Novel Precursor-Derived Meso-/Macroporous TiO2/SiOC Nanocomposites with Highly Stable Anatase Nanophase Providing Visible Light Photocatalytic Activity and Superior Adsorption of Organic Dyes
Abstract
:1. Introduction
2. Experiment
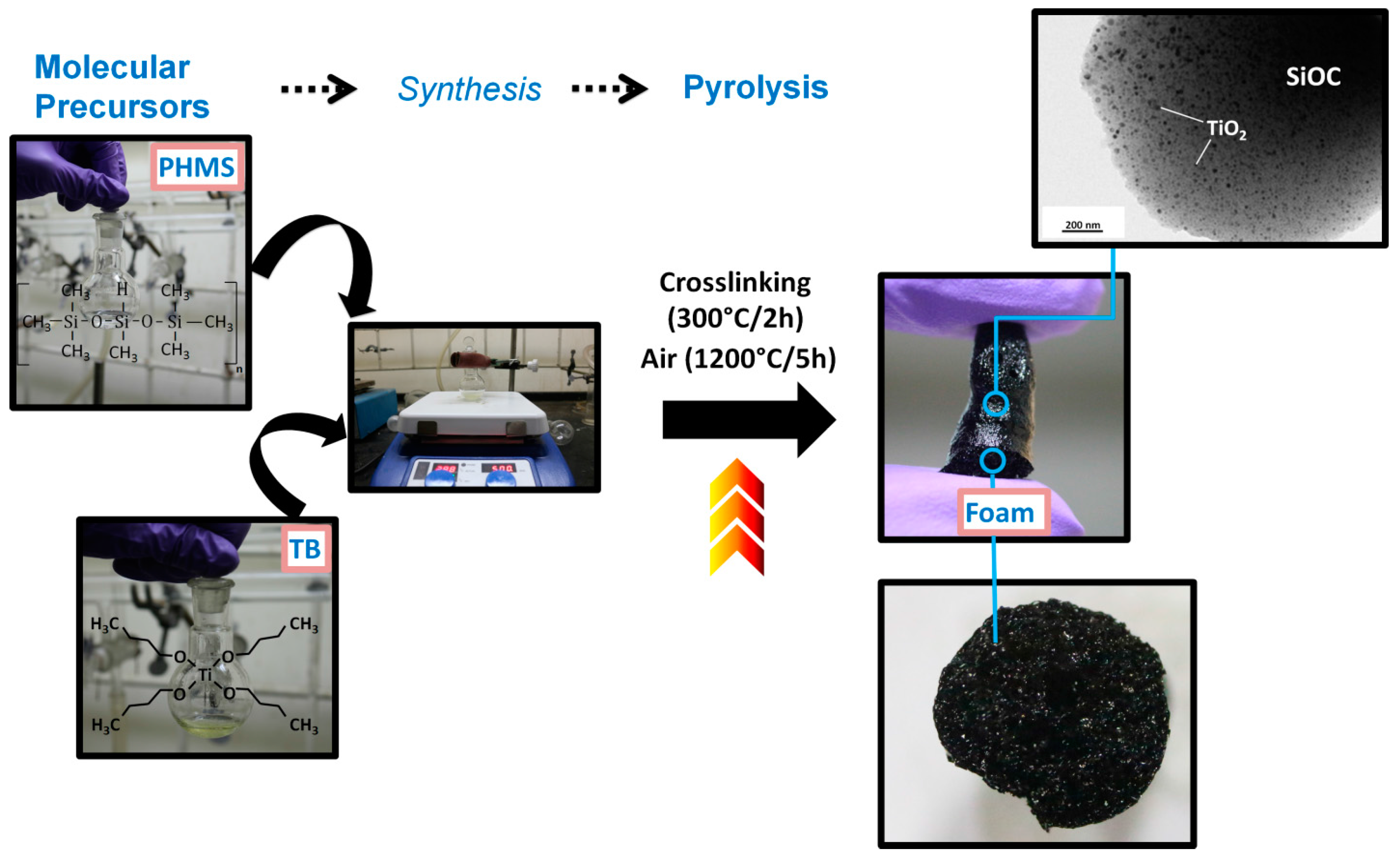
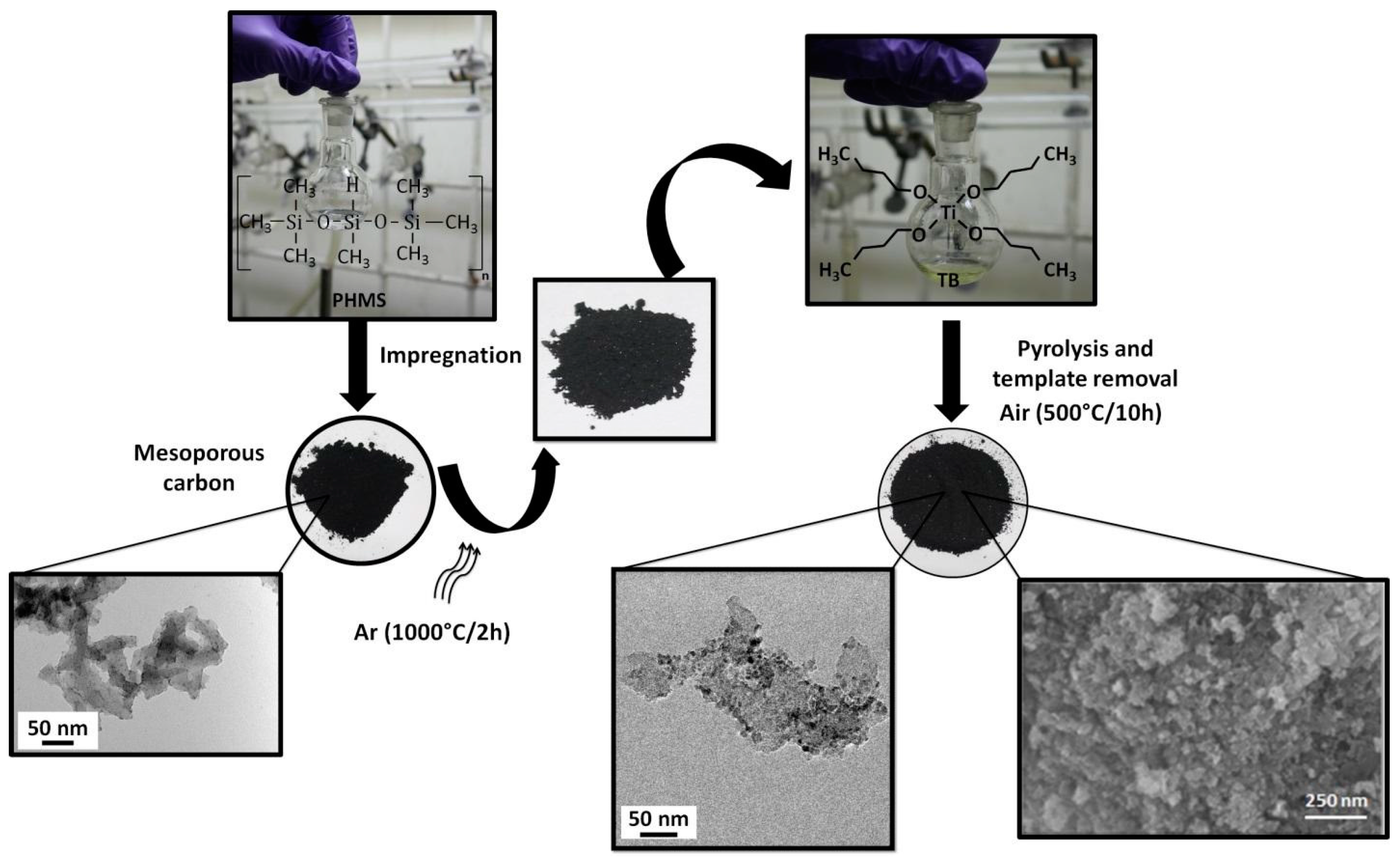
2.1. Design of Meso-/Macroporous Compounds
2.1.1. Synthesis of Macroporous Nanocomposite
2.1.2. Synthesis of Mesoporous Nanocomposites
2.2. Characterizations
2.3. Photocatalytic Studies
3. Results and Discussion
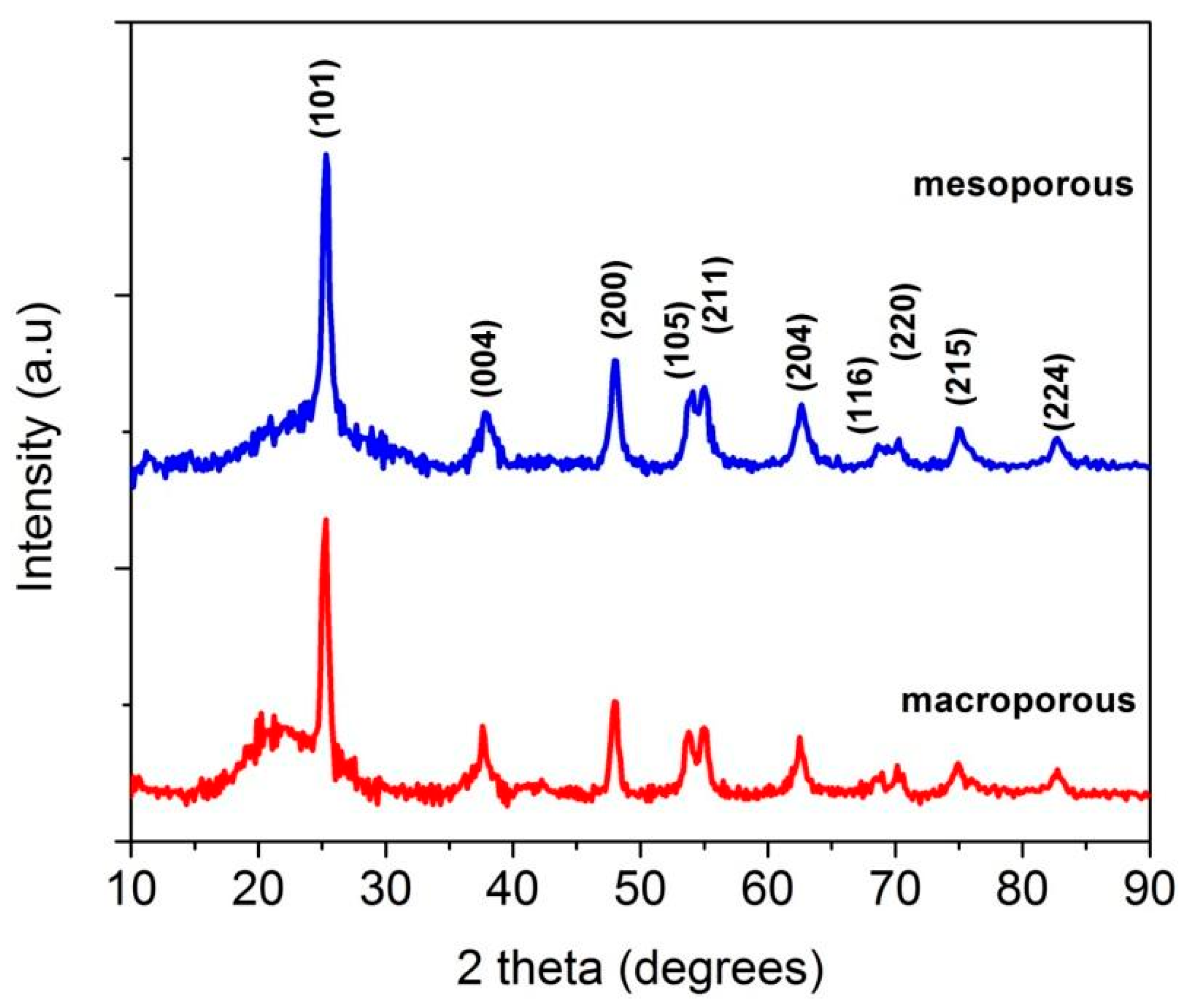
3.1. Phase Evolution
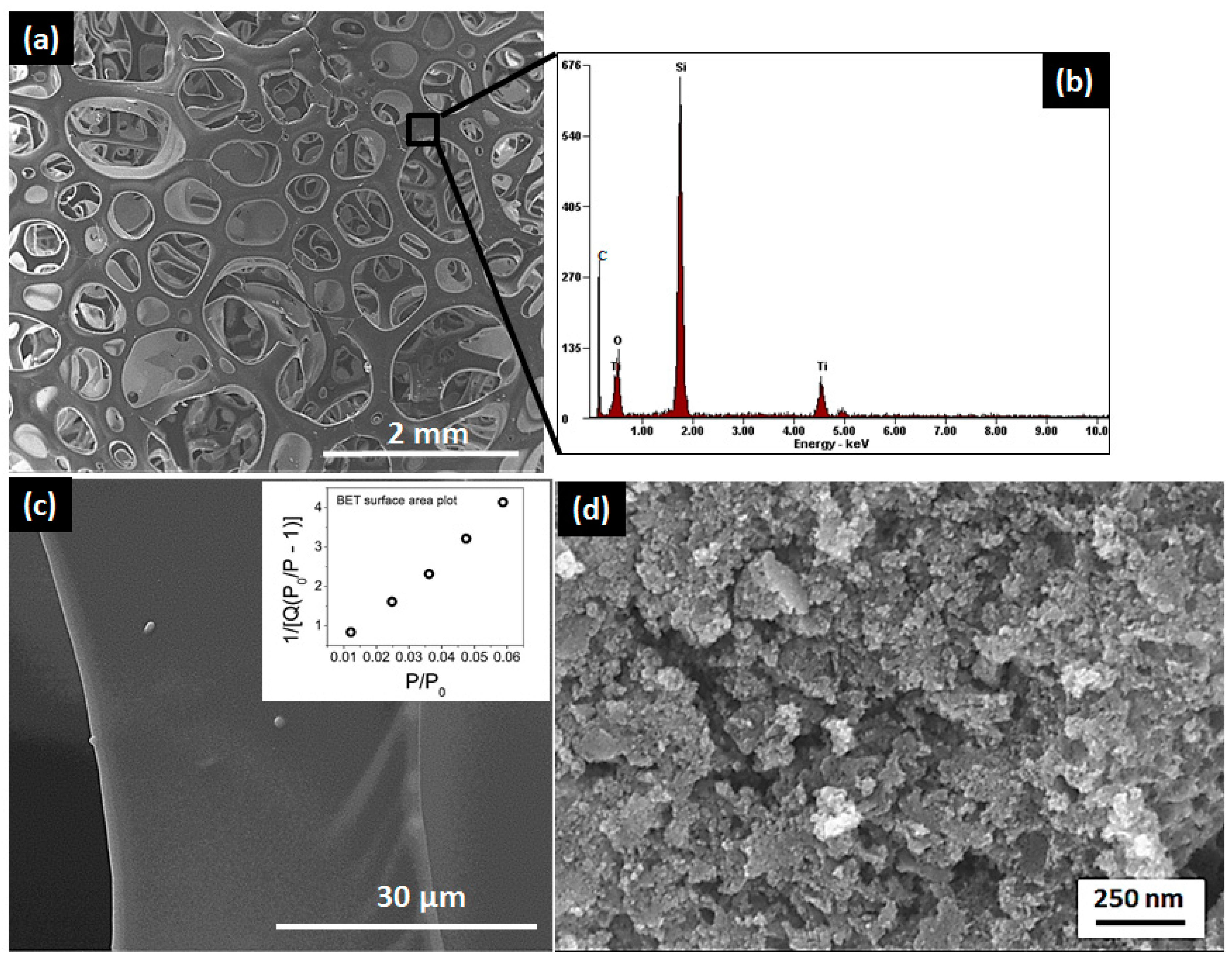
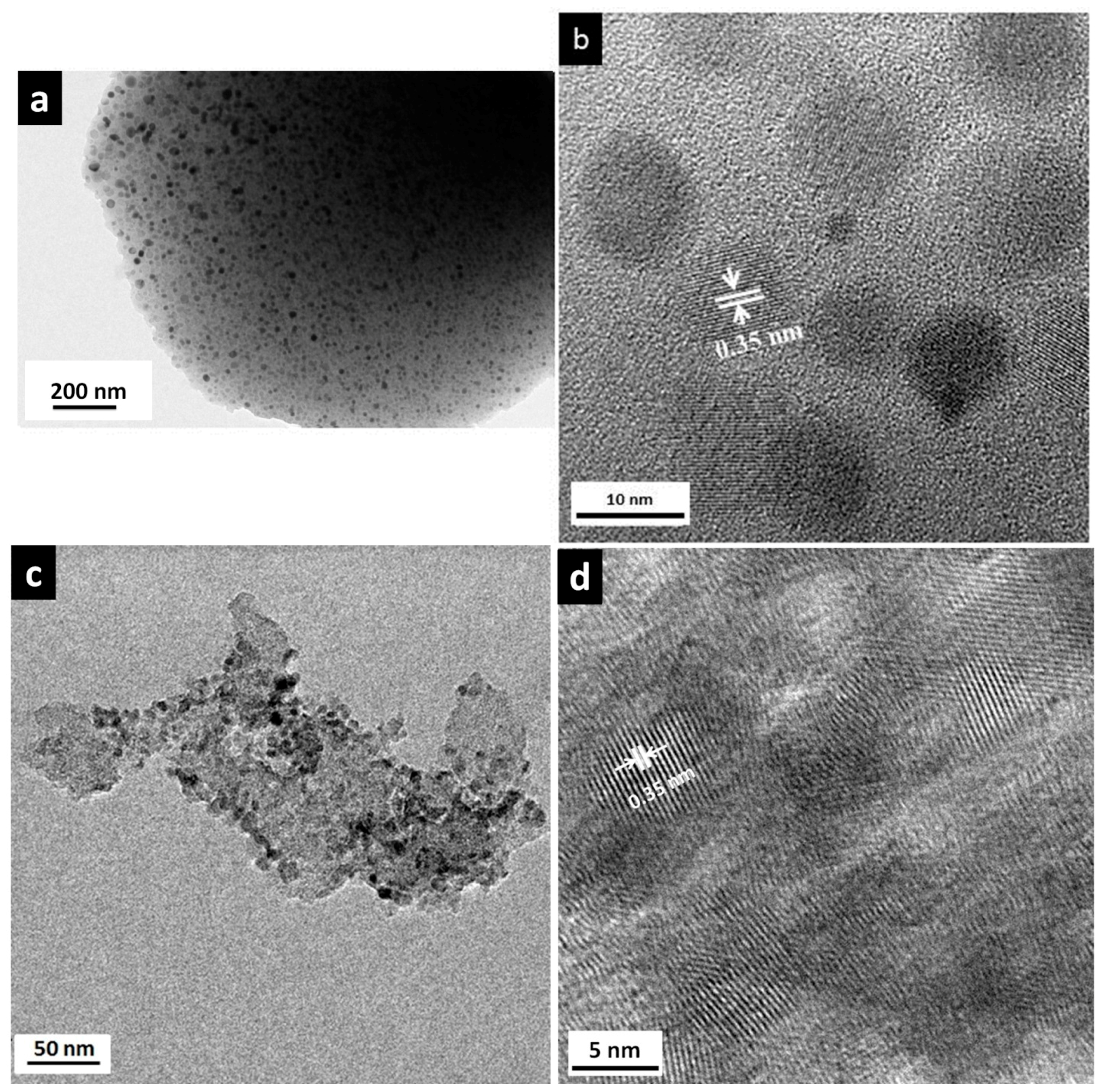
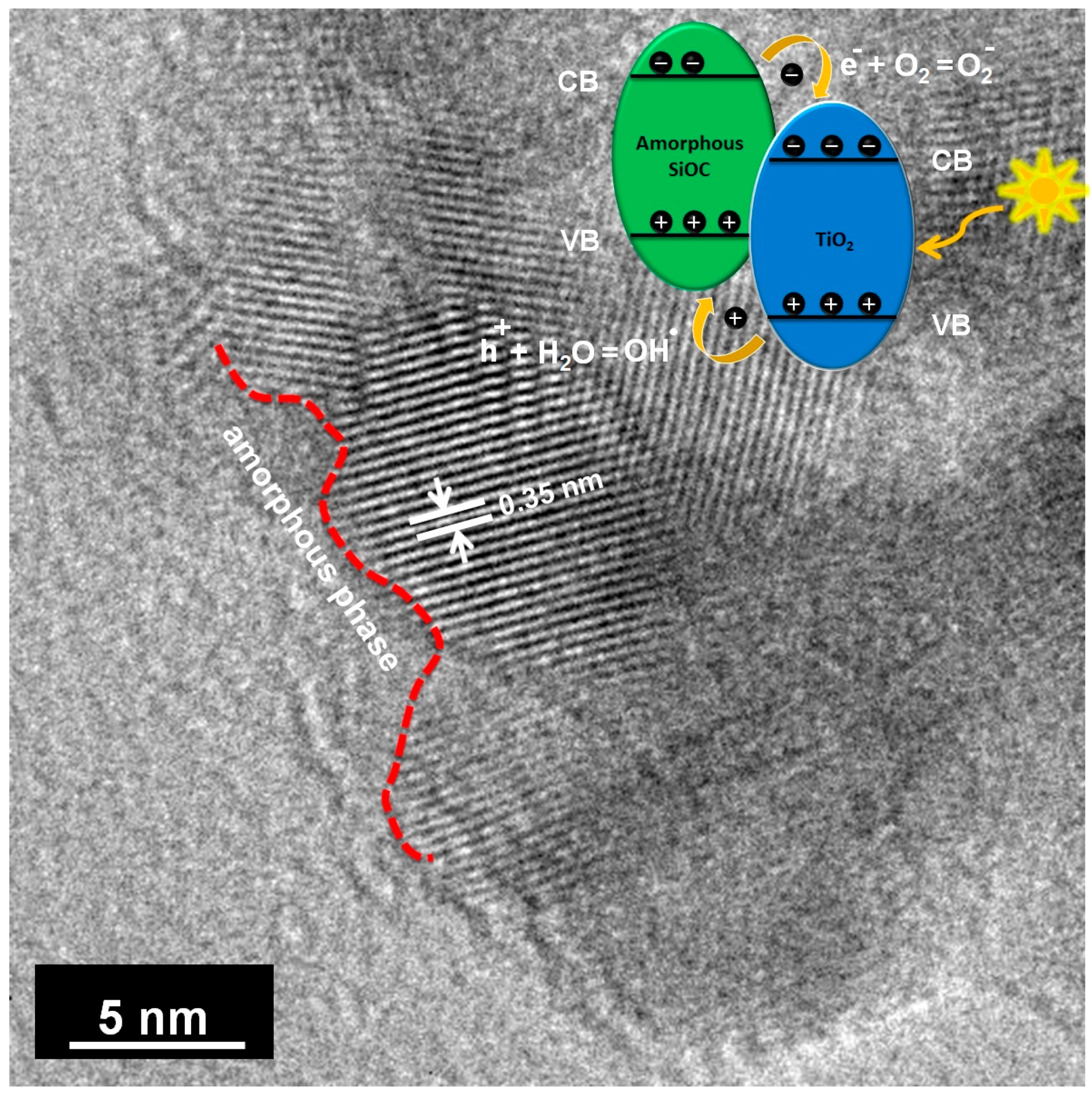
3.2. Microstructural and Nanostructural Features
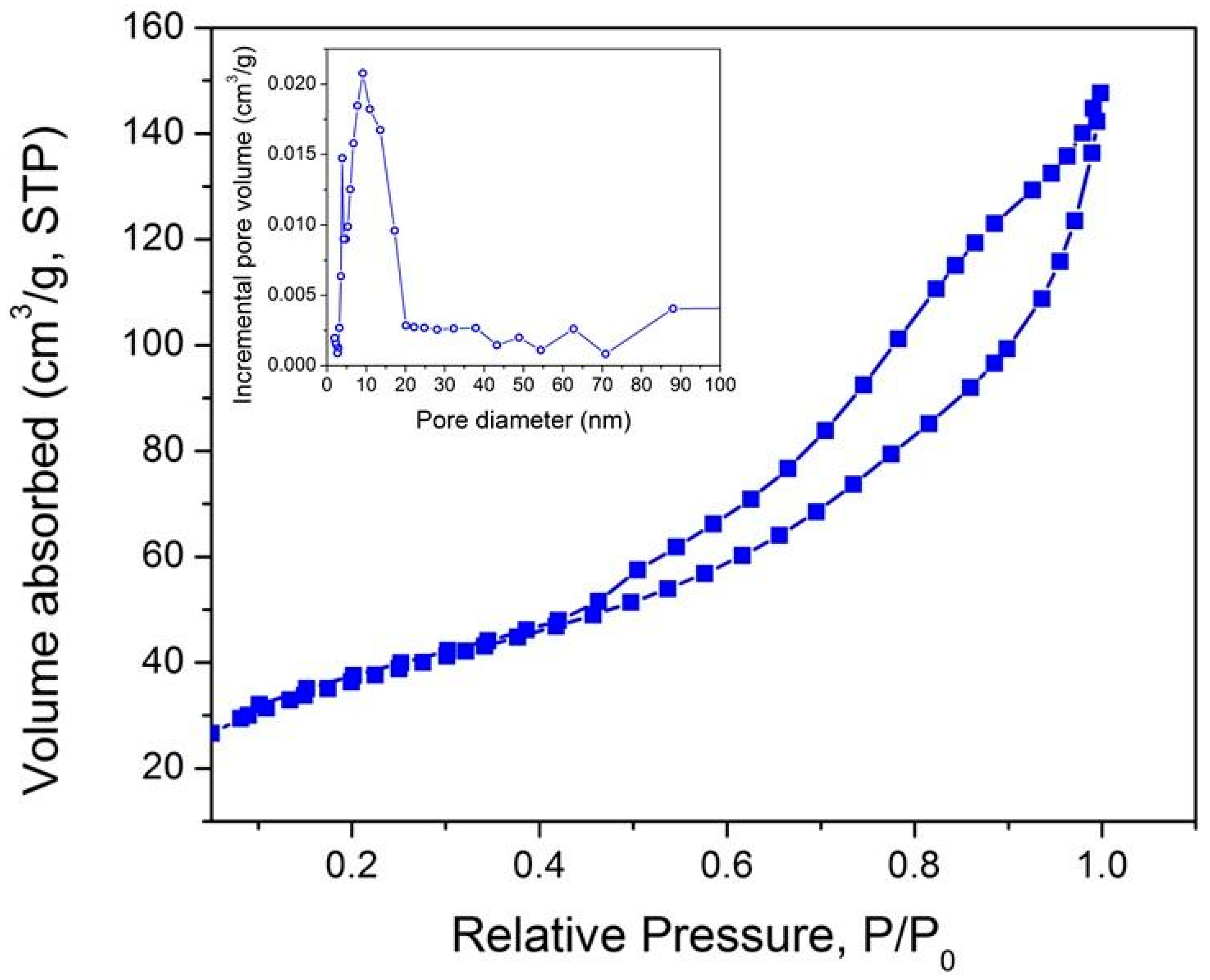
3.3. Characterization of Nanocomposites at the Meso-/Macroscopic Scales
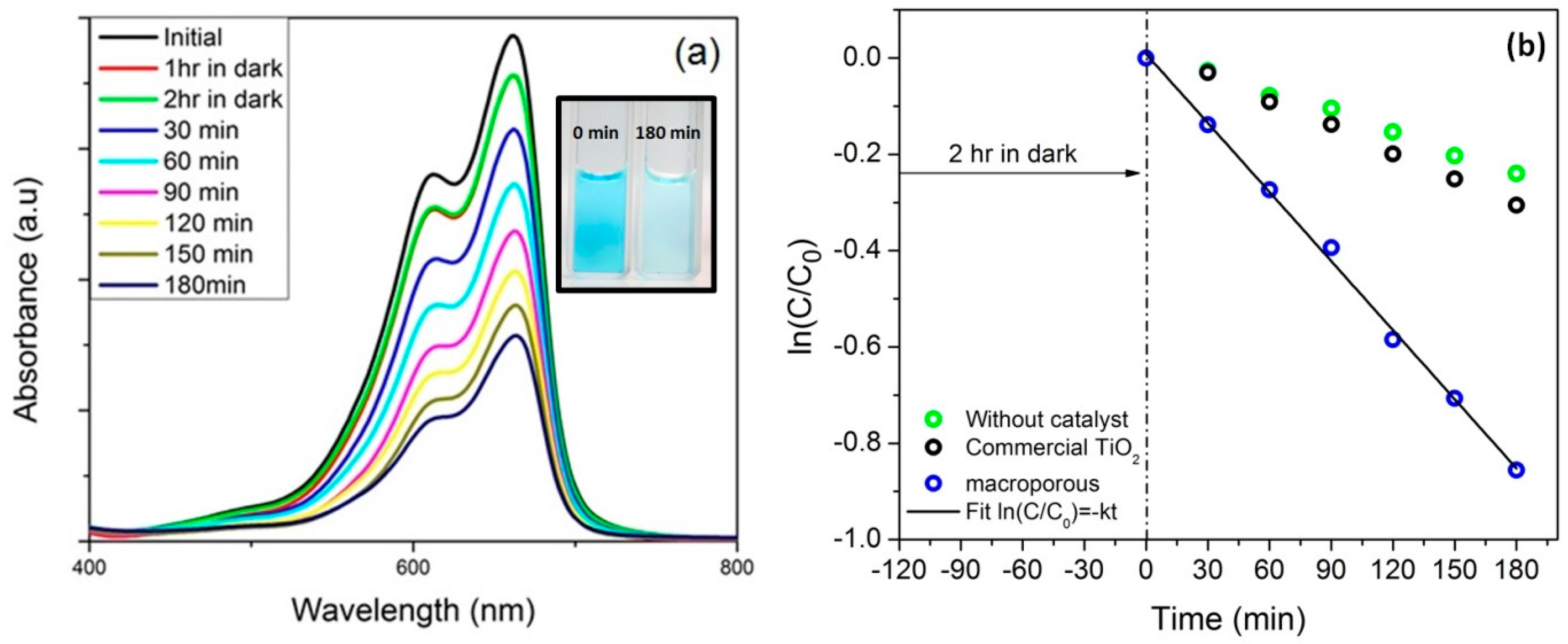
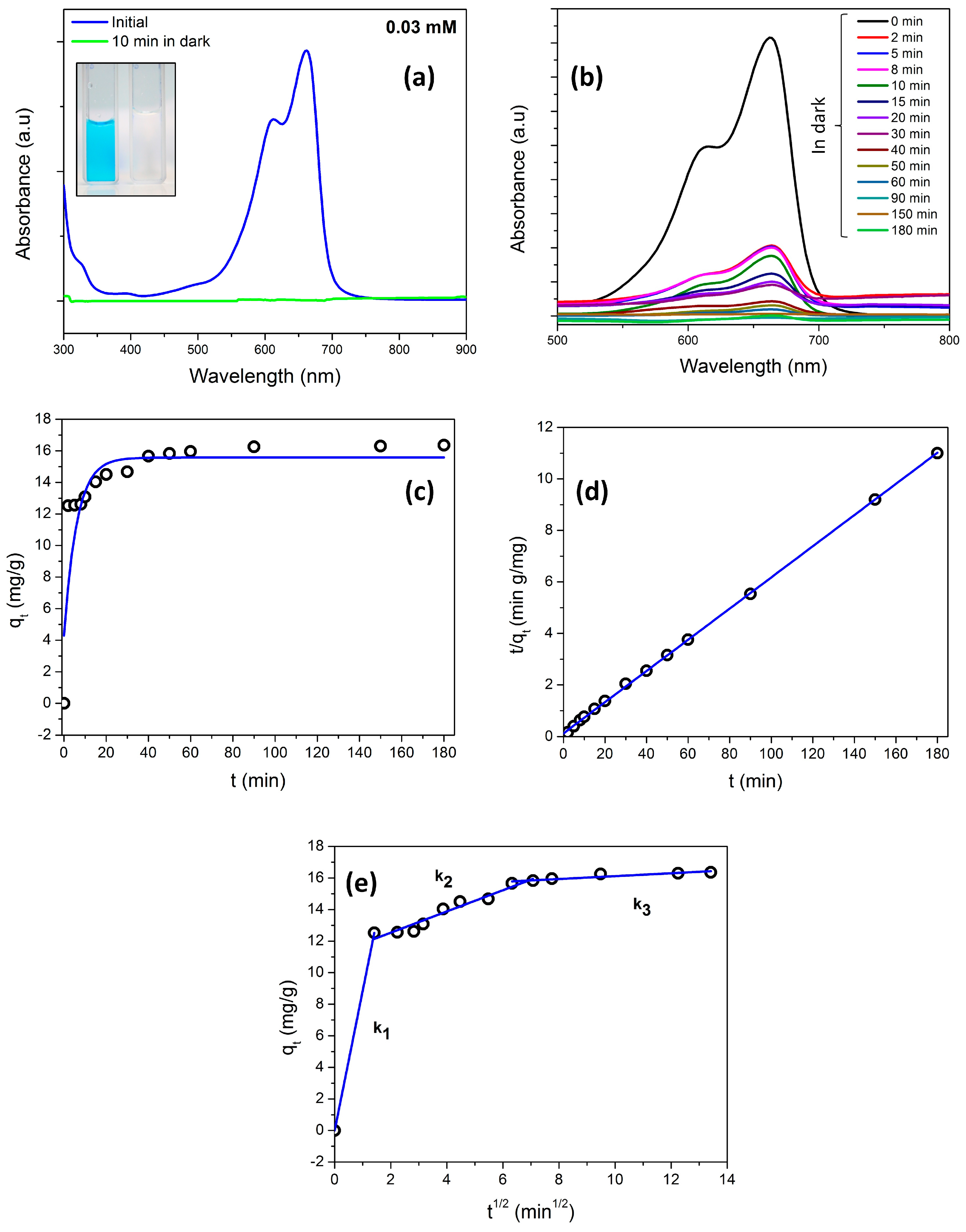
3.4. Adsorption/Photocatalyic Degradation Studies
3.5. Band Gap Measurement
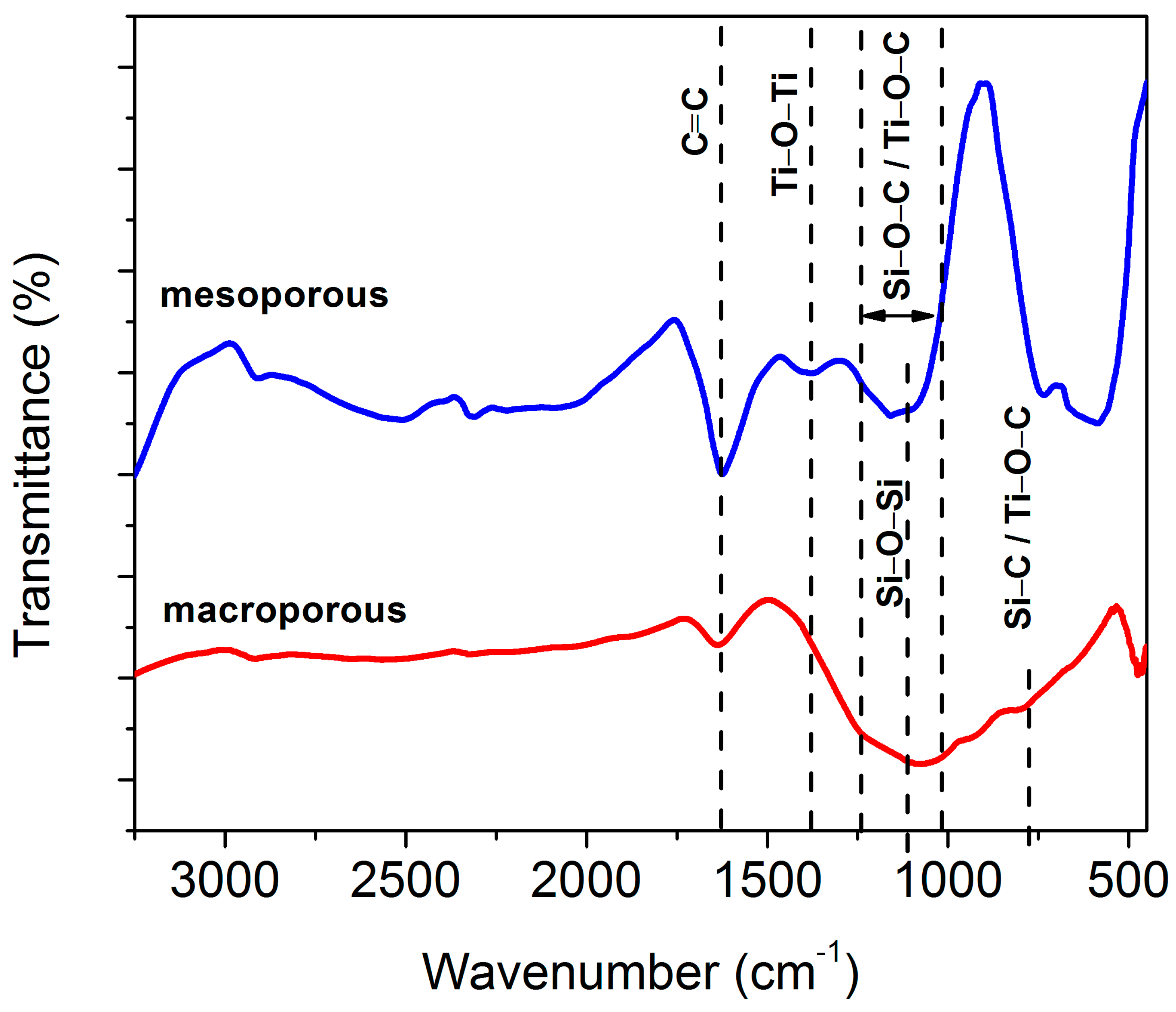
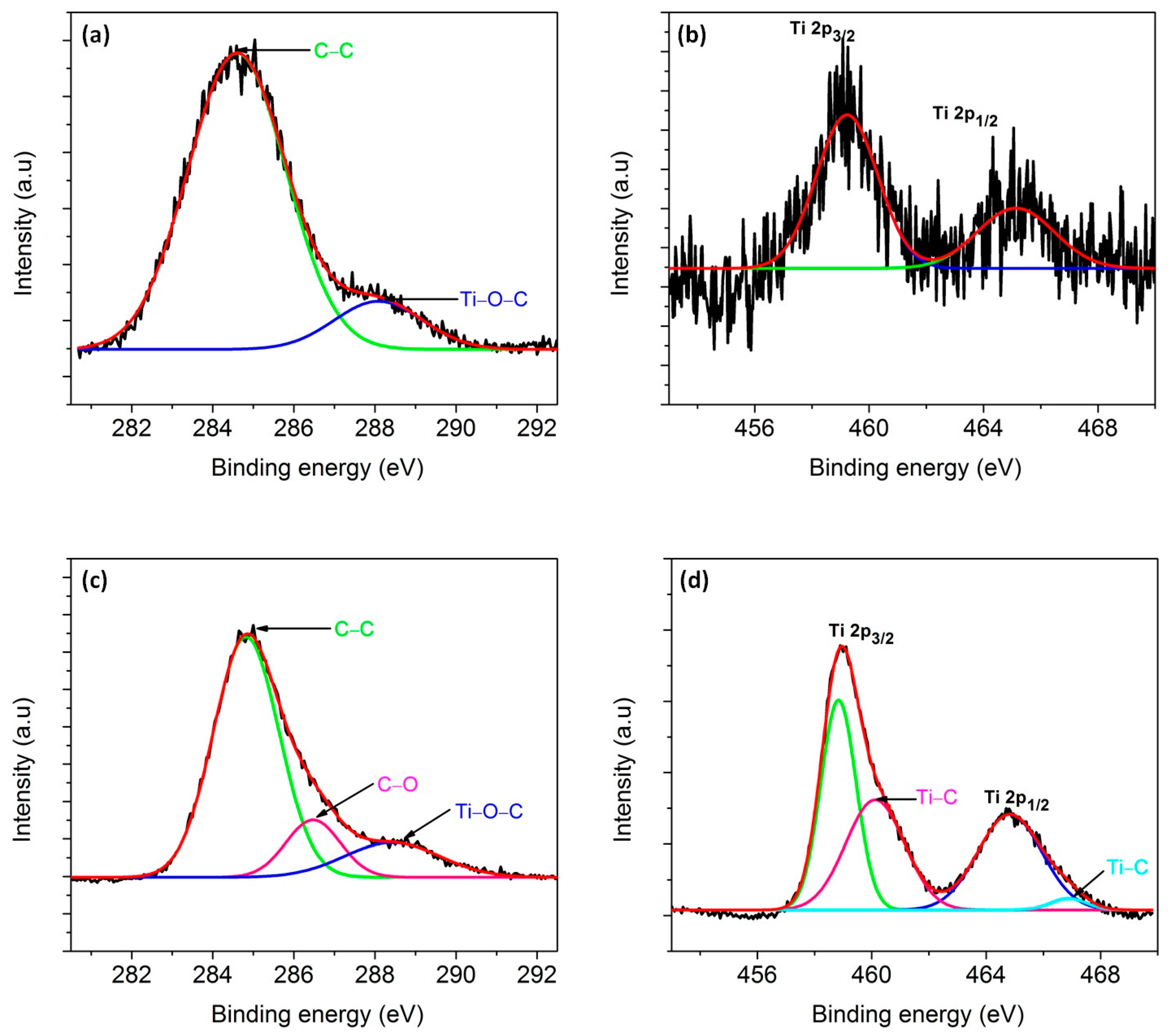
3.6. Bonding Characteristics
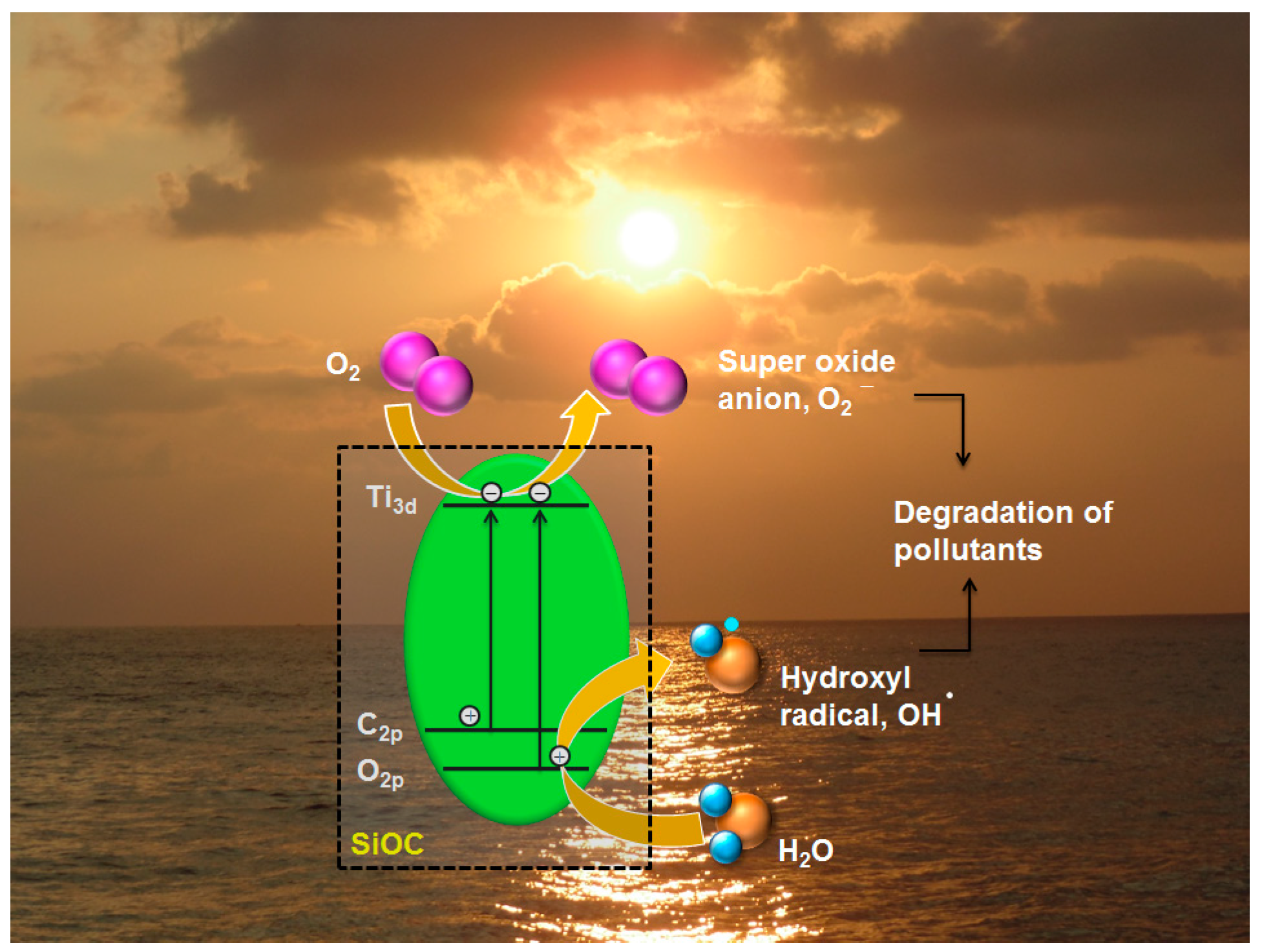
3.7. Photodegradation Mechanism
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Soraru, G.D.; Andrea, G.D.; Glisenti, A. XPS Characterization of Gel-Derived Silicon Oxycarbide Glasses. Mater. Lett. 1996, 27, l–5. [Google Scholar] [CrossRef]
- Du, P.; Wang, X.; Lin, I.-K.; Zhang, X. Effects of Composition and Thermal Annealing on the Mechanical Properties of Silicon Oxycarbide Films. Sens. Actuators A Phys. 2012, 176, 90–98. [Google Scholar] [CrossRef]
- Sorarù, G.D.; Pederiva, L.; Latournerie, J.; Raj, R. Pyrolysis Kinetics for the Conversion of a Polymer into an Amorphous Silicon Oxycarbide Ceramic. J. Am. Ceram. Soc. 2002, 85, 2181–2187. [Google Scholar] [CrossRef]
- Xu, T.; Ma, Q.; Chen, Z. High-Temperature Behavior of Silicon Oxycarbide Glasses in Air Environment. Ceram. Int. 2011, 37, 2555–2559. [Google Scholar] [CrossRef]
- Narisawa, M.; Ryu’ichi, S.; Ken’ichiro, K. Evaluation of Oxidation Resistance of Thin Continuous Silicon Oxycarbide Fiber Derived from Silicone Resin with Low Carbon Content. J. Mater. Sci. 2010, 45, 5642–5648. [Google Scholar] [CrossRef]
- Ionescu, E.; Kleebe, H.J.; Riedel, R. Silicon-Containing Polymer-Derived Ceramic Nanocomposites (PDC-NCs): Preparative Approaches and Properties. Chem. Soc. Rev. 2012, 41, 5032–5052. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.A.; Dulay, M.T. Heterogeneous Photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A Low-Cost, High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2 Films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Kay, A.; Rodicio, I.; Humphry-Baker, R.; Mueller, E.; Liska, P.; Vlachopoulos, N.; Graetzel, M. Conversion of Light to Electricity by Cis-X2bis(2,2′-bipyridyl-4,4′-dicarboxylate)ruthenium(II) Charge-Transfer Sensitizers (X = Cl−, Br−, I−, CN−, and SCN−) on Nanocrystalline Titanium Dioxide Electrodes. J. Am. Chem. Soc. 1993, 115, 6382–6390. [Google Scholar] [CrossRef]
- Barbé, C.J.; Arendse, F.; Comte, P.; Jirousek, M.; Lenzmann, F.; Shklover, V.; Grätzel, M. Nanocrystalline Titanium Oxide Electrodes for Photovoltaic Applications. J. Am. Ceram. Soc. 2005, 80, 3157–3171. [Google Scholar] [CrossRef]
- Hu, Y.; Tsai, H.-L.; Huang, C.-L. Effect of Brookite Phase on the Anatase–rutile Transition in Titania Nanoparticles. J. Eur. Ceram. Soc. 2003, 23, 691–696. [Google Scholar] [CrossRef]
- Aguado, J.; van Grieken, R.; López-Muñoz, M.; Marugán, J. Removal of Cyanides in Wastewater by Supported TiO2-Based Photocatalysts. Catal. Today 2002, 75, 95–102. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An Overview on Limitations of TiO2-Based Particles for Photocatalytic Degradation of Organic Pollutants and the Corresponding Countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Irie, H.; Watanabe, Y.; Hashimoto, K. Carbon-Doped Anatase TiO2 Powders as a Visible-Light Sensitive Photocatalyst. Chem. Lett. 2003, 32, 772–773. [Google Scholar] [CrossRef]
- Sakthivel, S.; Kisch, H. Daylight Photocatalysis by Carbon-Modified Titanium Dioxide. Angew. Chem. Int. Ed. 2003, 42, 4908–4911. [Google Scholar] [CrossRef] [PubMed]
- Czoska, A.M.; Livraghi, S.; Chiesa, M.; Giamello, E.; Agnoli, S.; Granozzi, G.; Finazzi, E.; Di Valentin, C.; Pacchioni, G. The Nature of Defects in Fluorine-Doped TiO2. J. Phys. Chem. C 2008, 112, 8951–8956. [Google Scholar] [CrossRef]
- Mizukoshi, Y.; Ohtsu, N.; Semboshi, S.; Masahashi, N. Visible Light Responses of Sulfur-Doped Rutile Titanium Dioxide Photocatalysts Fabricated by Anodic Oxidation. Appl. Catal. B Environ. 2009, 91, 152–156. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, Y.; Zhao, X. Low-Temperature Preparation and Visible-Light-Induced Catalytic Activity of Anatase F–N-Codoped TiO2. J. Mol. Catal. A Chem. 2007, 277, 119–126. [Google Scholar] [CrossRef]
- Liu, S.; Yu, J.; Wang, W.; Rodriguez, J.A.; Sanz, J.F.; Cappelletti, G.; Bianchi, C.L.; Lu, G.Q. Effects of Annealing on the Microstructures and Photoactivity of Fluorinated N-Doped TiO2. Phys. Chem. Chem. Phys. 2010, 12, 12308. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Seery, M.K.; Hinder, S.J.; Pillai, S.C. Oxygen Rich Titania: A Dopant Free, High Temperature Stable, and Visible-Light Active Anatase Photocatalyst. Adv. Funct. Mater. 2011, 21, 3744–3752. [Google Scholar] [CrossRef] [Green Version]
- Serpone, N. Is the Band Gap of Pristine TiO2 Narrowed by Anion- and Cation-Doping of Titanium Dioxide in Second-Generation Photocatalysts? J. Phys. Chem. B 2006, 110, 24287–24293. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.R.; Xia, X.H.; Guo, M.L.; Gao, Y.; Shao, G. Mn-Doped TiO2 Nanopowders with Remarkable Visible Light Photocatalytic Activity. Mater. Lett. 2011, 65, 2051–2054. [Google Scholar] [CrossRef]
- Gomathi Devi, L.; Narasimha Murthy, B. Characterization of Mo Doped TiO2 and Its Enhanced Photo Catalytic Activity Under Visible Light. Catal. Lett. 2008, 125, 320–330. [Google Scholar] [CrossRef]
- Ould-Chikh, S.; Proux, O.; Afanasiev, P.; Khrouz, L.; Hedhili, M.N.; Anjum, D.H.; Harb, M.; Geantet, C.; Basset, J.-M.; Puzenat, E. Photocatalysis with Chromium-Doped TiO2: Bulk and Surface Doping. ChemSusChem 2014, 7, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Khurana, C.; Pandey, O.P.; Chudasama, B. Synthesis of Visible Light-Responsive Cobalt-Doped TiO2 Nanoparticles with Tunable Optical Band Gap. J. Sol-Gel Sci. Technol. 2015, 75, 424–435. [Google Scholar] [CrossRef]
- Kim, S.; Hwang, S.-J.; Choi, W. Visible Light Active Platinum-Ion-Doped TiO2 Photocatalyst. J. Phys. Chem. B 2005, 109, 24260–24267. [Google Scholar] [CrossRef] [PubMed]
- Wetchakun, N.; Pirakitikulr, P.; Chiang, K.; Phanichphant, S. Visible Light-Active Nano-Sized Fe-Doped TiO2 Photocatalysts and Their Characterization. In Proceedings of the 2nd IEEE International Nanoelectronics Conference, Shanghai, China, 24–27 March 2008; pp. 836–841. [Google Scholar]
- Ohno, T.; Tanigawa, F.; Fujihara, K.; Izumi, S.; Matsumura, M. Photocatalytic Oxidation of Water by Visible Light Using Ruthenium-Doped Titanium Dioxide Powder. J. Photochem. Photobiol. A Chem. 1999, 127, 107–110. [Google Scholar] [CrossRef]
- Yu, Y.; Wen, W.; Qian, X.-Y.; Liu, J.-B.; Wu, J.-M. UV and Visible Light Photocatalytic Activity of Au/TiO2 Nanoforests with Anatase/Rutile Phase Junctions and Controlled Au Locations. Sci. Rep. 2017, 7, 41253. [Google Scholar] [CrossRef] [PubMed]
- Michalow, K.A.; Vital, A.; Heel, A.; Graule, T.; Reifler, F.A.; Ritter, A.; Zakrzewska, K.; Rekas, M. Photocatalytic Activity of W-Doped TiO2 Nanopowders. J. Adv. Oxid. Technol. 2008, 11, 56–64. [Google Scholar] [CrossRef]
- Wu, T.; Lin, T.; Zhao, J.; Hidaka, H.; Serpone, N. TiO2-Assisted Photodegradation of Dyes. 9. Photooxidation of a Squarylium Cyanine Dye in Aqueous Dispersions under Visible Light Irradiation. Environ. Sci. Technol. 1999, 33, 1379–1387. [Google Scholar] [CrossRef]
- Wu, T.; Liu, G.; Zhao, J.; Hidaka, H.; Serpone, N. Photoassisted Degradation of Dye Pollutants. V. Self-Photosensitized Oxidative Transformation of Rhodamine B under Visible Light Irradiation in Aqueous TiO2 Dispersions. J. Phys. Chem. B 1998, 102, 5845–5851. [Google Scholar] [CrossRef]
- Xu, Y.; Langford, C.H. UV- or Visible-Light-Induced Degradation of X3B on TiO2 Nanoparticles: The Influence of Adsorption. Langmuir 2001, 17, 897–902. [Google Scholar] [CrossRef]
- Marcì, G.; Augugliaro, V.; López-Muñoz, M.J.; Martín, C.; Palmisano, L.; Rives, V.; Schiavello, M.; Tilley, R.J.D.; Venezia, A.M. Preparation Characterization and Photocatalytic Activity of Polycrystalline ZnO/TiO2 Systems. 1. Surface and Bulk Characterization. J. Phys. Chem. B 2001, 105, 1026–1032. [Google Scholar] [CrossRef]
- Brahimi, R.; Bessekhouad, Y.; Bouguelia, A.; Trari, M. Visible Light Induced Hydrogen Evolution over the Heterosystem Bi2S3/TiO2. Catal. Today 2007, 122, 62–65. [Google Scholar] [CrossRef]
- Dong, W.; Lee, C.W.; Lu, X.; Sun, Y.; Hua, W.; Zhuang, G.; Zhang, S.; Chen, J.; Hou, H.; Zhao, D. Synchronous Role of Coupled Adsorption and Photocatalytic Oxidation on Ordered Mesoporous Anatase TiO2–SiO2 Nanocomposites Generating Excellent Degradation Activity of RhB Dye. Appl. Catal. B Environ. 2010, 95, 197–207. [Google Scholar] [CrossRef]
- Yoneyama, H.; Torimoto, T. Titanium Dioxide/adsorbent Hybrid Photocatalysts for Photodestruction of Organic Substances of Dilute Concentrations. Catal. Today 2000, 58, 133–140. [Google Scholar] [CrossRef]
- Xu, D.; Huang, Z.-H.; Kang, F.; Inagaki, M.; Ko, T.-H. Effect of Heat Treatment on Adsorption Performance and Photocatalytic Activity of TiO2-Mounted Activated Carbon Cloths. Catal. Today 2008, 139, 64–68. [Google Scholar] [CrossRef]
- Gómez-Solís, C.; Juárez-Ramírez, I.; Moctezuma, E.; Torres-Martínez, L.M. Photodegradation of Indigo Carmine and Methylene Blue Dyes in Aqueous Solution by SiC–TiO2 Catalysts Prepared by Sol–gel. J. Hazard. Mater. 2012, 217, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Kouamé, N.A.; Robert, D.; Keller, V.; Keller, N.; Pham, C.; Nguyen, P. Preliminary Study of the Use of β-SiC Foam as a Photocatalytic Support for Water Treatment. Catal. Today 2011, 161, 3–7. [Google Scholar] [CrossRef]
- Liu, Y.; Xin, F.; Wang, F.; Luo, S.; Yin, X. Synthesis, Characterization, and Activities of Visible Light-Driven Bi2O3–TiO2 Composite Photocatalysts. J. Alloys Compd. 2010, 498, 179–184. [Google Scholar] [CrossRef]
- He, J.; Cai, Q.Z.; Ji, Y.G.; Luo, H.H.; Li, D.J.; Yu, B. Influence of Fluorine on the Structure and Photocatalytic Activity of TiO2 Film Prepared in Tungstate-Electrolyte via Micro-Arc Oxidation. J. Alloys Compd. 2009, 482, 476–481. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, X.; Pan, C. “In Situ” Preparation of a TiO2/Eu2O3 Composite Film upon Ti Alloy Substrate by Micro-Arc Oxidation and Its Photo-Catalytic Property. J. Alloys Compd. 2012, 538, 16–20. [Google Scholar] [CrossRef]
- Hojamberdiev, M.; Prasad, R.M.; Morita, K.; Zhu, Y.; Schiavon, M.A.; Gurlo, A.; Riedel, R. Template-Free Synthesis of Polymer-Derived Mesoporous SiOC/TiO2 and SiOC/N-Doped TiO2 Ceramic Composites for Application in the Removal of Organic Dyes from Contaminated Water. Appl. Catal. B Environ. 2012, 115, 303–313. [Google Scholar] [CrossRef]
- Li, Z.; Hou, B.; Xu, Y.; Wu, D.; Sun, Y. Hydrothermal Synthesis, Characterization, and Photocatalytic Performance of Silica-Modified Titanium Dioxide Nanoparticles. J. Colloid Interface Sci. 2005, 288, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Osterlund, L.; Ljungstrom, S.; Palmqvist, A. Preparation of Nanosize Anatase and Rutile TiO2 by Hydrothermal Treatment of Microemulsions and Their Activity for Photocatalytic Wet Oxidation of Phenol. J. Phys. Chem. B 2002, 106, 10674–10679. [Google Scholar] [CrossRef]
- Yu, J.C.; Zhang, L.; Zheng, Z.; Zhao, J. Synthesis and Characterization of Phosphated Mesoporous Titanium Dioxide with High Photocatalytic Activity. Chem. Mater. 2003, 15, 2280–2286. [Google Scholar] [CrossRef]
- Elder, S.H.; Gao, Y.; Li, X.; Liu, J.; McCready, D.E.; Windisch, C.F. Zirconia-Stabilized 25-Å TiO2 Anatase Crystallites in a Mesoporous Structure. Chem. Mater. 1998, 10, 3140–3145. [Google Scholar] [CrossRef]
- Ovenstone, J.; Yanagisawa, K. Effect of Hydrothermal Treatment of Amorphous Titania on the Phase Change from Anatase to Rutile during Calcination. Chem. Mater. 1999, 11, 2770–2774. [Google Scholar] [CrossRef]
- Colombo, P.; Mera, G.; Riedel, R.; Soraru, G.D. Polymer-Derived Ceramics: 40 Years of Research and Innovation in Advanced Ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Colombo, P. (Ed.) Polymer Derived Ceramics: From Nano-Structure to Applications; DEStech Publications: Lancaster, PA, USA, 2010. [Google Scholar]
- Mera, G.; Gallei, M.; Bernard, S.; Ionescu, E. Ceramic Nanocomposites from Tailor-Made Preceramic Polymers. Nanomaterials 2015, 5, 468–540. [Google Scholar] [CrossRef] [PubMed]
- Viard, A.; Miele, P.; Bernard, S. Polymer-Derived Ceramics Route toward SiCN and SiBCN Fibers: From Chemistry of Polycarbosilazanes to the Design and Characterization of Ceramic Fibers. J. Ceram. Soc. Jpn. 2016, 124, 967–980. [Google Scholar] [CrossRef]
- Bechelany, M.C.; Proust, V.; Gervais, C.; Ghisleni, R.; Bernard, S.; Miele, P. In Situ Controlled Growth of Titanium Nitride in Amorphous Silicon Nitride: A General Route Toward Bulk Nitride Nanocomposites with Very High Hardness. Adv. Mater. 2014, 26, 6548–6553. [Google Scholar] [CrossRef] [PubMed]
- Bechelany, M.C.; Proust, V.; Lale, A.; Miele, P.; Malo, S.; Gervais, C.; Bernard, S. Nanocomposites through the Chemistry of Single-Source Precursors: Understanding the Role of Chemistry behind the Design of Monolith-Type Nanostructured Titanium Nitride/Silicon Nitride. Chem. A Eur. J. 2017, 23, 832–845. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, J.; Terzioglu, C.; Ionescu, E.; Graczyk-Zajac, M.; Hapis, S.; Kleebe, H.-J.; Riedel, R. Stable SiOC/Sn Nanocomposite Anodes for Lithium-Ion Batteries with Outstanding Cycling Stability. Adv. Funct. Mater. 2014, 24, 4097–4104. [Google Scholar] [CrossRef]
- Wen, Q.; Xu, Y.; Xu, B.; Fasel, C.; Guillon, O.; Buntkowsky, G.; Yu, Z.; Riedel, R.; Ionescu, E. Single-source-precursor synthesis of dense SiC/HfCxN1−x-based ultrahigh-temperature ceramic nanocomposites. Nanoscale 2014, 6, 13678–13689. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, M.; Schmalz, T.; Motz, G.; Kempe, R. Polymer Derived Non-Oxide Ceramics Modified with Late Transition Metals. Chem. Soc. Rev. 2012, 41, 5102–5116. [Google Scholar] [CrossRef] [PubMed]
- Lutterotti, L.; Matthies, S.; Wenk, H.R. MAUD: A Friendly Java Program for Material Analysis Using Diffraction. CPD Newsl. 1999, 21, 14–15. [Google Scholar]
- Byrne, C.; Fagan, R.; Hinder, S.; McCormack, D.; Pillai, S. New Approach of Modifying the Anatase to Rutile Transition Temperature in TiO2 Photocatalysts. RSC Adv. 2016, 6, 95232–95238. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the Enhanced Photocatalytic Activity of Degussa P25 Mixed-Phase TiO2 Using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Shao, X.; Lu, W.; Zhang, R.; Pan, F. Enhanced Photocatalytic Activity of TiO2-C Hybrid Aerogels for Methylene Blue Degradation. Sci. Rep. 2013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.F.; He, Y.L.; Zhang, M.S.; Yin, Z.; Chen, Q. Raman Scattering Study on Anatase TiO2 Nanocrystals. J. Phys. D Appl. Phys. 2000, 33, 912–916. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman Spectroscopy of Graphene and Graphite: Disorder, Electron–Phonon Coupling, Doping and Nonadiabatic Effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Duan, L.; Ma, Q. Effect of Pyrolysis Temperature on the Pore Structure Evolution of Polysiloxane-Derived Ceramics. Ceram. Int. 2012, 38, 2667–2671. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the Characterization of Porous Solids. Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Grosman, A.; Ortega, C. Nature of Capillary Condensation and Evaporation Processes in Ordered Porous Materials. Langmuir 2005, 21, 10515–10521. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.R.; Kruk, M.; Mercuri, L.P.; Jaroniec, M.; Zhao, L.; Kamiyama, T.; Terasaki, O.; Pinnavaia, T.J.; Liu, Y. Ordered Mesoporous Silica with Large Cage-Like Pores: Structural Identification and Pore Connectivity Design by Controlling the Synthesis Temperature and Time. J. Am. Chem. Soc. 2003, 125, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zou, Z.; Ye, J. Efficient Photocatalysis on BaBiO3 Driven by Visible Light. Phys. Chem. C 2007, 111, 12779–12785. [Google Scholar] [CrossRef]
- Tang, J.; Zou, Z.; Ye, J. Efficient Photocatalytic Decomposition of Organic Contaminants over CaBi2O4 under Visible-Light Irradiation. Angew. Chem. Int. Ed. 2004, 43, 4463–4466. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.V.; Porkodi, K.; Rocha, F. Langmuir–Hinshelwood Kinetics—A Theoretical Study. Catal. Commun. 2008, 9, 82–84. [Google Scholar] [CrossRef]
- Wang, R.; Cai, X.; Shen, F. TiO2 Hollow Microspheres with Mesoporous Surface: Superior Adsorption Performance for Dye Removal. Appl. Surf. Sci. 2014, 305, 352–358. [Google Scholar] [CrossRef]
- Ho, Y.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Han, S.; Liu, K.; Hu, L.; Teng, F.; Yu, P.; Zhu, Y. Superior Adsorption and Regenerable Dye Adsorbent Based on Flower-Like Molybdenum Disulfide Nanostructure. Sci. Rep. 2017, 7, 43599. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yin, S.-F.; Luo, S.-L.; Huang, R.; Zhang, Q.; Hong, T.; Au, P.C.T. Bi2O2CO3/BiOI Photocatalysts with Heterojunctions Highly Efficient for Visible-Light Treatment of Dye-Containing Wastewater. Ind. Eng. Chem. Res. 2012, 51, 6760–6768. [Google Scholar] [CrossRef]
- Huang, H.; He, Y.; Lin, Z.; Kang, L.; Zhang, Y. Two Novel Bi-Based Borate Photocatalysts: Crystal Structure, Electronic Structure, Photoelectrochemical Properties, and Photocatalytic Activity under Simulated Solar Light Irradiation. J. Phys. Chem. C 2013, 117, 22986–22994. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, G.; Naushad, M.; Singh, P.; Kalia, S. Polyacrylamide/Ni0.02Zn0.98O Nanocomposite with High Solar Light Photocatalytic Activity and Efficient Adsorption Capacity for Toxic Dye Removal. Ind. Eng. Chem. Res. 2014, 53, 15549–15560. [Google Scholar] [CrossRef]
- Reddy, K.M.; Manorama, S.V.; Reddy, A.R. Bandgap Studies on Anatase Titanium Dioxide Nanoparticles. Mater. Chem. Phys. 2003, 78, 239–245. [Google Scholar] [CrossRef]
- Wu, G.; Nishikawa, T.; Ohtani, B.; Chen, A. Synthesis and Characterization of Carbon-Doped TiO2 Nanostructures with Enhanced Visible Light Response. Chem. Mater. 2007, 19, 4530–4537. [Google Scholar] [CrossRef]
- Kaspar, J.; Storch, M.; Schitco, C.; Riedel, R.; Graczyk-Zajac, M. SiOC(N)/Hard Carbon Composite Anodes for Na-Ion Batteries: Influence of Morphology on the Electrochemical Properties. J. Electrochem. Soc. 2016, 163, A156–A162. [Google Scholar] [CrossRef]
- Lui, G.; Liao, J.Y.; Duan, A.; Zhang, Z.; Fowler, M.; Yu, A. Graphene-Wrapped Hierarchical TiO2 Nanoflower Composites with Enhanced Photocatalytic Performance. J. Mater. Chem. A 2013, 1, 12255–12262. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, X.; Tang, Y.; Su, K.; Kong, J. Hierarchically Porous Silicon-Carbon-Nitrogen Hybrid Materials towards Highly Efficient and Selective Adsorption of Organic Dyes. Sci. Rep. 2015, 5, 7910. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, K. Highly Porous SiOC Bulk Ceramics with Water Vapor Assisted Pyrolysis. J. Am. Ceram. Soc. 2015, 98, 2357–2365. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, S. Photodegradation of Methyl Orange by Photocatalyst of CNTs/P-TiO2 under UV and Visible-Light Irradiation. J. Hazard. Mater. 2011, 185, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E.; Rahimi, K. Adverse Effects of Graphene Incorporated in TiO2 Photocatalyst on Minuscule Animals under Solar Light Irradiation. J. Mater. Chem. 2012, 22, 23260–23266. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, C. TiO2/graphene Composite from Thermal Reaction of Graphene Oxide and Its Photocatalytic Activity in Visible Light. J. Mater. Sci. 2011, 46, 2622–2626. [Google Scholar] [CrossRef]
- Ingo, G.M.; Dire, S.; Babonneau, F. XPS studies of SiO2-TiO2 powders prepared by sol-gel process. Appl. Surf. Sci. 1993, 70, 230–234. [Google Scholar] [CrossRef]
- Corriu, R.J.P.; Leclercq, D.; Mutin, P.H.; Vioux, A. Preparation and Structure of Silicon Oxycarbide Glasses Derived from Polysiloxane Precursors. J. Sol-Gel Sci. Technol. 1997, 8, 327–330. [Google Scholar] [CrossRef]
- Dire, S.; Ceccato, R. Structural and Microstructural Evolution during Pyrolysis of Hybrid Polydimethylsiloxane-Titania Nanocomposites. J. Sol-Gel Sci. Technol. 2005, 34, 53–62. [Google Scholar] [CrossRef]
- Chandra, C.; Kim, J. Silicon Oxycarbide Produced from Silicone Oil for High-Performance Anode Material in Sodium Ion Batteries. J. Chem. Eng. 2018, 338, 126–136. [Google Scholar] [CrossRef]
- Halim, M.; Hudaya, C.; Kim, A.Y.; Lee, J.K. Phenyl-Rich Silicone Oil as a Precursor for SiOC Anode Materials for Long-Cycle and High-Rate Lithium Ion Batteries. J. Mater. Chem. A 2016, 4, 2651–2656. [Google Scholar]
- Kamisaka, H.; Adachi, T.; Yamashita, K. Theoretical Study of the Structure and Optical Properties of Carbon-Doped Rutile and Anatase Titanium Oxides. J. Chem. Phys. 2005, 123, 084704. [Google Scholar] [CrossRef] [PubMed]
- Yijun, Y.; Dawei, N.; Ye, Y.; Yeteng, Z.; Ying, M.; Jiannian, Y. High photocatalytic activity of carbon doped TiO2 prepared by fast combustion of organic capping ligands. RSC Adv. 2015, 5, 93635–93643. [Google Scholar]
- Nagaveni, K.; Hegde, M.S.; Ravishankar, N.; Subbanna, G.N.; Madras, G. Synthesis and Structure of Nanocrystalline TiO2 with Lower Band Gap Showing High Photocatalytic Activity. Langmuir 2004, 20, 2900–2907. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, J.; Feng, W.; An, L. Effect of Pyrolysis Temperature on the Piezoresistivity of Polymer-Derived Ceramics. J. Am. Ceram. Soc. 2011, 94, 359–362. [Google Scholar] [CrossRef]
- Zou, T.; Xie, C.; Liu, Y.; Zhang, S.; Zou, Z.; Zhang, S. Full Mineralization of Toluene by Photocatalytic Degradation with Porous TiO2/SiC Nanocomposite Film. J. Alloys Compd. 2013, 552, 504–510. [Google Scholar] [CrossRef]




| Material | K (10−3 × min−1) |
|---|---|
| SiOC | 1.3 [47] (0.03 mM of MB) |
| SiOC/TiO2 | 2.2 [47] (0.03 mM of MB) |
| SiOC/N-doped TiO2 | 3.4 [47] (0.03 mM of MB) |
| Macroporous TiO2/SiOC | 4.8 [present work] (0.03 mM of MB) |
| Mesoporous TiO2/SiOC | 3.0 [present work] (0.06 mM of MB) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasan Awin, E.; Lale, A.; Kumar, K.C.N.H.; Bilge Demirci, U.; Bernard, S.; Kumar, R. Novel Precursor-Derived Meso-/Macroporous TiO2/SiOC Nanocomposites with Highly Stable Anatase Nanophase Providing Visible Light Photocatalytic Activity and Superior Adsorption of Organic Dyes. Materials 2018, 11, 362. https://doi.org/10.3390/ma11030362
Wasan Awin E, Lale A, Kumar KCNH, Bilge Demirci U, Bernard S, Kumar R. Novel Precursor-Derived Meso-/Macroporous TiO2/SiOC Nanocomposites with Highly Stable Anatase Nanophase Providing Visible Light Photocatalytic Activity and Superior Adsorption of Organic Dyes. Materials. 2018; 11(3):362. https://doi.org/10.3390/ma11030362
Chicago/Turabian StyleWasan Awin, Eranezhuth, Abhijeet Lale, Kollamala Chellappan Nair Hari Kumar, Umit Bilge Demirci, Samuel Bernard, and Ravi Kumar. 2018. "Novel Precursor-Derived Meso-/Macroporous TiO2/SiOC Nanocomposites with Highly Stable Anatase Nanophase Providing Visible Light Photocatalytic Activity and Superior Adsorption of Organic Dyes" Materials 11, no. 3: 362. https://doi.org/10.3390/ma11030362






