Effect of Silver-Emitting Filler on Antimicrobial and Mechanical Properties of Soft Denture Lining Material
Abstract
:1. Introduction
2. Results
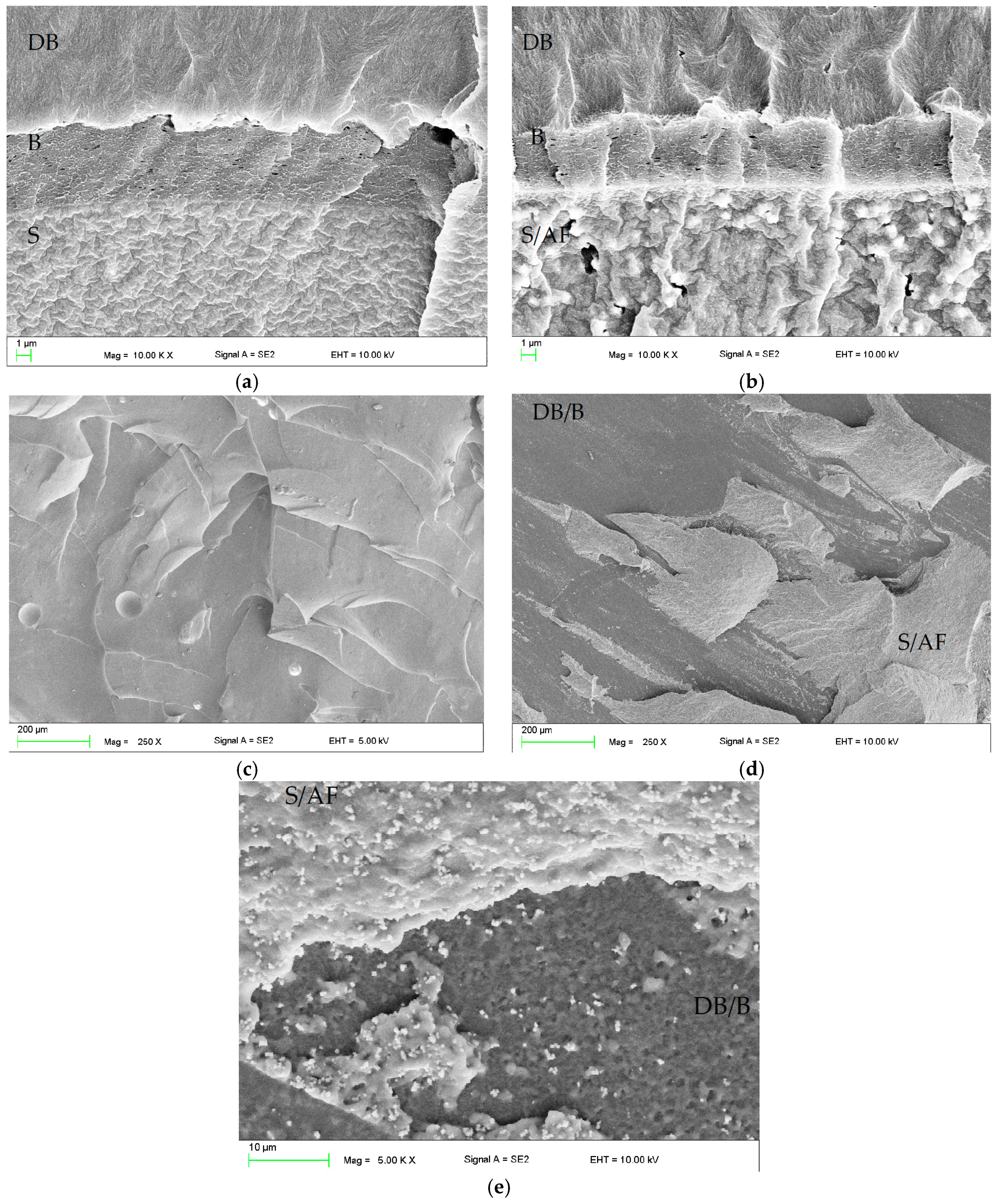
2.1. Scanning Electron Microscopy (SEM) Investigations
2.2. Microbiological Tests
2.3. Shore A Hardness
2.4. Tensile Strength
2.5. Tensile Bond Strength
2.6. Sorption and Solubility
3. Discussion
4. Materials and Methods
4.1. Materials Preparation
4.2. SEM Investigations
4.3. Microbiological Tests
4.4. Hardness Tests
4.5. Tensile Strength Tests
4.6. Tensile Bond Strength Tests
4.7. Sorption and Solubility
4.8. Statistical Analyzes
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Braden, M.; Wright, P.S.; Parker, S. Soft lining materials—A review. Eur. J. Prosthodont. Restor. Dent. 1995, 3, 163–174. [Google Scholar] [PubMed]
- Santawisuk, W.; Kanchanavasita, W.; Sirisinha, C.; Harnirattisai, C. Dynamic viscoelastic properties of experimental silicone soft lining materials. Dent. Mater. J. 2010, 29, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Chladek, G.; Żmudzki, J.; Kasperski, J. Long-Term Soft Denture Lining Materials. Materials 2014, 7, 5816–5842. [Google Scholar] [CrossRef] [PubMed]
- Mack, P.J. Denture soft lining materials: Clinical indications. Aust. Dent. J. 1989, 34, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Hashem, M.I. Advances in Soft Denture Liners: An Update. J. Contemp. Dent. Pract. 2015, 16, 314–318. [Google Scholar] [CrossRef] [PubMed]
- ELsyad, M.A.; Shaheen, N.H.; Ashmawy, T.M. Long-term clinical and prosthetic outcomes of soft liner and clip attachments for bar/implant overdentures: A randomised controlled clinical trial. J. Oral Rehabil. 2017, 44, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Chladek, G.; Wrzuś-Wieliński, M. The evaluation of selected attachment systems for implant-retained overdenture based on retention characteristics analysis. Acta Bioeng. Biomech. 2010, 12, 75–83. [Google Scholar] [PubMed]
- Anusavice, K.; Shen, C.; Rawls, H.R. Phillips’ Science of Dental Materials, 12th ed.; Saunders: St. Louis, MO, USA, 2013; ISBN 978-0-323-24205-9. [Google Scholar]
- Kimoto, S.; Kimoto, K.; Gunji, A.; Kawai, Y.; Murakami, H.; Tanaka, K.; Syu, K.; Aoki, H.; Tani, M.; Toyoda, M.; Kobayashi, K. Effects of resilient denture liner in mandibular complete denture on the satisfaction ratings of patients at the first appointment following denture delivery. Nihon Hotetsu Shika Gakkai Zasshi 2008, 52, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Kimoto, S.; Kimoto, K.; Gunji, A.; Kawai, Y.; Murakami, H.; Tanaka, K.; Syu, K.; Aoki, H.; Toyoda, M.; Kobayashi, K. Clinical effects of acrylic resilient denture liners applied to mandibular complete dentures on the alveolar ridge. J. Oral Rehabil. 2007, 34, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Pisani, M.X.; Malheiros-Segundo, A.D.L.; Balbino, K.L.; Souza, R.D.F.; Paranhos, H.D.F.O.; Lovato da Silva, C.H. Oral health related quality of life of edentulous patients after denture relining with a silicone-based soft liner. Gerodontology 2012, 29, e474–e480. [Google Scholar] [CrossRef] [PubMed]
- Palla, E.S.; Karaoglani, E.; Naka, O.; Anastassiadou, V. Soft denture liners’ effect on the masticatory function in patients wearing complete dentures: A systematic review. J. Dent. 2015, 43, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Mutluay, M.M.; Oguz, S.; Fløystrand, F.; Saxegaard, E.; Dogan, A.; Bek, B.; Ruyter, I.E. A prospective study on the clinical performance of polysiloxane soft liners: One-year results. Dent. Mater. J. 2008, 27, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Boscato, N.; Radavelli, A.; Faccio, D.; Loguercio, A.D. Biofilm formation of Candida albicans on the surface of a soft denture-lining material. Gerodontology 2009, 26, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.-B.; Lim, Y.; Youn, H.-I.; Chang, B.M.; Lee, J.-Y.; Shin, S.-W. Effect of denture cleansers on Candida albicans biofilm formation over resilient liners. J. Adv. Prosthodont. 2014, 6, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Nikawa, H.; Jin, C.; Makihira, S.; Egusa, H.; Hamada, T.; Kumagai, H. Biofilm formation of Candida albicans on the surfaces of deteriorated soft denture lining materials caused by denture cleansers in vitro. J. Oral Rehabil. 2003, 30, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Nikawa, H.; Jin, C.; Hamada, T.; Murata, H. Interactions between thermal cycled resilient denture lining materials, salivary and serum pellicles and Candida albicans in vitro. Part I. Effects on fungal growth. J. Oral Rehabil. 2000, 27, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Nikawa, H.; Jin, C.; Hamada, T.; Makihira, S.; Kumagai, H.; Murata, H. Interactions between thermal cycled resilient denture lining materials, salivary and serum pellicles and Candida albicans in vitro. Part II. Effects on fungal colonization. J. Oral Rehabil. 2000, 27, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Rodger, G.; Taylor, R.L.; Pearson, G.J.; Verran, J. In vitro colonization of an experimental silicone by Candida albicans. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Nevzatoğlu, E.U.; Ozcan, M.; Kulak-Ozkan, Y.; Kadir, T. Adherence of Candida albicans to denture base acrylics and silicone-based resilient liner materials with different surface finishes. Clin. Oral Investig. 2007, 11, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Tari, B.F.; Nalbant, D.; Dogruman Al, F.; Kustimur, S. Surface roughness and adherence of Candida albicans on soft lining materials as influenced by accelerated aging. J. Contemp. Dent. Pract. 2007, 8, 18–25. [Google Scholar] [PubMed]
- Taylor, R.L.; Bulad, K.; Verran, J.; McCord, J.F. Colonization and deterioration of soft denture lining materials in vivo. Eur. J. Prosthodont. Restor. Dent. 2008, 16, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Pavan, S.; dos Santos, P.H.; Filho, J.N.A.; Spolidorio, D.M.P. Colonisation of soft lining materials by micro-organisms. Gerodontology 2010, 27, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Skupien, J.A.; Valentini, F.; Boscato, N.; Pereira-Cenci, T. Prevention and treatment of Candida colonization on denture liners: A systematic review. J. Prosthet. Dent. 2013, 110, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Bulad, K.; Taylor, R.L.; Verran, J.; McCord, J.F. Colonization and penetration of denture soft lining materials by Candida albicans. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2004, 20, 167–175. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Hallikerimath, R.B. An In-vitro Evaluation of Retention, Colonization and Penetration of Commonly Used Denture Lining Materials By Candida albicans. J. Clin. Diagn. Res. JCDR 2016, 10, ZC84–ZC88. [Google Scholar] [CrossRef] [PubMed]
- Nawasrah, A.; AlNimr, A.; Ali, A.A. Antifungal Effect of Henna against Candida albicans Adhered to Acrylic Resin as a Possible Method for Prevention of Denture Stomatitis. Int. J. Environ. Res. Public. Health 2016, 13, 520. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Han, Q.; Zhou, X.; Zhang, K.; Wang, S.; Xu, H.H.K.; Weir, M.D.; Feng, M.; Li, M.; Peng, X.; et al. Heat-Polymerized Resin Containing Dimethylaminododecyl Methacrylate Inhibits Candida albicans Biofilm. Materials 2017, 10, 431. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Al-Kheraif, A.A.; Kellesarian, S.V.; Vohra, F.; Romanos, G.E. Oral Candida carriage and species prevalence in denture stomatitis patients with and without diabetes. J. Biol. Regul. Homeost. Agents 2017, 31, 343–346. [Google Scholar] [PubMed]
- Mothibe, J.V.; Patel, M. Pathogenic characteristics of Candida albicans isolated from oral cavities of denture wearers and cancer patients wearing oral prostheses. Microb. Pathog. 2017, 110, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Gleiznys, A.; Zdanavičienė, E.; Žilinskas, J. Candida albicans importance to denture wearers. A literature review. Stomatologija 2015, 17, 54–66. [Google Scholar] [PubMed]
- Al-hebshi, N.; Al-haroni, M.; Skaug, N. In vitro antimicrobial and resistance-modifying activities of aqueous crude khat extracts against oral microorganisms. Arch. Oral Biol. 2006, 51, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Iyer, M.; Gujjari, A.K.; Gowda, V.; Angadi, S. Antifungal response of oral-associated candidal reference strains (American Type Culture Collection) by supercritical fluid extract of nutmeg seeds for geriatric denture wearers: An in vitro screening study. J. Indian Prosthodont. Soc. 2017, 17, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, C.A.; Wataha, J.C.; Cibirka, R.M.; Schuster, G.S.; Parr, G.R. Effects of triclosan on the cytotoxicity and fungal growth on a soft denture liner. J. Prosthet. Dent. 2001, 85, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Price, C.; Waters, M.G.J.; Williams, D.W.; Lewis, M.A.O.; Stickler, D. Surface modification of an experimental silicone rubber aimed at reducing initial candidal adhesion. J. Biomed. Mater. Res. 2002, 63, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Garner, S.J.; Nobbs, A.H.; McNally, L.M.; Barbour, M.E. An antifungal coating for dental silicones composed of chlorhexidine nanoparticles. J. Dent. 2015, 43, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Chladek, G.; Mertas, A.; Barszczewska-Rybarek, I.; Nalewajek, T.; Żmudzki, J.; Król, W.; Łukaszczyk, J. Antifungal Activity of Denture Soft Lining Material Modified by Silver Nanoparticles—A Pilot Study. Int. J. Mol. Sci. 2011, 12, 4735–4744. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Chu, L.; Rawls, H.R.; Norling, B.K.; Cardenas, H.L.; Whang, K. Development of an antimicrobial resin—A pilot study. Dent. Mater. 2011, 27, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Nuñez-Anita, R.E.; Acosta-Torres, L.S.; Vilar-Pineda, J.; Martínez-Espinosa, J.C.; de la Fuente-Hernández, J.; Castaño, V.M. Toxicology of antimicrobial nanoparticles for prosthetic devices. Int. J. Nanomed. 2014, 9, 3999–4006. [Google Scholar] [CrossRef]
- Chladek, G.; Basa, K.; Mertas, A.; Pakieła, W.; Żmudzki, J.; Bobela, E.; Król, W. Effect of Storage in Distilled Water for Three Months on the Antimicrobial Properties of Poly(methyl methacrylate) Denture Base Material Doped with Inorganic Filler. Materials 2016, 9, 328. [Google Scholar] [CrossRef] [PubMed]
- Chladek, G.; Kasperski, J.; Barszczewska-Rybarek, I.; Żmudzki, J. Sorption, Solubility, Bond Strength and Hardness of Denture Soft Lining Incorporated with Silver Nanoparticles. Int. J. Mol. Sci. 2012, 14, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Pachava, K.R.; Nadendla, L.K.; Alluri, L.S.C.; Tahseen, H.; Sajja, N.P. Invitro Antifungal Evaluation of Denture Soft Liner Incorporated with Tea Tree Oil: A New Therapeutic Approach Towards Denture Stomatitis. J. Clin. Diagn. Res. JCDR 2015, 9, ZC62–ZC64. [Google Scholar] [CrossRef] [PubMed]
- Perchyonok, T. Bio-Active Denture Soft Liner Materials from Design to Application: In Vitro Approach. J. Dent. Health Oral Disord. Ther. 2017, 6, 1–5. [Google Scholar] [CrossRef]
- Kampmann, Y.; De Clerck, E.; Kohn, S.; Patchala, D.K.; Langerock, R.; Kreyenschmidt, J. Study on the antimicrobial effect of silver-containing inner liners in refrigerators. J. Appl. Microbiol. 2008, 104, 1808–1814. [Google Scholar] [CrossRef] [PubMed]
- Kulak, Y.; Arikan, A.; Kazazoglu, E. Existence of Candida albicans and microorganisms in denture stomatitis patients. J. Oral Rehabil. 1997, 24, 788–790. [Google Scholar] [CrossRef] [PubMed]
- Mirizadeh, A.; Atai, M.; Ebrahimi, S. Fabrication of denture base materials with antimicrobial properties. J. Prosthet. Dent. 2017. [Google Scholar] [CrossRef] [PubMed]
- Gupta, L.; Aparna, I.N.; Bhat, S.; Ginjupalli, K. Effect of comonomer of methacrylic acid on flexural strength and adhesion of Staphylococcus aureus to heat polymerized poly (methyl methacrylate) resin: An in vitro study. J. Indian Prosthodont. Soc. 2017, 17, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Sumi, Y.; Miura, H.; Sunakawa, M.; Michiwaki, Y.; Sakagami, N. Colonization of denture plaque by respiratory pathogens in dependent elderly. Gerodontology 2002, 19, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Shim, G.-I.; Kim, S.-H.; Eom, H.-W.; Kim, K.-M.; Choi, S.-Y. Development of a transparent, non-cytotoxic, silver ion-exchanged glass with antimicrobial activity and low ion elution. Enzyme Microb. Technol. 2015, 72, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Wang, Q.; Chang, Z.; Wang, Z.; Zheng, X.; Shao, D.; Dong, W.; Zhou, Y. Synergistic bactericidal activity of chlorhexidine-loaded, silver-decorated mesoporous silica nanoparticles. Int. J. Nanomed. 2017, 12, 3577–3589. [Google Scholar] [CrossRef] [PubMed]
- Pokrowiecki, R.; Zaręba, T.; Szaraniec, B.; Pałka, K.; Mielczarek, A.; Menaszek, E.; Tyski, S. In vitro studies of nanosilver-doped titanium implants for oral and maxillofacial surgery. Int. J. Nanomed. 2017, 12, 4285–4297. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, S.; Koul, V. Assessment of PVA/silver nanocomposite hydrogel patch as antimicrobial dressing scaffold: Synthesis, characterization and biological evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, R.; Palanisamy, S.; Chen, S.-M.; Chelladurai, K.; Padmavathy, S.; Saravanan, M.; Prakash, P.; Ajmal Ali, M.; Al-Hemaid, F.M.A. Antimicrobial efficacy of green synthesized drug blended silver nanoparticles against dental caries and periodontal disease causing microorganisms. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 56, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Malanovic, N.; Lohner, K. Gram-positive bacterial cell envelopes: The impact on the activity of antimicrobial peptides. Biochim. Biophys. Acta Biomembr. 2016, 1858, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Dentistry—Soft Lining Materials for Removable Dentures—Part 2: Materials for Long-Term Use; EN ISO 10139-2:2016; ISO International Organization for Standardization: Geneva, Switzerland, 2016.
- Kim, B.-J.; Yang, H.-S.; Chun, M.-G.; Park, Y.-J. Shore hardness and tensile bond strength of long-term soft denture lining materials. J. Prosthet. Dent. 2014, 112, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Mese, A.; Guzel, K.G. Effect of storage duration on the hardness and tensile bond strength of silicone- and acrylic resin-based resilient denture liners to a processed denture base acrylic resin. J. Prosthet. Dent. 2008, 99, 153–159. [Google Scholar] [CrossRef]
- Han, Y.; Kiat-amnuay, S.; Powers, J.M.; Zhao, Y. Effect of nano-oxide concentration on the mechanical properties of a maxillofacial silicone elastomer. J. Prosthet. Dent. 2008, 100, 465–473. [Google Scholar] [CrossRef]
- Iwaki, M.; Akiba, N.; Minakuchi, S.; Takahashi, H. Influence of methyl mercaptan at early setting stages on the properties of self curing addition silicone resilient denture lining materials. J. Prosthodont. Res. 2009, 53, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, D.N.; Goiato, M.C.; Zuccolotti, B.C.R.; Moreno, A.; dos Santos, D.M.; Pesqueira, A.A. Effect of thermocycling on hardness, absorption, solubility and colour change of soft liners. Gerodontology 2012, 29, e215–e219. [Google Scholar] [CrossRef] [PubMed]
- Mutluay, M.M.; Ruyter, I.E. Evaluation of bond strength of soft relining materials to denture base polymers. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2007, 23, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Zayed, S.M.; Alshimy, A.M.; Fahmy, A.E. Effect of Surface Treated Silicon Dioxide Nanoparticles on Some Mechanical Properties of Maxillofacial Silicone Elastomer. Int. J. Biomater. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- El-Hadary, A.; Drummond, J.L. Comparative study of water sorption, solubility, and tensile bond strength of two soft lining materials. J. Prosthet. Dent. 2000, 83, 356–361. [Google Scholar] [CrossRef]
- Jabbal, R.S.; Datta, K. Comparative evaluation of water sorption and solubility of two autopolymerizing soft denture liners in distilled water and artificial saliva. Indian J. Dent. Sci. 2016, 8, 208–214. [Google Scholar] [CrossRef]
- Pisani, M.X.; Leite, V.M.F.; Badaró, M.M.; Malheiros-Segundo, A.d.L.; Paranhos, H.d.F.d.O.; Silva, C.H.L. da Soft denture liners and sodium perborate: Sorption, solubility and color change. Braz. J. Oral Sci. 2015, 14, 219–223. [Google Scholar] [CrossRef]
- Silver Sodium Hydrogen Zirconium Phosphate; Full Public Report, File No STD/1081. National Industrial Chemicals Notification and Assessment Scheme (Nicnas), 11 March 2004. Available online: https://www.pharosproject.net/uploads/files/sources/1185/alphasan-std1081fr.pdf (accessed on 21 February 2018).
- Mutluay, M.M.; Tezvergil-Mutluay, A. The influence of cyclic stress on surface properties of soft liners. Odontology 2017, 105, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Melaiye, A.; Sun, Z.; Hindi, K.; Milsted, A.; Ely, D.; Reneker, D.H.; Tessier, C.A.; Youngs, W.J. Silver(I)-imidazole cyclophane gem-diol complexes encapsulated by electrospun tecophilic nanofibers: Formation of nanosilver particles and antimicrobial activity. J. Am. Chem. Soc. 2005, 127, 2285–2291. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, Q.; Wang, Y.; Yu, H.; Chen, X.; Jing, X. Biodegradable electrospun poly(l-lactide) fibers containing antibacterial silver nanoparticles. Eur. Polym. J. 2006, 42, 2081–2087. [Google Scholar] [CrossRef]
- Taylor, P.L.; Ussher, A.L.; Burrell, R.E. Impact of heat on nanocrystalline silver dressings. Part I: Chemical and biological properties. Biomaterials 2005, 26, 7221–7229. [Google Scholar] [CrossRef] [PubMed]
- Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties; EN ISO 37:2017; ISO International Organization for Standardization: Geneva, Switzerland, 2017.
- Parker, S.; Meththananda, I.; Braden, M.; Pearson, G.J. Characterisation of some experimental silicones. J. Mater. Sci. Mater. Med. 2006, 17, 1255–1258. [Google Scholar] [CrossRef] [PubMed]






| Filler Concentration, % | Antimicrobial Efficacy, % | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Candida albicans ATCC 10231 | Staphylococcus aureus ATCC 25923 | Escherichia coli ATCC 25922 | |||||||
| Med | Max | Min | Med | Max | Min | Med | Max | Min | |
| 0 | −7.5 | 3.7 | −18.5 | 0.0 | 1.8 | 0.0 | 0.0 | 0.0 | 0.0 |
| 0.25 | 4.8 | 7.2 | −15.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 0.5 | −6.3 | 0.6 | −16.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 1 | 48.4 | 51.7 | 29.2 | 0.0 | 24.6 | 0.0 | 0.0 | 0.0 | 0.0 |
| 2 | 56.6 | 70.9 | 30.7 | 58.7 | 100.0 | 23.5 | >99.9 | >99.9 | >99.9 |
| 4 | 50.6 | 53.9 | 45.5 | 71.6 | 90.4 | 39.5 | 100.0 | 100.0 | 100.0 |
| 6 | 63.1 | 71.4 | 56.0 | 65.1 | 90.4 | 56.4 | 100.0 | 100.0 | 100.0 |
| 8 | 45.6 | 63.1 | 34.9 | 92.9 | 96.2 | 81.0 | 100.0 | 100.0 | 100.0 |
| 10 | 68.5 | 82.2 | 47.4 | 95.2 | 97.0 | 87.9 | 100.0 | 100.0 | 100.0 |
| 12 | 90.8 | 99.1 | 65.7 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| 14 | 98.8 | 99.3 | 98.6 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabłońska-Stencel, E.; Pakieła, W.; Mertas, A.; Bobela, E.; Kasperski, J.; Chladek, G. Effect of Silver-Emitting Filler on Antimicrobial and Mechanical Properties of Soft Denture Lining Material. Materials 2018, 11, 318. https://doi.org/10.3390/ma11020318
Jabłońska-Stencel E, Pakieła W, Mertas A, Bobela E, Kasperski J, Chladek G. Effect of Silver-Emitting Filler on Antimicrobial and Mechanical Properties of Soft Denture Lining Material. Materials. 2018; 11(2):318. https://doi.org/10.3390/ma11020318
Chicago/Turabian StyleJabłońska-Stencel, Ewa, Wojciech Pakieła, Anna Mertas, Elżbieta Bobela, Jacek Kasperski, and Grzegorz Chladek. 2018. "Effect of Silver-Emitting Filler on Antimicrobial and Mechanical Properties of Soft Denture Lining Material" Materials 11, no. 2: 318. https://doi.org/10.3390/ma11020318





