Recent Evidence on Bioactive Glass Antimicrobial and Antibiofilm Activity: A Mini-Review
Abstract
:1. Overview of Bioactive Glass
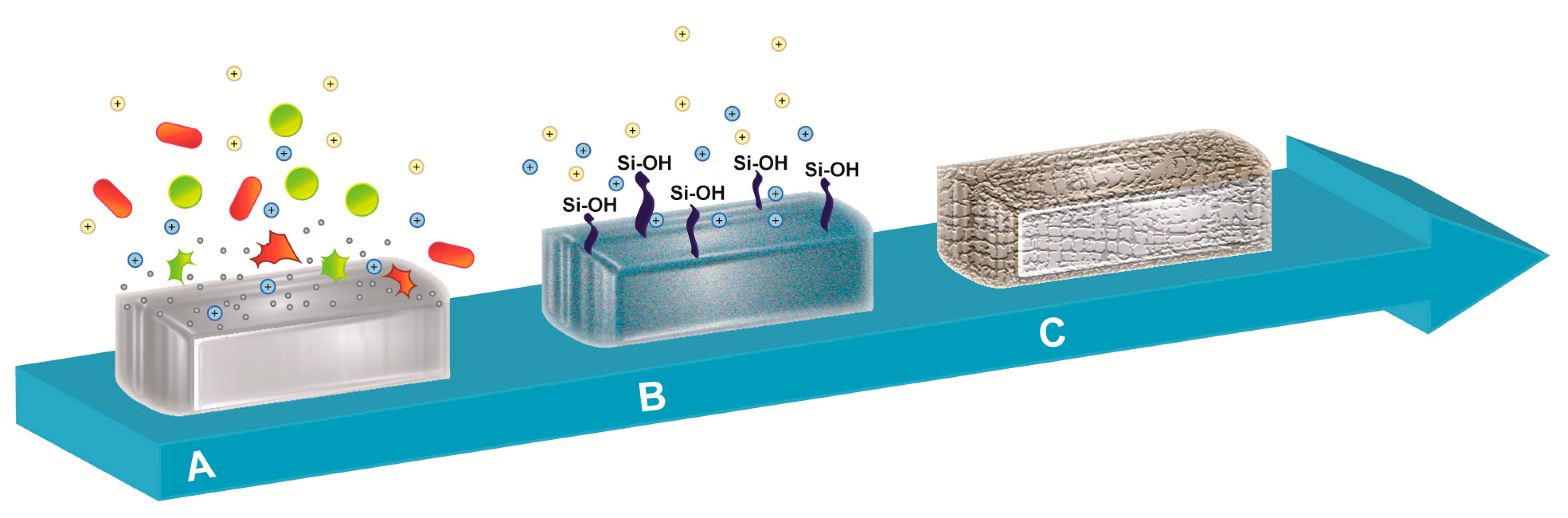
2. Reaction of Bioactive Glass with Body Fluids
3. Antibacterial Activity of Bioactive Glass
4. Bioactive Glass against Multidrug-Resistant (MDR) Bacteria
5. Antibiofilm Activity of Bioactive Glass
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Bottagisio, M.; Lovati, A.B.; Lopa, S.; Moretti, M. Osteogenic Differentiation of Human and Ovine Bone Marrow Stromal Cells in response to β-Glycerophosphate and Monosodium Phosphate. Cell Reprogr. 2015, 17, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Jones, J.R. Bioactive Glasses: Frontiers and Challenges. Front. Bioeng. Biotechnol. 2015, 3, 194. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Välimäki, V.V.; Aro, H.T. Molecular basis for action of bioactive glasses as bone graft substitute. Scand. J. Surg. 2006, 95, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Putzer, D.; Fuchs, J.; Coraca-Huber, D.; Christoph, A.; Liebensteiner, M.; Nogler, M. BAG-S53P4 as an additive to bone allografts: A laboratory study using an uniaxial compression test. J. Orthop. Res. 2015, 33, 1875–1879. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. Symp. 1971, 334, 117–141. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics. J. Am. Ceram. Soc. 1998, 81, 1705–1728. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- Day, R.M. Bioactive glass stimulates the secretion of angiogenic growth factors and angiogenesis in vitro. Tissue Eng. 2005, 11, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Leu, A.; Stieger, S.M.; Dayton, P.; Ferrara, K.W.; Leach, J.K. Angiogenic response to bioactive glass promotes bone healing in an irradiated calvarial defect. Tissue Eng. Part A 2009, 15, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Turunen, T.; Peltola, J.; Yli-Urpo, A.; Happonen, R.P. Bioactive glass granules as a bone adjunctive material in maxillary sinus floor augmentation. Clin. Oral Implants Res. 2004, 15, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Peltola, M.; Aitasalo, K.; Suonpää, J.; Varpula, M.; Yli-Urpo, A. Bioactive glass S53P4 in frontal sinus obliteration: A long-term clinical experience. Head Neck 2006, 28, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Profeta, A.C.; Huppa, C. Bioactive-glass in Oral and Maxillofacial Surgery. Craniomaxillofac. Trauma Reconstr. 2016, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rantakokko, J.; Frantzén, J.P.; Heinänen, J.; Kajander, S.; Kotilainen, E.; Gullichsen, E.; Lindfors, N.C. Posterolateral spondylodesis using bioactive glass S53P4 and autogenous bone in instrumented unstable lumbar spine burst fractures. A prospective 10-year follow-up study. Scand. J. Surg. 2012, 101, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Sarin, J.; Grénman, R.; Aitasalo, K.; Pulkkinen, J. Bioactive glass S53P4 in mastoid obliteration surgery for chronic otitis media and cerebrospinal fluid leakage. Ann. Otol. Rhinol. Laryngol. 2012, 121, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Pernaa, K.; Koski, I.; Mattila, K.; Gullichsen, E.; Heikkila, J.; Aho, A.; Lindfors, N. Bioactive glass S53P4 and autograft bone in treatment of depressed tibial plateau fractures—A prospective randomized 11-year follow-up. J. Long-Term Eff. Med. Implants 2011, 21, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Lindfors, N.C.; Heikkilä, J.T.; Koski, I.; Mattila, K.; Aho, A.J. Bioactive glass and autogenous bone as bone graft substitutes in benign bone tumors. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Polo, L.; Gómez-Cerezo, N.; Aznar, E.; Vivancos, J.L.; Sancenón, F.; Arcos, D.; Vallet-Regí, M.; Martínez-Máñez, R. Molecular gates in mesoporous bioactive glasses for the treatment of bone tumors and infection. Acta Biomater. 2017, 50, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Gubler, M.; Brunner, T.J.; Zehnder, M.; Waltimo, T.; Sener, B.; Stark, W.J. Do bioactive glasses convey a disinfecting mechanism beyond a mere increase in pH? Int. Endod. J. 2008, 41, 670–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Munukka, E.; Leppäranta, O.; Hupa, L.; Ylänen, H.; Salonen, J.; Eerola, E.; Viljanen, M.K.; Hupa, M. Comparison of antibacterial effect of three bioactive glasses. Key Eng. Mater. 2006, 309, 345–348. [Google Scholar] [CrossRef]
- Zhang, D.; Hupa, M.; Hupa, L. In situ pH within particle beds of bioactive glasses. Acta Biomater. 2008, 4, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Leppäranta, O.; Munukka, E.; Ylänen, H.; Viljanen, M.K.; Eerola, E.; Hupa, M.; Hupa, L. Antibacterial effects and dissolution behavior of six bioactive glasses. J. Biomed. Mater. Res. A 2010, 93, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Leppäranta, O.; Vaahtio, M.; Peltola, T.; Zhang, D.; Hupa, L.; Hupa, M.; Ylänen, H.; Salonen, J.I.; Viljanen, M.K.; Eerola, E. Antibacterial effect of bioactive glasses on clinically important anaerobic bacteria in vitro. J. Mater. Sci. Mater. Med. 2008, 19, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Vassena, C.; Fenu, S.; De Vecchi, E.; Signori, V.; De Francesco, R.; Romanò, C.L. In vitro antibiofilm activity of bioactive glass S53P4. Future Microbiol. 2014, 9, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Aurégan, J.C.; Bégué, T. Bioactive glass for long bone infection: A systematic review. Injury 2015, 46, S3–S7. [Google Scholar] [CrossRef]
- Lindfors, N.C.; Hyvönen, P.; Nyyssönen, M.; Kirjavainen, M.; Kankare, J.; Gullichsen, E.; Salo, J. Bioactive glass S53P4 as bone graft substitute in treatment of osteomyelitis. Bone 2010, 47, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Lindfors, N.C.; Koski, I.; Heikkilä, J.T.; Mattila, K.; Aho, A.J. A prospective randomized 14-year follow-up study of bioactive glass and autogenous bone as bone graft substitutes in benign bone tumors. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Romanò, D.; De Vecchi, E.; Vassena, C.; Logoluso, N.; Mattina, R.; Romanò, C.L. Bioactive glass BAG-S53P4 for the adjunctive treatment of chronic osteomyelitis of the long bones: An in vitro and prospective clinical study. BMC Infect. Dis. 2013, 13, 584. [Google Scholar] [CrossRef] [PubMed]
- McAndrew, J.; Efrimescu, C.; Sheehan, E.; Niall, D. Through the looking glass; bioactive glass S53P4 (BonAlive®) in the treatment of chronic osteomyelitis. Ir. J. Med. Sci. 2013, 182, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Romanò, C.L.; Logoluso, N.; Meani, E.; Romanò, D.; De Vecchi, E.; Vassena, C.; Drago, L. A comparative study of the use of bioactive glass S53P4 and antibiotic-loaded calcium-based bone substitutes in the treatment of chronic osteomyelitis: A retrospective comparative study. Bone Jt. J. 2014, 96, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Van Gestel, N.A.; Geurts, J.; Hulsen, D.J.; van Rietbergen, B.; Hofmann, S.; Arts, J.J. Clinical Applications of S53P4 Bioactive Glass in Bone Healing and Osteomyelitic Treatment: A Literature Review. Biomed. Res. Int. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Ylänen, H. Bioactive Glasses: Materials, Properties and Applications, 2nd ed.; Elsevier Woodhead Publishing: Cambridge, UK, 2011; ISBN 9780081009369. [Google Scholar]
- Turdean-Ionescu, C.; Stevensson, B.; Izquierdo-Barba, I.; García, A.; Arcos, D.; Vallet-Regí, M.; Edén, M. Surface Reactions of Mesoporous Bioactive Glasses Monitored by Solid-State NMR: Concentration Effects in Simulated Body Fluid. J. Phys. Chem. C 2016, 120, 4961–4974. [Google Scholar] [CrossRef]
- Rabiee, S.M.; Nazparvar, N.; Azizian, M.; Vashaee, D.; Tayebi, L. Effect of ion substitution on properties of bioactive glasses: A review. Ceram. Int. 2015, 41, 7241–7251. [Google Scholar] [CrossRef]
- Heikkila, J.T. Use of bioactive glasses as bone substitutes in orthopeadics and traumatology. In Bioactive Glasses: Materials, Properties and Applications, 2nd ed.; Ylanen, H., Ed.; Elsevier Woodhead Publishing: Cambridge, UK, 2011; pp. 189–208. ISBN 9780081009369. [Google Scholar]
- Hupa, L. Melt-derived bioactive glasses. In Bioactive Glasses: Materials, Properties and Applications, 2nd ed.; Ylanen, H., Ed.; Elsevier Woodhead Publishing: Cambridge, UK, 2011; pp. 3–28. ISBN 9780081009369. [Google Scholar]
- Begum, S.; Johnson, W.E.; Worthington, T.; Martin, R.A. The influence of pH and fluid dynamics on the antibacterial efficacy of 45S5 Bioglass. Biomed. Mater. 2016, 11, 015006. [Google Scholar] [CrossRef] [PubMed]
- Leu, A.; Leach, J.K. Proangiogenic potential of a collagen/bioactive glass substrate. Pharm. Res. 2008, 25, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Munukka, E.; Leppäranta, O.; Korkeamäki, M.; Vaahtio, M.; Peltola, T.; Zhang, D.; Hupa, L.; Ylänen, H.; Salonen, J.I.; Viljanen, M.K.; et al. Bactericidal effects of bioactive glasses on clinically important aerobic bacteria. J. Mater. Sci. Mater. Med. 2008, 19, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.N.; Bal, B.S.; Huang, W. Review: Emerging developments in the use of bioactive glasses for treating infected prosthetic joints. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 41, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Ran, S.; He, Z.; Liang, J. Survival of Enterococcus faecalis during alkaline stress: Changes in morphology, ultrastructure, physiochemical properties of the cell wall and specific gene transcripts. Arch. Oral Biol. 2013, 58, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Allan, I.; Newman, H.; Wilson, M. Antibacterial activity of particulate Bioglass against supra- and subgingival bacteria. Biomaterials 2001, 22, 1683–1687. [Google Scholar] [CrossRef]
- Bortolin, M.; Romanò, C.L.; Bidossi, A.; De Vecchi, E.; Mattina, R.; Drago, L. BAG-S53P4 as bone graft extender and antimicrobial activity against gentamicin and vancomycin resistant bacteria. Future Microbiol. 2018, in press. [Google Scholar]
- Lindfors, N.; Geurts, J.; Drago, L.; Arts, J.J.; Juutilainen, V.; Hyvönen, P.; Suda, A.J.; Domenico, A.; Artiaco, S.; Alizadeh, C.; et al. Erratum: Antibacterial Bioactive Glass, S53P4, for Chronic Bone Infections—A Multinational Study. Adv. Exp. Med. Biol. 2017, 971, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Pilizota, T.; Shaevitz, J.W. Plasmolysis and cell shape depend on solute outer-membrane permeability during hyperosmotic shock in E. coli. Biophys. J. 2013, 104, 2733–2742. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; De Vecchi, E.; Bortolin, M.; Toscano, M.; Mattina, R.; Romanò, C.L. Antimicrobial activity and resistance selection of different bioglass S53P4 formulations against multidrug resistant strains. Future Microbiol. 2015, 10, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Stoor, P.; Söderling, E.; Salonen, J.I. Antibacterial effects of a bioactive glass paste on oral microorganisms. Acta Odontol. Scand. 1998, 56, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, M.; Waltimo, T.; Sener, B.; Söderling, E. Dentin enhances the effectiveness of bioactive glass S53P4 against a strain of Enterococcus faecalis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Waltimo, T.; Brunner, T.J.; Vollenweider, M.; Stark, W.J.; Zehnder, M. Antimicrobial effect of nanometric bioactive glass 45S5. J. Dent. Res. 2007, 86, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, V.; Nahrkhalaji, M.M.; Fathi, M.H.; Mousavi, S.B.; Esfahani, B.N. Antibacterial effects of sol-gel-derived bioactive glass nanoparticle on aerobic bacteria. J. Biomed. Mater. Res. A 2010, 94, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Coraça-Huber, D.C.; Fille, M.; Hausdorfer, J.; Putzer, D.; Nogler, M. Efficacy of antibacterial bioactive glass S53P4 against S. aureus biofilms grown on titanium discs in vitro. J. Orthop. Res. 2014, 32, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Cacciotti, I. Bivalent cationic ions doped bioactive glasses: The influence of magnesium, zinc, strontium and copper on the physical and biological properties. J. Mater. Sci. Mater. Med. 2017, 52, 8812–8831. [Google Scholar] [CrossRef]
- Neut, D.; van der Mei, H.C.; Bulstra, S.K.; Busscher, H.J. The role of small-colony variants in failure to diagnose and treat biofilm infections in orthopedics. Acta Orthop. 2007, 78, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Darouiche, R.O. Treatment of infections associated with surgical implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Peel, T.N.; Buising, K.L.; Choong, P.F. Diagnosis and management of prosthetic joint infection. Curr. Opin. Infect. Dis. 2012, 25, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Lipsky, B.A. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin. Infect. Dis. 2012, 54, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Lalidou, F.; Kolios, G.; Tavridou, A.; Drosos, G.I. Bone grafts as carriers for local antibiotic delivery for the treatment and prevention of bone infections. Surg. Technol. Int. 2014, 25, 239–245. [Google Scholar] [PubMed]
- Pacheco, H.; Vedantham, K.; Aniket; Young, A.; Marriott, I.; El-Ghannam, A. Tissue engineering scaffold for sequential release of vancomycin and rhBMP2 to treat bone infections. J. Biomed. Mater. Res. A 2014, 102, 4213–4223. [Google Scholar] [CrossRef] [PubMed]
- Van de Belt, H.; Neut, D.; Schenk, W.; Horn, J. Infection of orthopedic implants and the use of antibiotic-loaded bone cements: A review. Acta Orthop. Scand. 2001, 72, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.; Kolár, M.; Florschütz, A.V.; Novotný, R.; Pantůcek, R.; Kesselová, M. In vitro testing of gentamicin-vancomycin loaded bone cement to prevent prosthetic joint infection. Biomed. Pap. 2005, 149, 153–158. [Google Scholar] [CrossRef]
- Jiranek, W.A.; Hanssen, A.D.; Greenwald, A.S. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J. Bone Jt. Surg. Am. 2006, 887, 2487–2500. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Kretlow, J.D.; Nguyen, A.; Young, S.; Baggett, L.S.; Wong, M.E.; Kasper, F.K.; Mikos, A.G. Antibiotic-releasing porous polymethylmethacrylate constructs for osseous space maintenance and infection control. Biomaterials 2010, 31, 4146–4156. [Google Scholar] [CrossRef] [PubMed]
- Gergely, I.; Zazgyva, A.; Man, A.; Zuh, S.G.; Pop, T.S. The in vitro antibacterial effect of S53P4 bioactive glass and gentamicin impregnated polymethylmethacrylate beads. Acta Microbiol. Immunol. Hung. 2014, 61, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Parsons, B.; Strauss, E. Surgical management of chronic osteomyelitis. Am. J. Surg. 2004, 188, 57–66. [Google Scholar] [CrossRef]
- Calhoun, J.H.; Manring, M.M.; Shirtliff, M. Osteomyelitis of the long bones. Semin. Plast. Surg. 2009, 23, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Righi, E. Multidrug-resistant bacteria: What is the threat? Hematol. Am. Soc. Hematol. Educ. Program 2013, 2013, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.C.; Gonçalves, L.M.; Rijo, P.; Vaz, M.A.; Almeida, A.J.; Bettencourt, A.F. A novel modified acrylic bone cement matrix. A step forward on antibiotic delivery against multiresistant bacteria responsible for prosthetic joint infections. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 38, 218–226. [Google Scholar] [CrossRef] [PubMed]
- El-Tablawy, S.Y.; Abd-Allah, W.M.; Araby, E. Efficacy of Irradiated Bioactive Glass 45S5 on Attenuation of Microbial Growth and Eradication of Biofilm from AISI 316 L Discs: In-vitro Study. Silicon 2017, 1–12. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Sameni, M.; Hashemi, A.; Zamani, F.; Rostami, A.; Mozafari, M. Silver- and fluoride-containing mesoporous bioactive glasses versus commonly used antibiotics: Activity against multidrug-resistant bacterial strains isolated from patients with burns. Burns 2016, 42, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Galarraga-Vinueza, M.E.; Mesquita-Guimarães, J.; Magini, R.S.; Souza, J.C.; Fredel, M.C.; Boccaccini, A.R. Anti-biofilm properties of bioactive glasses embedding organic active compounds. J. Biomed. Mater. Res. A 2017, 105, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Elgharably, H.; Hussain, S.T.; Shrestha, N.K.; Blackstone, E.H.; Pettersson, G.B. Current Hypotheses in Cardiac Surgery: Biofilm in Infective Endocarditis. Semin. Thorac. Cardiovasc. Surg. Spring 2016, 28, 56–59. [Google Scholar] [CrossRef] [PubMed]
- McConoughey, S.J.; Howlin, R.; Granger, J.F.; Manring, M.M.; Calhoun, J.H.; Shirtliff, M.; Kathju, S.; Stoodley, P. Biofilms in periprosthetic orthopedic infections. Future Microbiol. 2014, 9, 987–1007. [Google Scholar] [CrossRef] [PubMed]
- Beikler, T.; Flemming, T.F. Oral biofilm-associated diseases: Trends and implications for quality of life, systemic health and expenditures. Periodontology 2011, 55, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Borlee, B.R.; Goldman, A.D.; Murakami, K.; Samudrala, R.; Wozniak, D.J.; Parsek, M.R. Pseudomonas aeruginosa uses a cyclic-di-GMP-regulated adhesin to reinforce the biofilm extracellular matrix. Mol. Microbiol. 2010, 75, 827–842. [Google Scholar] [CrossRef] [PubMed]
- Roilides, E.; Simitsopoulou, M.; Katragkou, A.; Walsh, T.J. How Biofilms Evade Host Defenses. Microbiol. Spectr. 2015, 3, 1–10. [Google Scholar] [CrossRef]
- Uppuluri, P.; Chaturvedi, A.K.; Srinivasan, A.; Banerjee, M.; Ramasubramaniam, A.K.; Köhler, J.R.; Kadosh, D.; Lopez-Ribot, J.L. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog. 2010, 26, e1000828. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. Am. 2007, 89, 780–785. [Google Scholar]
- Xu, Y.T.; Wu, Q.; Chen, Y.M.; Smales, R.J.; Shi, S.Y.; Wang, M.T. Antimicrobial effects of a bioactive glass combined with fluoride or triclosan on Streptococcus mutans biofilm. Arch. Oral Biol. 2015, 60, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Bari, A.; Bloise, N.; Fiorilli, S.; Novajra, G.; Vallet-Regí, M.; Bruni, G.; Torres-Pardo, A.; González-Calbet, J.M.; Visai, L.; Vitale-Brovarone, C. Copper-containing mesoporous bioactive glass nanoparticles as multifunctional agent for bone regeneration. Acta Biomater. 2017, 55, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Bortolin, M.; De Vecchi, E.; Romanò, C.L.; Toscano, M.; Mattina, R.; Drago, L. Antibiofilm agents against MDR bacterial strains: Is bioactive glass BAG-S53P4 also effective? J. Antimicrob. Chemother. 2016, 71, 123–127. [Google Scholar] [CrossRef] [PubMed]
| Bioglass | SiO2 | Na2O | CaO | P2O5 | Other |
|---|---|---|---|---|---|
| 45S5 | 45 | 24.5 | 24.5 | 6 | - |
| 42S5 | 42.1 | 26.3 | 29 | 2.6 | - |
| S53P4 | 53 | 23 | 20 | 4 | - |
| 55S4 | 52.1 | 21.5 | 23.8 | 2.6 | - |
| 58S | 60 | 0 | 36 | 4 | - |
| 70S30C | 70 | 30 | 0 | 0 | - |
| 45S5F | 45 | 24.5 | 12.25 | 6 | 12.5 CaF2 |
| 40S5B5 | 40 | 24.5 | 24.5 | 6 | 5 B2O3 |
| Methodology | Methodology Description | References |
|---|---|---|
| Bacterial cultivation test | Direct culture of bacteria with powdered bioglass for 1–4 days. The antibacterial activity of bioglass was correlated with the ions concentration and the pH change in the medium. | [22,23,40] |
| Direct culture | Evaluation of bacterial growth in the presence of bioglass under static and shaking conditions for 24–96 h. | [38,43] |
| Indirect culture | Evaluation of bacterial growth in medium conditioned with bioglass under static and shaking conditions for 24–96 h. | [38,43] |
| Indirect culture with adjusted pH | The pH of bioglass-conditioned supernatants was adjusted by adding HCl to give a final pH of 7.2. | [43] |
| Time-kill curves | The antibacterial activity of bioglass was tested with morselized bone graft to mimic the in vivo buffering-conditions after 0, 24, 48 and 72 h. | [44] |
| Methodology | Methodology Description | References |
|---|---|---|
| Activity against mature biofilm | Following bacterial biofilm formation, titanium discs were placed in direct contact with bioactive glass from 24 h to five days. | [52] |
| Crystal violet | The biofilm formation on titanium disks was observed by means of a colorimetric assay. | [24] |
| Confocal Laser Microscopy (CLM) | The biofilm biomass on titanium disks was observed by means of CLM. | [24] |
| Scanning Electron Microscopy (SEM) | The antibacterial activity against immature biofilm grown on coverslips was assessed by means of SEM. | [80,81] |
| MTT test | Following biofilm formation on a 96-well plate after incubation with bioglass, the viability of bacteria was evaluated by means of an MTT test. | [81] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drago, L.; Toscano, M.; Bottagisio, M. Recent Evidence on Bioactive Glass Antimicrobial and Antibiofilm Activity: A Mini-Review. Materials 2018, 11, 326. https://doi.org/10.3390/ma11020326
Drago L, Toscano M, Bottagisio M. Recent Evidence on Bioactive Glass Antimicrobial and Antibiofilm Activity: A Mini-Review. Materials. 2018; 11(2):326. https://doi.org/10.3390/ma11020326
Chicago/Turabian StyleDrago, Lorenzo, Marco Toscano, and Marta Bottagisio. 2018. "Recent Evidence on Bioactive Glass Antimicrobial and Antibiofilm Activity: A Mini-Review" Materials 11, no. 2: 326. https://doi.org/10.3390/ma11020326





