Fabrication and Anti-Oxidation Ability of SiC-SiO2 Coated Carbon Fibers Using Sol-Gel Method
Abstract
:1. Introduction
2. Experiment
2.1. Preparation of Modified SiC/SiO2 Sol and Emulstion
2.2. Coating Process
2.3. Oxidation
2.4. Characterization
3. Result and Discussion
3.1. Microstructure Analysis of Coatings

3.2. Mechanical Property of Coated CF
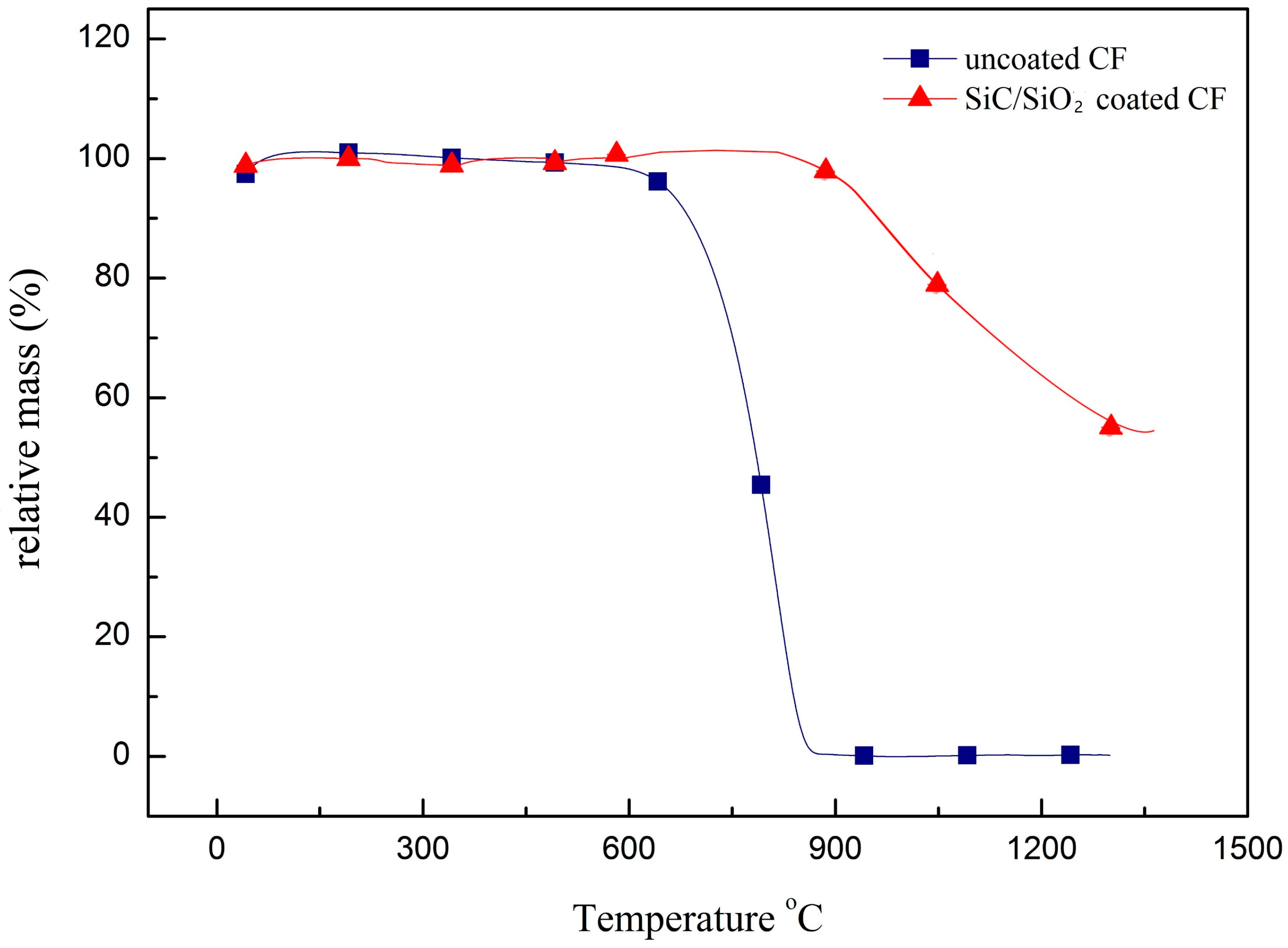
3.3. Anti-Oxidation Performance
3.4. Oxidation Mechanism of Coated CF
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Yuan, Y.; Sun, Y.; Yan, S.; Zhao, J.; Liu, S.; Zhang, M.; Zheng, X.; Jia, L. Multiply fully recyclable carbon fibre reinforced heat-resistant covalent thermosetting advanced composites. Nat. Commun. 2017, 8, 14657. [Google Scholar] [CrossRef] [PubMed]
- Toldy, A.; Niedermann, P.; Pomázi, Á.; Marosi, G.; Szolnoki, B. Flame Retardancy of Carbon Fibre Reinforced Sorbitol Based Bioepoxy Composites with Phosphorus-Containing Additives. Materials 2017, 10, 467. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X.; Miao, Y.; Li, Y.; Xi, X.; Zhong, X.; Pei, X.; He, L.; Huang, Q. The influences of carbon nanotubes introduced in three different phases of carbon fiber/pyrolytic carbon/silicon carbide composites on microstructure and properties of their composites. Carbon 2018, 129, 409–414. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G.; Hilmas, G.E. Ultra-High Temperature Ceramics: Materials for Extreme Environment Applications. Scr. Mater. 2017, 129, 94–99. [Google Scholar] [CrossRef]
- Yu, S.; Park, K.; Lee, J.W.; Hong, S.M.; Park, C.; Han, T.H.; Koo, C.M. Enhanced thermal conductivity of epoxy/Cu-plated carbon fiber fabric composites. Macromol. Res. 2017, 25, 559–564. [Google Scholar] [CrossRef]
- Zhuang, Q.; Zhang, P.; Li, M.; Yan, H.; Yu, Z.; Lu, Q. Microstructure, Wear Resistance and Oxidation Behavior of Ni-Ti-Si Coatings Fabricated on Ti6Al4V by Laser Cladding. Materials 2017, 10, 1248. [Google Scholar] [CrossRef] [PubMed]
- Zamani, P.; Valefi, Z. Microstructure, phase composition and mechanical properties of plasma sprayed Al2O3, Cr2O3 and Cr2O3-Al2O3 composite coatings. Surf. Coat. Technol. 2017, 316, 138–145. [Google Scholar] [CrossRef]
- Du, B.; Hong, C.; Qu, Q.; Zhou, S.; Liu, C.; Zhang, X. Oxidative protection of a carbon-bonded carbon fiber composite with double-layer coating of MoSi2-SiC whisker and TaSi2-MoSi2-SiC whisker by slurry method. Ceram. Int. 2017, 43, 9531–9537. [Google Scholar] [CrossRef]
- Xiaojun, M.; Zhou, W.; Yin, C. Structure and Photocatalytic Properties of Mn-Doped TiO2 Loaded on Wood-Based Activated Carbon Fiber Composites. Materials 2017, 10, 631. [Google Scholar] [CrossRef] [PubMed]
- Farrokhzad, M.A. High temperature oxidation behaviour of autocatalytic Ni-P-BN (h) coatings. Surf. Coat. Technol. 2017, 309, 390–400. [Google Scholar] [CrossRef]
- Jia, Y.; Li, H.; Feng, L.; Sun, J.; Li, K.; Fu, Q. Ablation behavior of rare earth La-modified ZrC coating for SiC-coated carbon/carbon composites under an oxyacetylene torch. Corros. Sci. 2016, 104, 61–70. [Google Scholar] [CrossRef]
- Huo, C.; Guo, L.; Feng, L.; Wang, C.; Li, Z.; Zhang, Y.; Kou, G. Improving the oxidation resistance under thermal shock condition of SiC-coated C/C composites with refined SiC grain size using ferrocene. Surf. Coat. Technol. 2017, 316, 39–47. [Google Scholar] [CrossRef]
- Doganli, G.; Yuzer, B.; Aydin, I.; Gultekin, T.; Con, A.H.; Selcuk, H.; Palamutcu, S. Functionalization of cotton fabric with nanosized TiO2 coating for self-cleaning and antibacterial property enhancement. J. Coat. Technol. Res. 2016, 13, 257–265. [Google Scholar] [CrossRef]
- Yong, X.; Cao, L.; Huang, J.; Kong, W.; Su, J.; Li, C.; Ouyang, H.; Zhou, L.; Liu, J. Microstructure and oxidation protection of a MoSi2/SiO2-B2O3-Al2O3, coating for SiC-coated carbon/carbon composites. Surf. Coat. Technol. 2017, 311, 63–69. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Shen, Y.H.; Chen, W.W.; Cheng, H.W.; Wang, L. Preparation of Continuous Al2O3/Y2O3 Coating on Carbon Fiber by a Novel Aqueous Plasma Electrolysis. Mater. Sci. Forum Trans. Tech. Publ. 2017, 898, 1575–1582. [Google Scholar] [CrossRef]
- Li, L. Damage evolution and life prediction of cross-ply C/SiC ceramic-matrix composite under cyclic fatigue loading at room temperature and 800 °C in air. Materials 2015, 8, 8539–8560. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Sun, S.; Tu, G.; Lu, S.; Li, K.; Zhu, X. The Microstructure of Nanocrystalline TiB2 Films Prepared by Chemical Vapor Deposition. Materials 2017, 10, 1425. [Google Scholar] [CrossRef] [PubMed]
- Szczurek, A.; Barcikowski, M.; Leluk, K.; Babiarczuk, B.; Kaleta, J.; Krzak, J. Improvement of Interaction in a Composite Structure by Using a Sol-Gel Functional Coating on Carbon Fibers. Materials 2017, 10, 990. [Google Scholar] [CrossRef] [PubMed]
- Tatarko, P.; Casalegno, V.; Hu, C.; Salvo, M.; Ferraris, M.; Reece, M.J. Joining of CVD-SiC coated and uncoated fibre reinforced ceramic matrix composites with pre-sintered Ti3SiC2 MAX phase using Spark Plasma Sintering. J. Eur. Ceram. Soc. 2016, 36, 3957–3967. [Google Scholar] [CrossRef]
- Januś, M.; Kyzioł, K.; Kluska, S.; Konefał-Góral, J.; Małek, A.; Jonas, S. Plasma Assisted Chemical Vapour Deposition—Technological Design of Functional Coatings. Arch. Metall. Mater. 2015, 60, 909–914. [Google Scholar] [CrossRef]
- Jonas, S.; Januś, M.; Jaglarz, J.; Kyzioł, K. Formation of SixNy (H) and C:N:H layers by Plasma-Assisted Chemical Vapor Deposition method. Thin Solid Films 2016, 600, 162–168. [Google Scholar] [CrossRef]
- Lee, E.S.; Lee, C.H.; Chun, Y.S.; Han, C.J.; Lim, D.S. Effect of hydrogen plasma-mediated surface modification of carbon fibers on the mechanical properties of carbon-fiber-reinforced polyetherimide composites. Compos. Part B Eng. 2017, 116, 451–458. [Google Scholar] [CrossRef]
- Guo, Z.; Sang, L.; Wang, Z.; Chen, Q.; Yang, L.; Liu, Z. Deposition of copper thin films by plasma enhanced pulsed chemical vapor deposition for metallization of carbon fiber reinforced plastics. Surf. Coat. Technol. 2016, 307, 1059–1064. [Google Scholar] [CrossRef]
- Kern, F.; Gadow, R. Liquid phase coating process for protective ceramic layers on carbon fibers. Surf. Coat. Technol. 2002, 151–152, 418–423. [Google Scholar] [CrossRef]
- Zhou, Q.; Peng, Z.; Yang, M.; Hu, H.; Song, N.; Ni, L. A linear polycarbosilane containing m-carborane units: Synthesis and characterization as precursor for SiC/B4C ceramics. High Perform. Polym. 2017, 0954008317746730. [Google Scholar] [CrossRef]
- Wang, J.; Lin, W.; Yan, X.; Wu, X.; Wu, F.; Yang, Y. Preparation and microstructure of Al2O3–SiO2–TiO2 coating on three-dimensional braided carbon fiber by sol–gel technology. Mater. Des. 2016, 89, 928–932. [Google Scholar] [CrossRef]
- Figueira, R.B.; Callone, E.; Silva, C.J.; Pereira, E.V.; Dirè, S. Hybrid coatings enriched with tetraethoxysilane for corrosion mitigation of hot-dip galvanized steel in chloride contaminated simulated concrete pore solutions. Materials 2017, 10, 306. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Li, X.; Du, A.; Wu, S.; Shen, J.; Zhou, B. Timing of polyethylene glycol addition for the control of SiO2 sol structure and sol–gel coating properties. J. Coat. Technol. Res. 2017, 14, 447–454. [Google Scholar] [CrossRef]
- Xia, K.; Lu, C.; Yang, Y. Preparation of anti-oxidative SiC/SiO2 coating on carbon fibers from vinyltriethoxysilane by sol–gel method. Appl. Surf. Sci. 2013, 265, 603–609. [Google Scholar] [CrossRef]
- Liu, H.; Liu, F.J.; Wang, H.J.; Wang, H.F.; Cheng, L.; Fan, L.D. Sample Preparation Method for Mechanical Property Test of Carbon Fiber. CN 101949791 A, 16 September 2010. [Google Scholar]
- Rubber and Plastics Test Equipment—Tensile, Flexural and Compression Types (Constant Rate of Traverse)—Specification; International Organization for Standardization: Geneva, Switzerland, 2002; ISO 5893:2002.
- Vautard, F.; Fioux, P.; Vidal, L.; Dentzer, J.; Schultz, J.; Nardin, M.; Defoort, B. Influence of an oxidation of the carbon fiber surface by boiling nitric acid on the adhesion strength in carbon fiber-acrylate composites cured by electron beam. Surf. Interface Anal. 2013, 45, 722–741. [Google Scholar] [CrossRef]
- Gao, S.L.; Mäder, E.; Plonka, R. Nanocomposite coatings for healing surface defects of glass fibers and improving interfacial adhesion. Compos. Sci. Technol. 2008, 68, 2892–2901. [Google Scholar] [CrossRef]
- Jiménez, C.; Mergia, K.; Lagos, M.; Yialouris, P.; Agote, I.; Liedtke, V.; Messoloras, S.; Panayiotatos, Y.; Padovano, E.; Badini, C.; et al. Joining of ceramic matrix composites to high temperature ceramics for thermal protection systems. J. Eur. Ceram. Soc. 2016, 36, 443–449. [Google Scholar] [CrossRef]
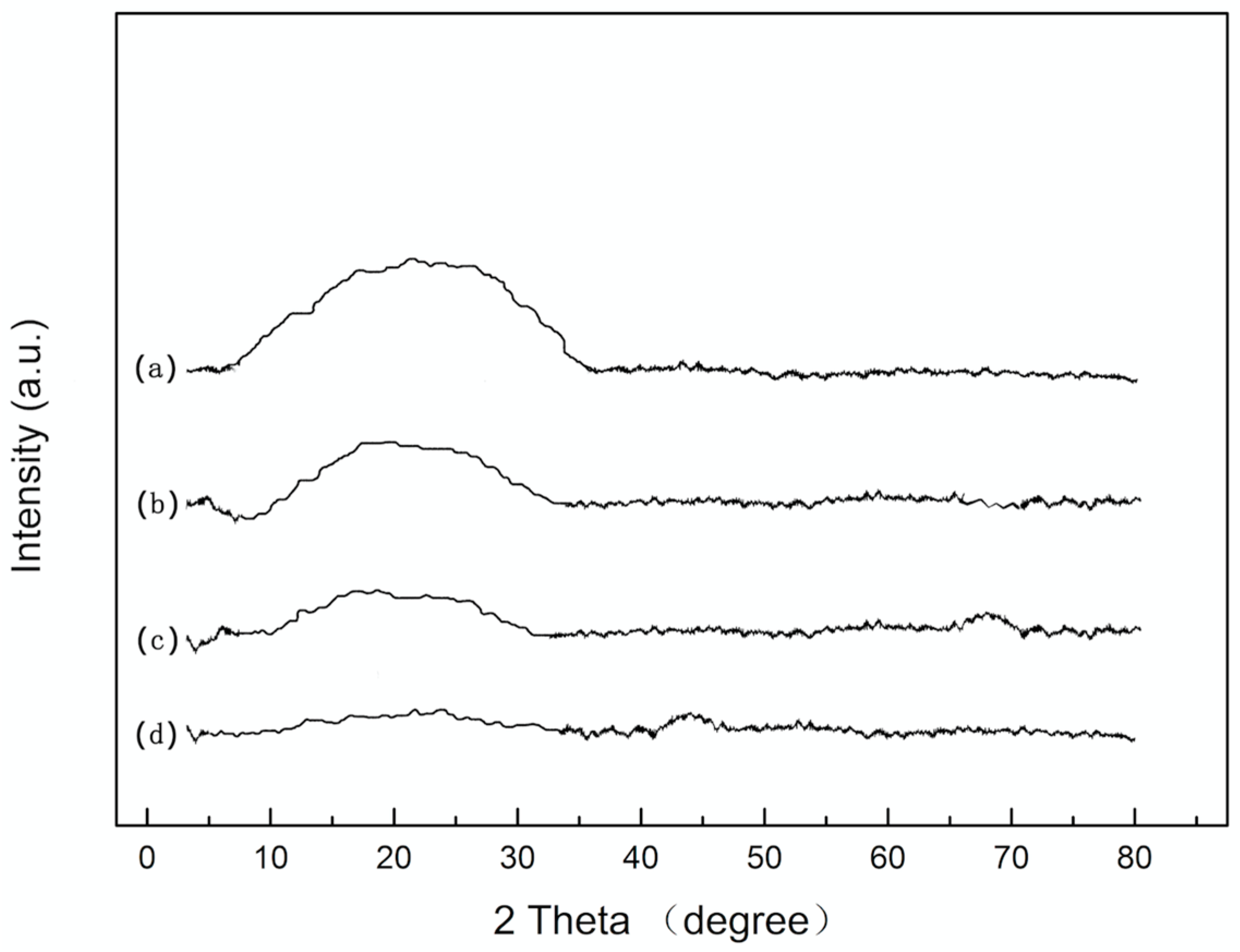
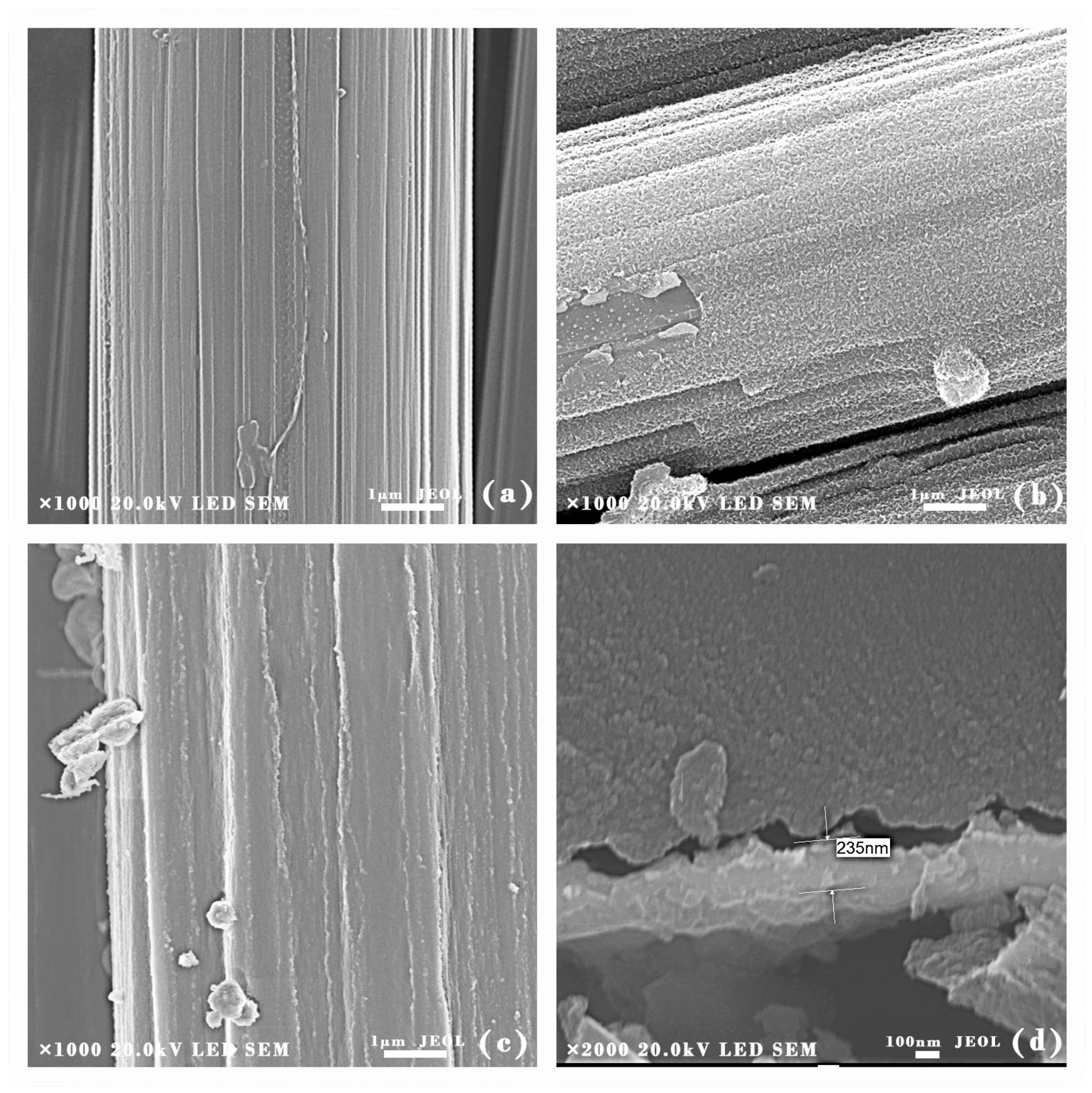
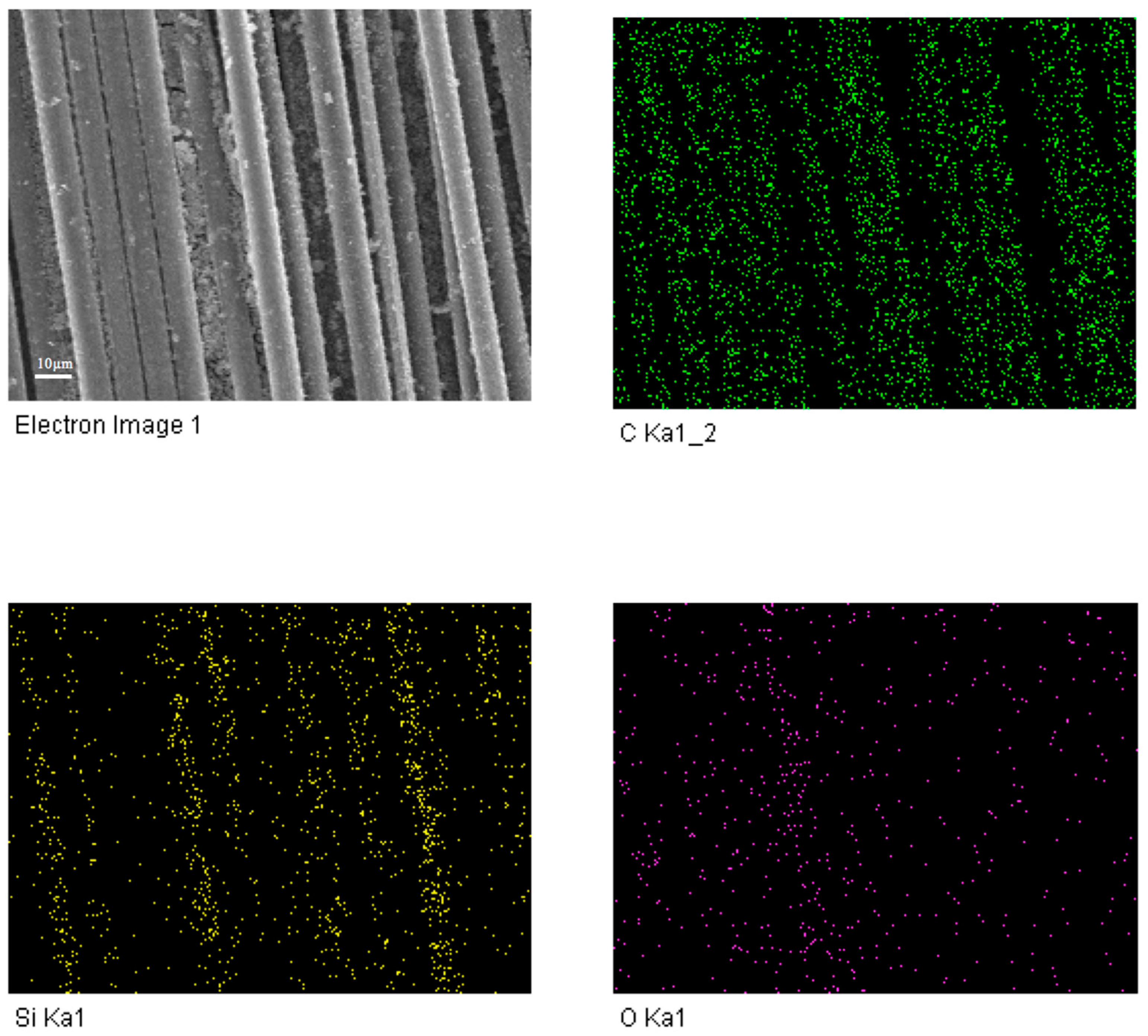


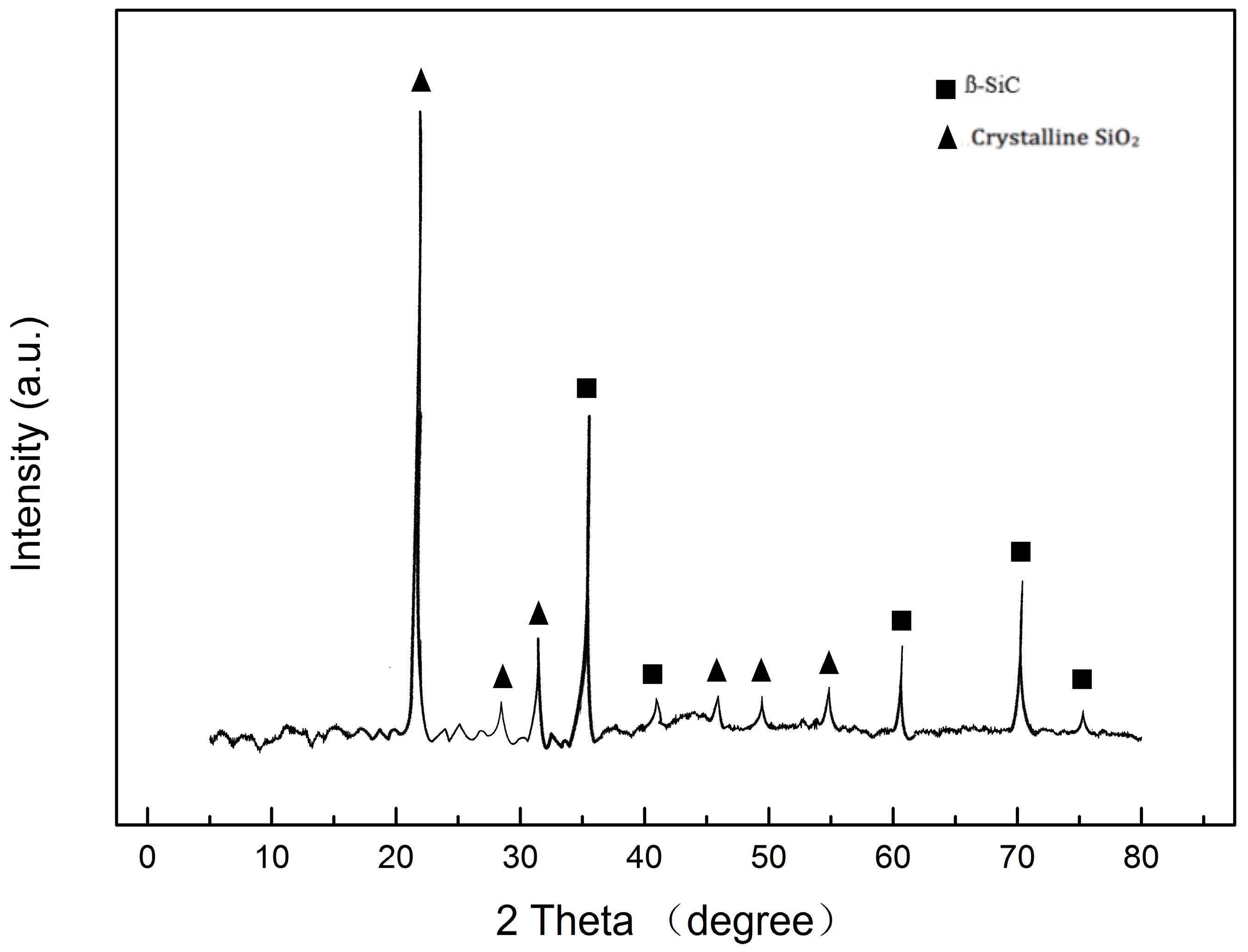

| Sample Number | Water/TEOS | SiO2 Coating |
|---|---|---|
| Sample 1 | 1/1 | The thickest coating with no cracks. The surface is not uniform. |
| Sample 2 | 1/3 | Thinner than Sample 1, and the surface is uniform. |
| Sample 3 | 1/5 | The surface is not uniform, and cracks appear. |
| Sample 4 | 1/7 | It cannot form SiO2 coating. |
| Sample | Strength MPa | Young’s Modulus GPa | Fracture Elongation % | Standard Deviation of Modulus |
|---|---|---|---|---|
| Uncoated CF | 2745 | 223 | 1.75 | 5.98 |
| SiO2 coated CF | 2980 | 207 | 1.40 | 4.24 |
| SiC/SiO2 coated CF | 3098 | 210 | 1.43 | 5.12 |
| 800 °C treated SiO2 coated CF | 2455 | 132 | 1.22 | 3.35 |
| 1000 °C treated SiO2 coated CF | 1832 | 101 | 1.16 | 4.31 |
| 1400 °C treated SiO2 coated CF | 1102 | 67 | 0.65 | 4.89 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Huang, Z.; Wang, X.; Wang, B. Fabrication and Anti-Oxidation Ability of SiC-SiO2 Coated Carbon Fibers Using Sol-Gel Method. Materials 2018, 11, 350. https://doi.org/10.3390/ma11030350
Yang G, Huang Z, Wang X, Wang B. Fabrication and Anti-Oxidation Ability of SiC-SiO2 Coated Carbon Fibers Using Sol-Gel Method. Materials. 2018; 11(3):350. https://doi.org/10.3390/ma11030350
Chicago/Turabian StyleYang, Guangyuan, Zhixiong Huang, Xu Wang, and Bo Wang. 2018. "Fabrication and Anti-Oxidation Ability of SiC-SiO2 Coated Carbon Fibers Using Sol-Gel Method" Materials 11, no. 3: 350. https://doi.org/10.3390/ma11030350




