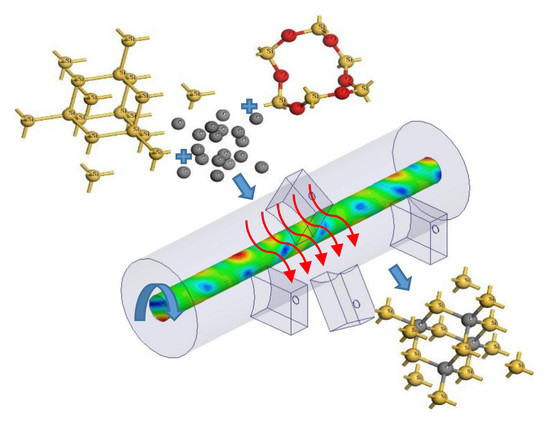
Ultrarapid Multimode Microwave Synthesis of Nano/Submicron β-SiC
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Devices
2.2. SiC Synthesis Using RMMC and CMMC Reactors
2.3. Characterisation
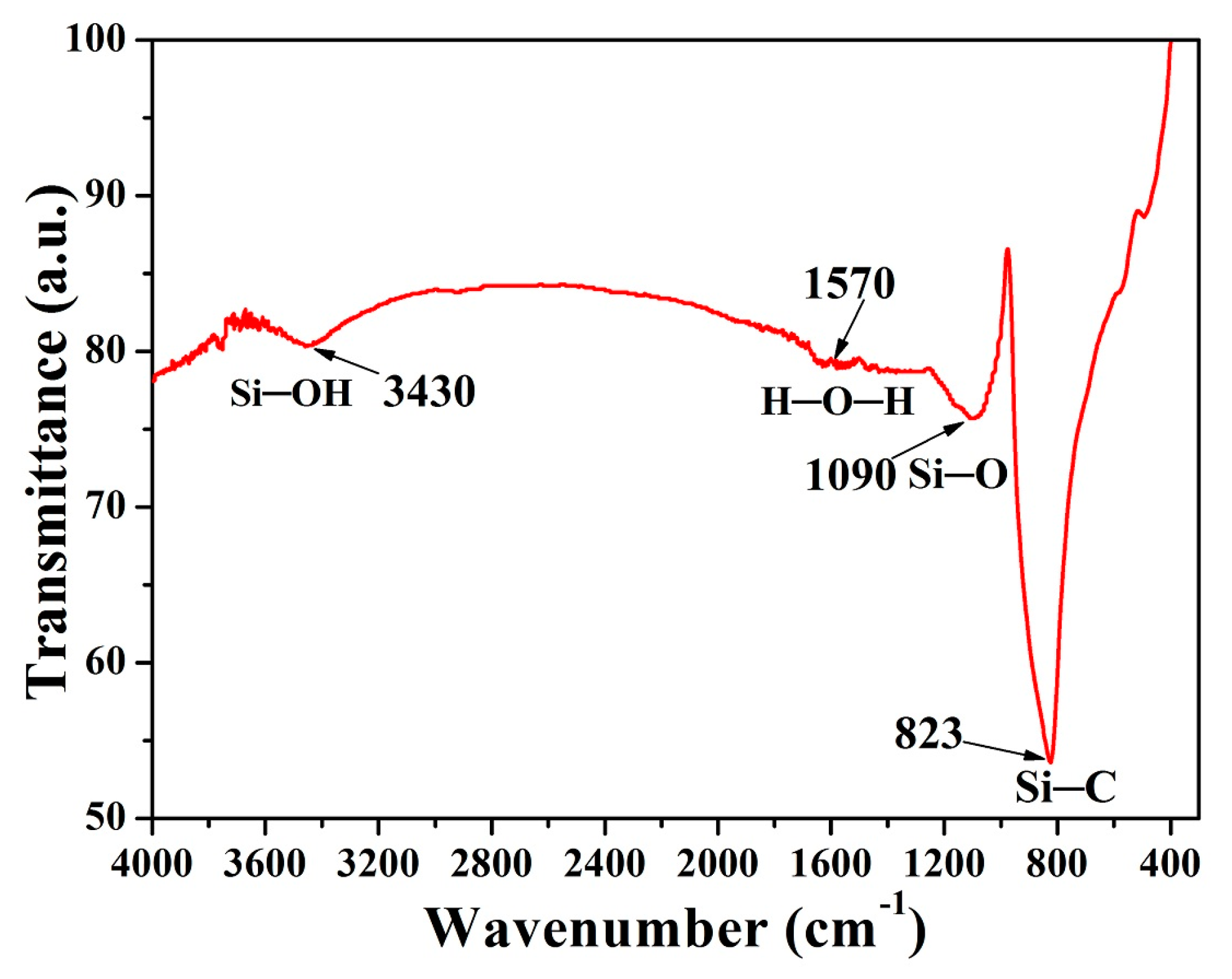
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kitchen, H.J.; Vallance, S.R.; Kennedy, J.L.; Tapia-Ruiz, N.; Carassiti, L.; Harrison, A.; Whittaker, A.G.; Drysdale, T.D.; Kingman, S.W.; Gregory, D.H. Modern microwave methods in solid state inorganic materials chemistry: From fundamentals to manufacturing. Chem. Rev. 2014, 114, 1170–1206. [Google Scholar] [CrossRef] [PubMed]
- Schwenke, A.M.; Stumpf, S.; Hoeppener, S.; Schubert, U.S. Free-standing carbon nanofibrous films prepared by a fast microwave-assisted synthesis process. Adv. Funct. Mater. 2014, 24, 1602–1608. [Google Scholar] [CrossRef]
- Kennedy, J.L.; Drysdale, T.D.; Gregory, D.H. Rapid, energy-efficient synthesis of the layered carbide, Al4C3. Green Chem. 2015, 17, 285–290. [Google Scholar] [CrossRef]
- Birkel, A.; Lee, Y.G.; Koll, D.; Meerbeek, X.V.; Frank, S.; Choi, M.J.; Kang, Y.S.; Char, K.H.; Tremel, W.F. Highly efficient and stable dye-sensitized solar cells based on SnO2 nanocrystals prepared by microwave-assisted synthesis. Energy Environ. Sci. 2012, 5, 5392–5400. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, W.C.; Liu, Y.C.; Zeng, L.K.; Sun, L.Y. The Microwave-Assisted Green Synthesis of TiC Powders. Materials 2016, 9, 904. [Google Scholar] [CrossRef] [PubMed]
- Moshtaghioun, B.M.; Poyato, R.; Cumbrera, F.L.; de Bernardi-Martin, S.; Monshi, A.; Abbasi, M.H.; Karimzadeh, F.; Dominguez-Rodriguez, A. Rapid carbothermic synthesis of silicon carbide nano powders by using microwave heating. J. Eur. Ceram. Soc. 2012, 32, 1787–1794. [Google Scholar] [CrossRef]
- Van Laar, J.H.; Slabber, J.F.M.; Meyer, J.P.; van der Walt, I.J.; Puts, G.J.; Crouse, P.L. Microwave-plasma synthesis of nano-sized silicon carbide at atmospheric pressure. Ceram. Int. 2015, 16, 4326–4333. [Google Scholar] [CrossRef]
- Zhao, M.; Johnson, M.; He, W.Z.; Li, G.M.; Zhao, C.; Huang, J.W.; Zhu, H.C. Transformation of waste crystalline silicon into submicro β-SiC by multimode microwave sintering with low carbon emissions. Powder Technol. 2017, 322, 290–295. [Google Scholar] [CrossRef]
- Omidi, Z.; Ghasemi, A.; Bakhshi, S.R. Synthesis and characterization of SiC ultrafine particles by means of sol-gel and carbothermal reduction methods. Ceram. Int. 2015, 41, 5779–5784. [Google Scholar] [CrossRef]
- Moskovskikh, D.O.; Song, Y.; Rouvimov, S.; Rogachev, A.S.; Mukasyan, A.S. Silicon carbide ceramics: Mechanical activation, combustion and spark plasma sintering. Ceram. Int. 2016, 42, 12686–12693. [Google Scholar] [CrossRef]
- Kuang, J.L.; Cao, W.B.; Elder, S. Synthesis of α-SiC particles at 1200 °C by microwave heating. Powder Technol. 2013, 247, 106–111. [Google Scholar] [CrossRef]
- Nam, D.H.; Kim, B.-G.; Yoon, J.-Y.; Lee, M.-H.; Seo, W.-S.; Jeong, S.-M.; Yang, C.-W.; Lee, W.-J. High-Temperature Chemical Vapor Deposition for SiC Single Crystal Bulk Growth Using Tetramethylsilane as a Precursor. Cryst. Growth Des. 2014, 14, 5569–5574. [Google Scholar] [CrossRef]
- Aldalbahi, A.; Li, E.; Rivera, M.; Velazquez, R.; Altalhi, T.; Peng, X.Y.; Feng, P.X. A new approach for fabrications of SiC based photodetectors. Sci. Rep. 2016, 6, 23457. [Google Scholar] [CrossRef] [PubMed]
- Pushpakaran, B.N.; Subburaj, A.S.; Bayne, S.B.; Mookken, J. Impact of silicon carbide semiconductor technology in Photovoltaic Energy System. Renew. Sustain. Energy Rev. 2016, 55, 971–989. [Google Scholar] [CrossRef]
- Seo, Y.-K.; Eom, J.-H.; Kim, Y.-W. Process-tolerant pressureless-sintered silicon carbide ceramics with alumina-yttria-calcia-strontia. J. Eur. Ceram. Soc. 2018, 38, 445–452. [Google Scholar] [CrossRef]
- Chen, J.H.; Liu, W.N.; Yang, T.; Lin, B.; Su, J.D.; Hou, X.M.; Chou, K.C. A Facile Synthesis of a Three-Dimensional Flexible 3C-SiC Sponge and Its Wettability. Cryst. Growth Des. 2014, 14, 4624–4630. [Google Scholar] [CrossRef]
- Xie, W.; Möbus, G.; Zhang, S.W. Molten salt synthesis of silicon carbide nanorods using carbon nanotubes as templates. J. Mater. Chem. 2011, 21, 18325–18330. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Zhang, X.Y.; Zhang, Z.Z.; Tong, Y.C.; Li, F.Y.; Wu, J.C.S.; Wang, X.X. Open mouthed β-SiC hollow-sphere with highly photocatalytic activity for reduction of CO2 with H2O. Appl. Catal. B 2017, 206, 158–167. [Google Scholar] [CrossRef]
- Pawbake, A.; Mayabadi, A.; Waykar, R.; Kulkarni, R.; Jadhavar, A.; Waman, V.; Parmar, J.; Bhattacharyya, S.; Ma, Y.R.; Devan, R.; et al. Growth of boron doped hydrogenated nanocrystalline cubic silicon carbide (3C-SiC) films by Hot Wire-CVD. Mater. Res. Bull. 2016, 76, 205–215. [Google Scholar] [CrossRef]
- Wang, S.H.; Wang, T.; Druzhinin, S.I.; Wesner, D.; Jiang, X.; Schönherr, H. Detailed Study of BSA Adsorption on Micro-and Nanocrystalline Diamond/β-SiC Composite Gradient Films by Time-Resolved Fluorescence Microscopy. Langmuir 2017, 33, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, J.; Li, Z.J.; Yu, H.Y.; Wang, Y.Q.; Meng, A.; Li, Q.D. Bamboo-like 3C-SiC nanowires with periodical fluctuating diameter: Homogeneous synthesis, synergistic growth mechanism, and their luminescence properties. J. Solid State Chem. 2016, 243, 247–252. [Google Scholar] [CrossRef]
- Mukasyan, A.S.; Rogachev, A.S. Combustion synthesis: Mechanically induced nanostructured materials. J. Mater. Sci. 2017, 52, 11826–11833. [Google Scholar] [CrossRef]
- Wang, P.; Cheng, L.F.; Zhang, Y.N.; Zhang, L.T. Synthesis of SiC nano bers with superior electromagnetic wave absorption performance by electrospinning. J. Alloys Compd. 2017, 716, 306–320. [Google Scholar] [CrossRef]
- Chen, J.J.; Ding, L.J.; Xin, L.P.; Zeng, F.; Chen, J. Thermochemistry and growth mechanism of SiC nanowires. J. Solid State Chem. 2017, 253, 282–286. [Google Scholar] [CrossRef]
- Kang, P.C.; Zhang, B.; Wu, G.H.; Gou, H.S.; Chen, G.Q.; Jiang, L.T.; Mula, S.H. Synthesis of β-SiC nanowires by ball milled nanoparticles of silicon and carbon. J. Alloys Compd. 2014, 604, 304–308. [Google Scholar] [CrossRef]
- Yazdanfar, M.; Pedersen, H.; Sukkaew, P.; Ivanov, I.G.; Danielsson, Ö.; Kordina, O.; Janzén, E. On the use of methane as a carbon precursor in Chemical Vapor Deposition of silicon carbide. J. Cryst. Growth 2014, 390, 24–29. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, Q.F.; Tu, R.; Goto, T.; Zhang, L.M. Growth Mechanism and Defects of <111>-Oriented β-SiC Films Deposited by Laser Chemical Vapor Deposition. J. Am. Ceram. Soc. 2015, 98, 236–241. [Google Scholar] [CrossRef]
- Su, J.J.; Gao, B.; Chen, Z.D.; Fu, J.J.; An, W.; Peng, X.; Zhang, X.M.; Wang, L.; Huo, K.F.; Chu, P.K. Large-Scale Synthesis and Mechanism of β-SiC Nanoparticles from Rice Husks by Low-Temperature Magnesiothermic Reduction. ACS Sustain. Chem. Eng. 2016, 4, 6600–6607. [Google Scholar] [CrossRef]
- Agathopoulos, S. Combustion synthesis of ultra-fine SiC powders in low pressure N2-atmosphere. Ceram. Int. 2012, 38, 4165–4171. [Google Scholar] [CrossRef]
- Meng, G.W.; Zhang, L.D.; Qin, Y.; Mo, C.M.; Phillipp, F. Synthesisof β-SiC nanowires with SiO2 wrappers. Nanostruct. Mater. 1999, 12, 1003–1006. [Google Scholar] [CrossRef]
- Meng, A.; Li, Z.J.; Zhang, J.L.; Gao, L.; Li, H.J. Synthesis and Raman scattering of β-SiC/SiO2 core-shell nanowires. J. Cryst. Growth 2007, 308, 263–268. [Google Scholar] [CrossRef]
- Seo, Y.-K.; KIM, Y.-W.; Nishimura, T.; Seo, W.-K. High thermal conductivity of spark plasma sintered silicon carbide ceramics with yttria and scandia. J. Am. Ceram. Soc. 2017, 100, 1290–1294. [Google Scholar] [CrossRef]
- Chabi, S.; Rocha, V.G.; Tuñón, E.G.; Ferraro, C.; Saiz, E.; Xia, Y.D.; Zhu, Y.Q. Ultralight, Strong, Three-Dimensional SiC Structures. ACS Nano 2016, 10, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.K.; Zhang, Y.Z.; Li, J.Y.; Zhang, H.J.; Song, S.P.; Zhang, S.W. Catalytic effect of cobalt on microwave synthesis of β-SiC powder. Powder Technol. 2017, 317, 209–215. [Google Scholar] [CrossRef]
- Jin, H.Q.; Dai, S.S.; Huang, K.M. Microwave Chemistry; Science Press: Beijing, China, 1999. [Google Scholar]
- Zhang, J.; Liu, X.H.; Jia, Q.L.; Huang, J.T.; Zhang, S.W. Novel synthesis of ultra-long single crystalline β-SiC nanofibers with strong blue/green luminescent properties. Ceram. Int. 2016, 42, 4600–4606. [Google Scholar] [CrossRef]
- Zhang, M. Quasi-monodisperse β-SiC nanospheres: Synthesis and application in chemical-mechanical polishing. J. Phys. Chem. Solids 2017, 103, 1–5. [Google Scholar] [CrossRef]
- Wang, F.; Cao, W.B.; Sun, J.L.; He, R.L. Synthesis and Preparation of SiC Powders at Low Temperature. Mater. Rev. 2008, 22, 126–128. [Google Scholar]
- Wang, Y.X.; Tan, S.H.; Jiang, D.L. Research and Development of Reaction Sintered Silicon Carbide. J. Inorg. Mater. 2004, 19, 456–462. [Google Scholar]
- Carassiti, L.; Jones, A.; Harrison, P.; Dobson, P.S.; Kingman, S.; MacLaren, I.; Gregory, D.H. Ultra-rapid, sustainable and selective synthesis of silicon carbide powders and nanomaterials via microwave heating. Energy Environ. Sci. 2011, 4, 1503–1510. [Google Scholar] [CrossRef]
- Li, Z.B.; Wang, Y.G.; An, L.N. Control of the thermal conductivity of SiC by modifying the polymer precursor. J. Eur. Ceram. Soc. 2017, 37, 61–67. [Google Scholar] [CrossRef]
- Fritz, G.; Matern, E. Carbosilanes: Syntheses and Reactions; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; Tokyo, Japan, 1986. [Google Scholar]
- Wu, R.B.; Yang, G.Y.; Pan, Y.; Chen, J.J.; Zhai, R.; Wu, L.L.; Lin, J. Prism-shaped SiC nanowhiskers. J. Alloys Compd. 2008, 453, 241–246. [Google Scholar] [CrossRef]
- Kumagai, T.; Izumi, S.; Hara, S.; Sakai, S. Development of bond-order potentials that can reproduce the elastic constants and melting point of silicon for classical molecular dynamics simulation. Comput. Mater. Sci. 2007, 39, 457–464. [Google Scholar] [CrossRef]
- Kim, Y.-W.; Lee, Y.-I.; Mitomo, M. Sinterability of Nano-Sized Silicon Carbide Powders. J. Ceram. Soc. Jpn. 2006, 114, 681–685. [Google Scholar] [CrossRef]
- Vix-Guterl, C.; Alix, I.; Gibot, P.; Ehrburger, P. Formation of tubular silicon carbide from a carbonesilica material by using a reactive replica technique: Infra-red characterization. Appl. Surf. Sci. 2003, 210, 329–337. [Google Scholar] [CrossRef]
- Zhou, L.J.; Huang, Y.; Xie, Z.P. Gelcasting of concentrated aqueous silicon carbide suspension. J. Eur. Ceram. Soc. 2000, 20, 85–90. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Jia, Q.L.; Lin, L.X.; Huang, J.T.; Zhang, S.W. Molten salt assisted synthesis of 3C–SiC nanowire and its photoluminescence properties. Ceram. Int. 2015, 41, 12614–12620. [Google Scholar] [CrossRef]
- Wu, R.B.; Zhou, K.; Yue, C.Y.; Wei, J.; Pan, Y. Recent progress in synthesis, properties and potential applications of SiC nanomaterials. Prog. Mater. Sci. 2015, 72, 1–60. [Google Scholar] [CrossRef]
- Yang, K.; Yang, Y.; Lin, Z.M.; Li, J.T.; Du, J.S. Mechanical-activation-assisted combustion synthesis of SiC powders with polytetrafluoroethylene as promoter. Mater. Res. Bull. 2007, 42, 1625–1632. [Google Scholar] [CrossRef]







| Sample Number | A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | A9 | A10 | |
| a | Silicon source | SiO2 | SiO2 | SiO2 | SiO2 | SiO2 | Si | Si | Si | Si | Si |
| Irradiation time (min) | 3 | 4 | 5 | 7 | 12 | 1 | 3 | 6 | 8 | 12 | |
| Sample Number | B1 | B2 | B3 | B4 | B5 | B6 | B7 | B8 | B9 | B10 | |
| b | Silicon source | SiO2 | SiO2 | SiO2 | SiO2 | SiO2 | Si | Si | Si | Si | Si |
| Irradiation time (min) | 2 | 4 | 5 | 7 | 10 | 1 | 4 | 5 | 6 | 8 | |
| Sample Number | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | |
| c | Silicon source | SiO2 | SiO2 | SiO2 | SiO2 | Si | Si | Si | Si |
| Irradiation time (s) | 50 | 70 | 100 | 120 | 20 | 40 | 70 | 100 | |
| Sample Number | D1 | D2 | D3 | D4 | D5 | D6 | D7 | D8 | |
| d | Silicon source | SiO2 | SiO2 | SiO2 | SiO2 | Si | Si | Si | Si |
| Irradiation time (s) | 50 | 70 | 80 | 100 | 20 | 30 | 50 | 100 | |
| Sample | A8 | B9 | B10 | D5 |
|---|---|---|---|---|
| Phases, wt % | β-SiC: 98.1(6.3)%; Si: 1.9(0.1)%; | β-SiC: 100(7.5)%; | β-SiC: 95.4(4.3)%; Graphite: 4.6(0.2)% | β-SiC: 100(7.4)%; |
| Cell formula units/Z | 4 | 4 | 4 | 4 |
| α-Parameter/Å | 4.3556 | 4.3584 | 4.3602 | 4.3600 |
| Unit cell vol/Å3 | 82.63 | 82.79 | 82.89 | 82.88 |
| Calculated density, ρ/g cm−3 | 3.233 | 3.217 | 3.213 | 3.213 |
| Residue factor/R | 18.66 | 18.98 | 19.69 | 17.77 |
| Residue factor/Rp | 2.52 | 3.07 | 2.73 | 2.57 |
| Residue factor/Rwp | 3.68 | 4.53 | 4.16 | 3.81 |
| Residue factor/Rexp | 3.14 | 4.40 | 3.77 | 3.41 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Johnson, M.; He, W.; Li, G.; Zhao, C.; Yu, L.; Huang, J.; Zhu, H. Ultrarapid Multimode Microwave Synthesis of Nano/Submicron β-SiC. Materials 2018, 11, 317. https://doi.org/10.3390/ma11020317
Zhao M, Johnson M, He W, Li G, Zhao C, Yu L, Huang J, Zhu H. Ultrarapid Multimode Microwave Synthesis of Nano/Submicron β-SiC. Materials. 2018; 11(2):317. https://doi.org/10.3390/ma11020317
Chicago/Turabian StyleZhao, Min, Michael Johnson, Wenzhi He, Guangming Li, Chen Zhao, Luling Yu, Juwen Huang, and Haochen Zhu. 2018. "Ultrarapid Multimode Microwave Synthesis of Nano/Submicron β-SiC" Materials 11, no. 2: 317. https://doi.org/10.3390/ma11020317