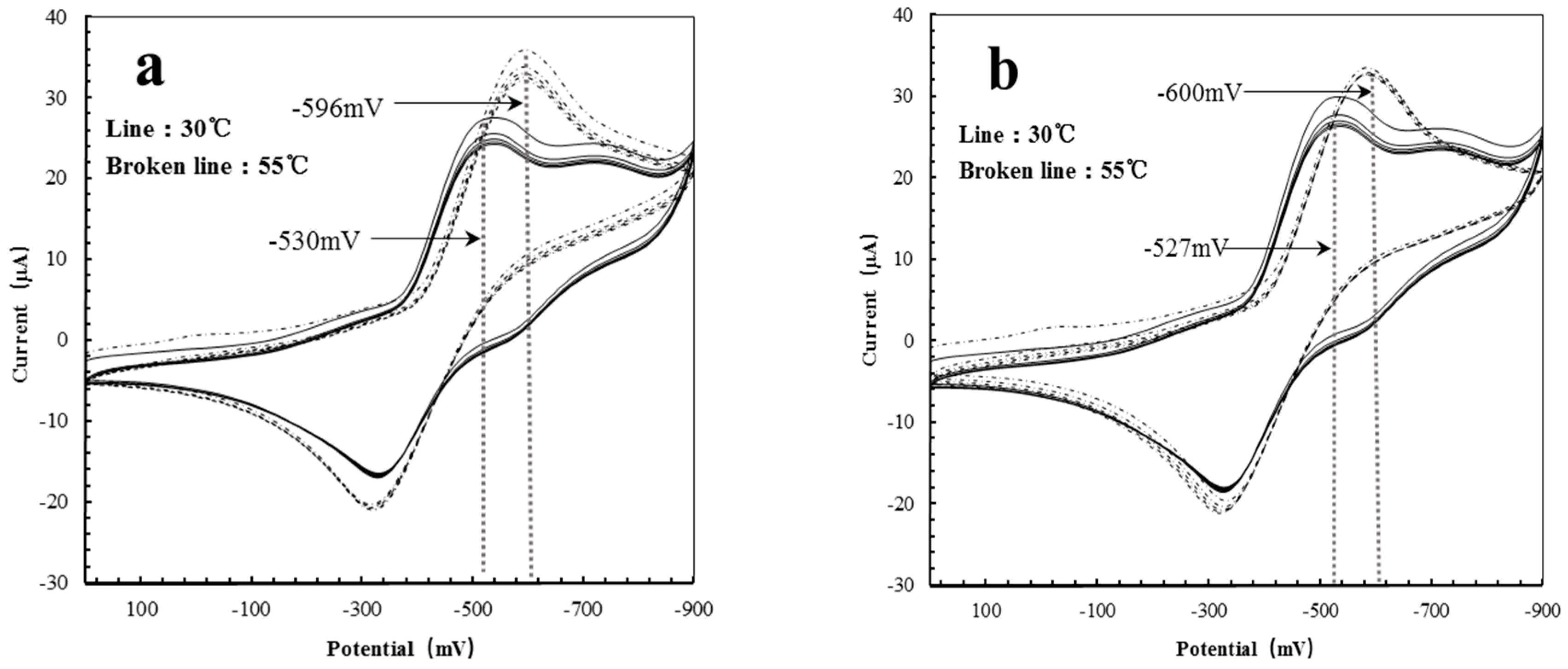
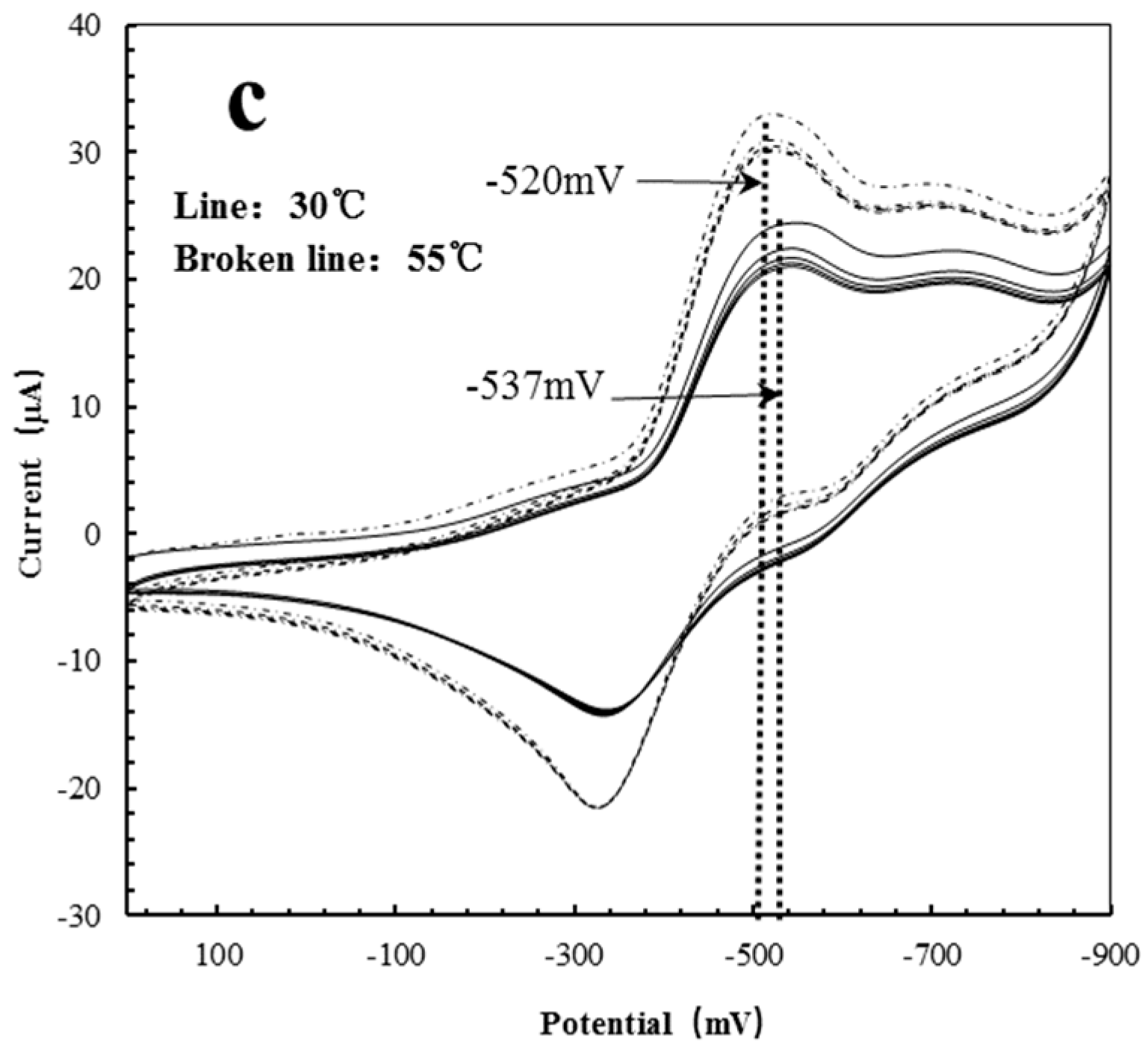
| Polymer Reactors | 30 °C (mV) | 50 °C (mV) | Delta (mV) |
|---|---|---|---|
| AgHBP-A | −530 | −596 | −66 |
| HBP-A | −527 | −600 | −73 |
| AgHBP-N | −537 | −520 | +17 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, R.; Yang, H.; Deng, X.; Jin, L.; Wang, Y.; Li, S. A Self-Switchable Polymer Reactor for Controlled Catalytic Chemistry Processes with a Hyperbranched Structure. Materials 2018, 11, 245. https://doi.org/10.3390/ma11020245
Luo R, Yang H, Deng X, Jin L, Wang Y, Li S. A Self-Switchable Polymer Reactor for Controlled Catalytic Chemistry Processes with a Hyperbranched Structure. Materials. 2018; 11(2):245. https://doi.org/10.3390/ma11020245
Chicago/Turabian StyleLuo, Rong, Hong Yang, Xiaobo Deng, Liqiang Jin, Yulu Wang, and Songjun Li. 2018. "A Self-Switchable Polymer Reactor for Controlled Catalytic Chemistry Processes with a Hyperbranched Structure" Materials 11, no. 2: 245. https://doi.org/10.3390/ma11020245




