Mg-MOF-74/MgF2 Composite Coating for Improving the Properties of Magnesium Alloy Implants: Hydrophilicity and Corrosion Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Instrumentation
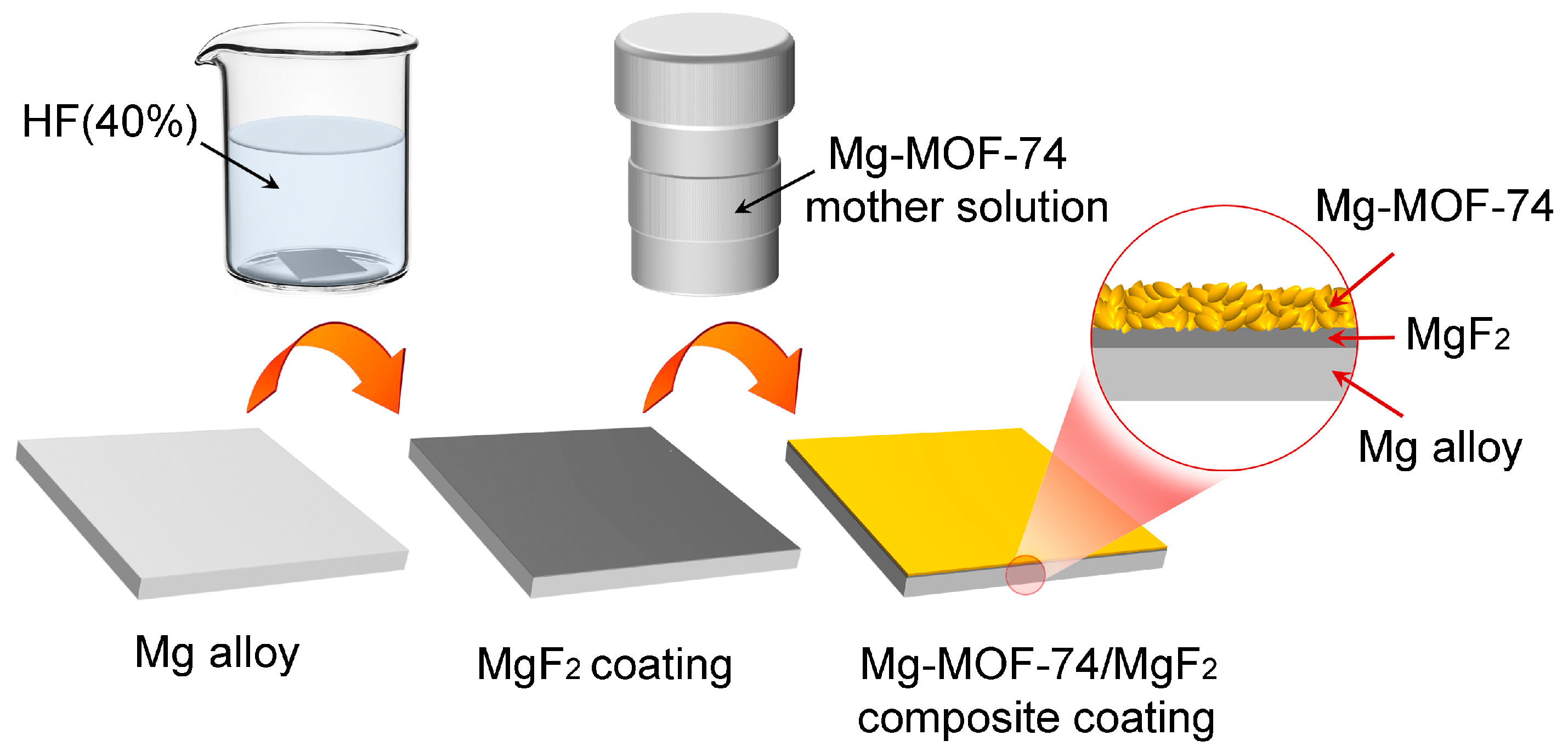
2.3. Sample Preparation
2.4. Corrosion Resistance Study
2.5. Hydrophilicity Study
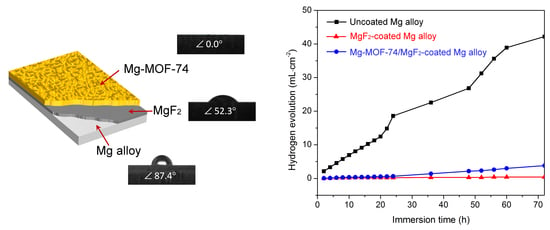
3. Results and Discussion
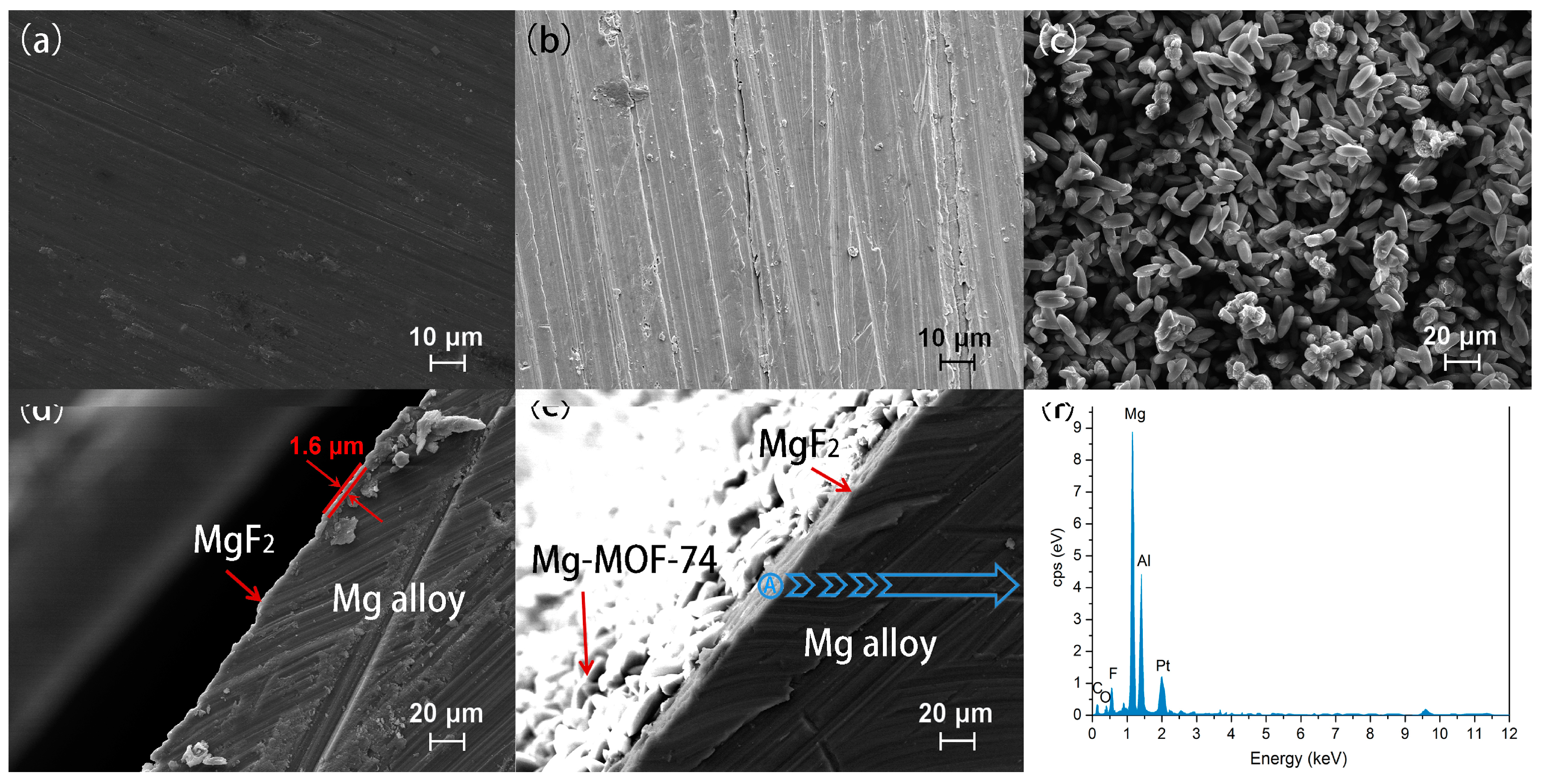
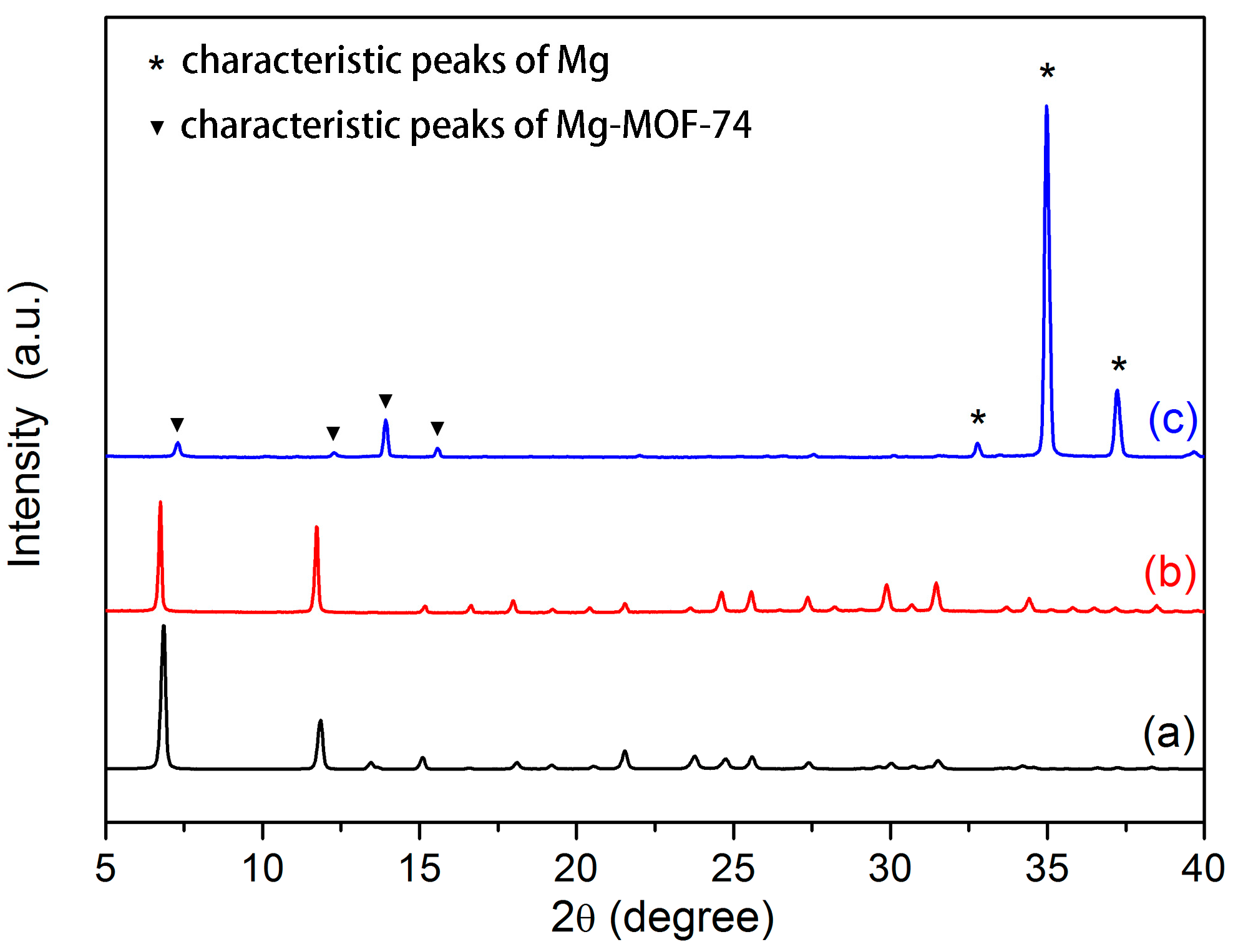
3.1. Microstructure and Composition of Coating
3.2. Corrosion Resistance Property
3.3. Hydrophilicity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Waizy, H.; Seitz, J.M.; Reifenrath, J.; Weizbauer, A.; Bach, F.W.; Meyer-Lindenberg, A.; Denkena, B.; Windhagen, H. Biodegradable magnesium implants for orthopedic applications. J. Mater. Sci. 2013, 48, 39–50. [Google Scholar] [CrossRef]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Fathi, M.; Savabi, O.; Razavi, S.M.; Heidari, F.; Manshaei, M.; Vashaee, D.; Tayebi, L. In vivo study of nanostructured diopside (CaMgSi2O6) coating on magnesium alloy as biodegradable orthopedic implants. Appl. Surf. Sci. 2014, 313, 60–66. [Google Scholar] [CrossRef]
- Shi, P.; Niu, B.; Shanshan, E.; Chen, Y.; Li, Q. Preparation and characterization of PLA coating and PLA/MAO composite coatings on AZ31 magnesium alloy for improvement of corrosion resistance. Surf. Coat. Technol. 2015, 262, 26–32. [Google Scholar] [CrossRef]
- Córdoba, L.C.; Montemor, M.F.; Coradin, T. Silane/TiO2 coating to control the corrosion rate of magnesium alloys in simulated body fluid. Corros. Sci. 2016, 104, 152–161. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Hu, T.; Chu, P.K. In vitro studies of biomedical magnesium alloys in a simulated physiological environment: A review. Acta Biomater. 2011, 7, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, E. Biocorrosion behavior of magnesium alloy in different simulated fluids for biomedical application. Mater. Sci. Eng. C 2009, 29, 1691–1696. [Google Scholar] [CrossRef]
- Song, G.; Song, S. A possible biodegradable magnesium implant material. Adv. Eng. Mater. 2007, 9, 298–302. [Google Scholar] [CrossRef]
- Saris, N.L.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium: An update on physiological, clinical and analytical aspects. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J.; Zhang, P.; Li, Y.; Wang, J.; Lai, Y.; Qin, L. Surface modification of magnesium alloys developed for bioabsorbable orthopedic implants: A general review. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100B, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Q.; Li, L.; Zhang, P.; Wang, Z.; Chen, F. Fabrication of hydroxyapatite/stearic acid composite coating and corrosion behavior of coated magnesium alloy. Mater. Lett. 2012, 88, 76–78. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, J.; Chen, M.; Liu, D. Biological activity evaluation of magnesium fluoride coated Mg-Zn-Zr alloy in vivo. Mater. Sci. Eng. C 2017, 75, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Han, Y.; Qi, K. Formation Mechanism, Degradation Behavior, and Cytocompatibility of a Nanorod-Shaped HA and Pore-Sealed MgO Bilayer Coating on Magnesium. ACS Appl. Mater. Interfaces 2014, 6, 18258–18274. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Hao, Q.; Peng, X.; Chu, P.K. Enhanced corrosion resistance and biocompatibility of PMMA-coated ZK60 magnesium alloy. Mater. Lett. 2016, 173, 178–181. [Google Scholar] [CrossRef]
- Makar, G.L.; Kruger, J. Corrosion of magnesium. Int. Mater. Rev. 1993, 38, 138–153. [Google Scholar] [CrossRef]
- Thomann, M.; Krause, C.; Angrisani, N.; Bormann, D.; Hassel, T.; Windhagen, H.; Meyer-Lindenberg, A. Influence of a magnesium-fluoride coating of magnesium-based implants (MgCa0.8) on degradation in a rabbit model. J. Biomed. Mater. Res. A 2010, 93A, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Drynda, A.; Seibt, J.; Hassel, T.; Bach, F.W.; Peuster, M. Biocompatibility of fluoride-coated magnesium-calcium alloys with optimized degradation kinetics in a subcutaneous mouse model. J. Biomed. Mater. Res. A 2013, 101, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Lu, Y.; Su, Y.; Hu, J.; Lian, J.; Li, G. Enhancing the corrosion resistance and surface bioactivity of a calcium-phosphate coating on a biodegradable AZ60 magnesium alloy via a simple fluorine post-treatment method. RSC Adv. 2015, 5, 56001–56010. [Google Scholar] [CrossRef]
- Ren, M.; Cai, S.; Liu, T.; Huang, K.; Wang, X.; Zhao, H.; Niu, S.; Zhang, R.; Wu, X. Calcium phosphate glass/MgF2 double layered composite coating for improving the corrosion resistance of magnesium alloy. J. Alloys Compd. 2014, 591, 34–40. [Google Scholar] [CrossRef]
- Feng, Y.; Zhu, S.; Wang, L.; Chang, L.; Yan, B.; Song, X.; Guan, S. Characterization and corrosion property of nano-rod-like HA on fluoride coating supported on Mg-Zn-Ca alloy. Bioact. Mater. 2017, 2, 63–70. [Google Scholar] [CrossRef]
- Zhang, Y.; Forsyth, M.; Hinton, B.R.W. The Effect of Treatment Temperature on Corrosion Resistance and Hydrophilicity of an lonic Liquid Coating for Mg-Based Stents. ACS Appl. Mater. Interfaces 2014, 6, 18989–18997. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Salvi, G.E.; Huyng-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin. Oral Implants Res. 2011, 22, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Cai, S.; Xu, G.; Dou, Y.; Hu, H. Synthesis of mesoporous hydroxyapatite thin films using F127 as templates for biomedical applications. Mater. Lett. 2012, 85, 64–67. [Google Scholar] [CrossRef]
- Rupp, F.; Scheideler, L.; Olshanska, N.; de Wild, M.; Wieland, M.; Geis-Gerstorfer, J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J. Biomed. Mater. Res. A 2006, 76, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.O.; Bijelic, A.; Ziebart, T.; Koch, F.; Kammerer, P.W.; Wieland, M.; Konerding, M.A.; Al-Nawas, B. Submicron Scale-Structured Hydrophilic Titanium Surfaces Promote Early Osteogenic Gene Response for Cell Adhesion and Cell Differentiation. Clin. Implant Dent. Relat. Res. 2013, 15, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Luo, C.; Zhang, Z.; Hermawan, H.; Zhu, D.; Huang, J.; Liang, Y.; Li, G.; Ren, L. Bioinspired surface functionalization of metallic biomaterials. J. Mech. Behav. Biomed. 2018, 77, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.W.; Zhao, Y.; Leung, F.K.L.; Xi, T.; Zhang, Z.; Zheng, Y.; Wu, S.; Luk, K.D.K.; Cheung, K.M.C.; Chu, P.; et al. Functionalized Polymeric Membrane with Enhanced Mechanical and Biological Properties to Control the Degradation of Magnesium Alloy. Adv. Healthc. Mater. 2017, 6, 1601269. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, W.; Yang, H.; Sun, T.; Liu, K.; Wang, Z.; Niu, C. Improved thermal dehydrogenation of ammonia borane by MOF-5. RSC Adv. 2015, 5, 10746–10750. [Google Scholar] [CrossRef]
- Rocca, J.D.; Liu, D.; Lin, W. Nanoscale Metal-Organic Frameworks for Biomedical Imaging and Drug Delivery. Acc. Chem. Res. 2011, 44, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal-Organic Frameworks in Biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Yu, J.; Liu, M.; Liu, A.; Dou, Z.; Yang, Y. A Low Cytotoxic Cationic Metal-Organic Framework Carrier for Controllable Drug Release. J. Med. Chem. 2014, 57, 5679–5685. [Google Scholar] [CrossRef] [PubMed]
- Mesbah, A.; Jacques, S.; Rocca, E.; Francois, M.; Steinmetz, J. Compact Metal-Organic Frameworks for Anti-Corrosion Applications: New Binary Linear Saturated Carboxylates of Zinc. Eur. J. Inorg. Chem. 2011, 8, 1315–1321. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Q.; Chen, R.; Liu, J.; Zhang, H.; Li, R.; Takahashi, K.; Liu, P.; Wang, J. Fabrication of ZIF-8@SiO2 Micro/Nano Hierarchical Superhydrophobic Surface on AZ31 Magnesium Alloy with Impressive Corrosion Resistance and Abrasion Resistance. ACS Appl. Mater. Interfaces 2017, 9, 11106–11115. [Google Scholar] [CrossRef] [PubMed]
- Bernini, M.C.; Fairen-Jimenez, D.; Pasinetti, M.; Ramirez-Pastor, A.J.; Snurr, R.Q. Screening of bio-compatible metal-organic frameworks as potential drug carriers using Monte Carlo simulations. J. Mater. Chem. B 2014, 2, 766–774. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Zeng, R.C.; Liu, C.L.; Gao, J.C. Comparison of calcium phosphate coatings on Mg-Al and Mg-Ca alloys and their corrosion behavior in Hank’s solution. Surf. Coat. Technol. 2010, 204, 3636–3640. [Google Scholar] [CrossRef]
- Ben, T.; Lu, C.; Pei, C.; Xu, S.; Qiu, S. Polymer-Supported and Free-Standing Metal-Organic Framework Membrane. Chem. Eur. J. 2012, 18, 10250–10253. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhu, G.; Hewitt, I.J.; Qiu, S. “Twin Copper Source” Growth of Metal-Organic Framework Membrane: Cu3(BTC)2 with High Permeability and Selectivity for Recycling H2. J. Am. Chem. Soc. 2009, 131, 1646–1647. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, F.X.; Zhang, F.; Wang, Z.; Zhang, R.; Wang, C.; Qiu, S.L. In situ growth of continuous thin metal-organic framework film for capacitive humidity sensing. J. Mater. Chem. 2011, 21, 3775–3778. [Google Scholar] [CrossRef]
- Caskey, S.R.; Wong-Foy, A.G.; Matzger, A.J. Dramatic Tuning of Carbon Dioxide Uptake via Metal Substitution in a Coordination polymer with Cylindrical Pores. J. Am. Chem. Soc. 2008, 130, 10870–10871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.Z.; Zhao, L.L.; Chen, H.Y.; Xu, S.L.; Evans, D.G.; Duan, X. Corrosion resistance of superhydrophobic layered double hydroxide films on aluminum. Angew. Chem. Int. Ed. 2008, 47, 2466–2469. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Schwartz, Z.; Olivares-Navarrete, R.; Boyan, B.; Tannenbaum, R. Enhancement of Surface Wettability via the Modification of Microtextured Titanium Implant Surfaces with Polyelectrolytes. Langmuir 2011, 27, 5976–5985. [Google Scholar] [CrossRef] [PubMed]
- Martines, E.; Seunarine, K.; Morgan, H.; Gadegaard, N.; Wilkinson, C.D.W.; Riehle, M.O. Superhydrophobicity and Superhydrophilicity of Regular Nanopatterns. Nano Lett. 2005, 5, 2097–2103. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Morelock, C.R.; Burtch, N.C.; Mounfield, W.P., III; Hungerford, J.T.; Walton, K.S. Tuning the Kinetic Water Stability and Adsorption Interactions of Mg-MOF-74 by Partial Substitution with Co or Ni. Ind. Eng. Chem. Res. 2015, 54, 12408–12414. [Google Scholar] [CrossRef]
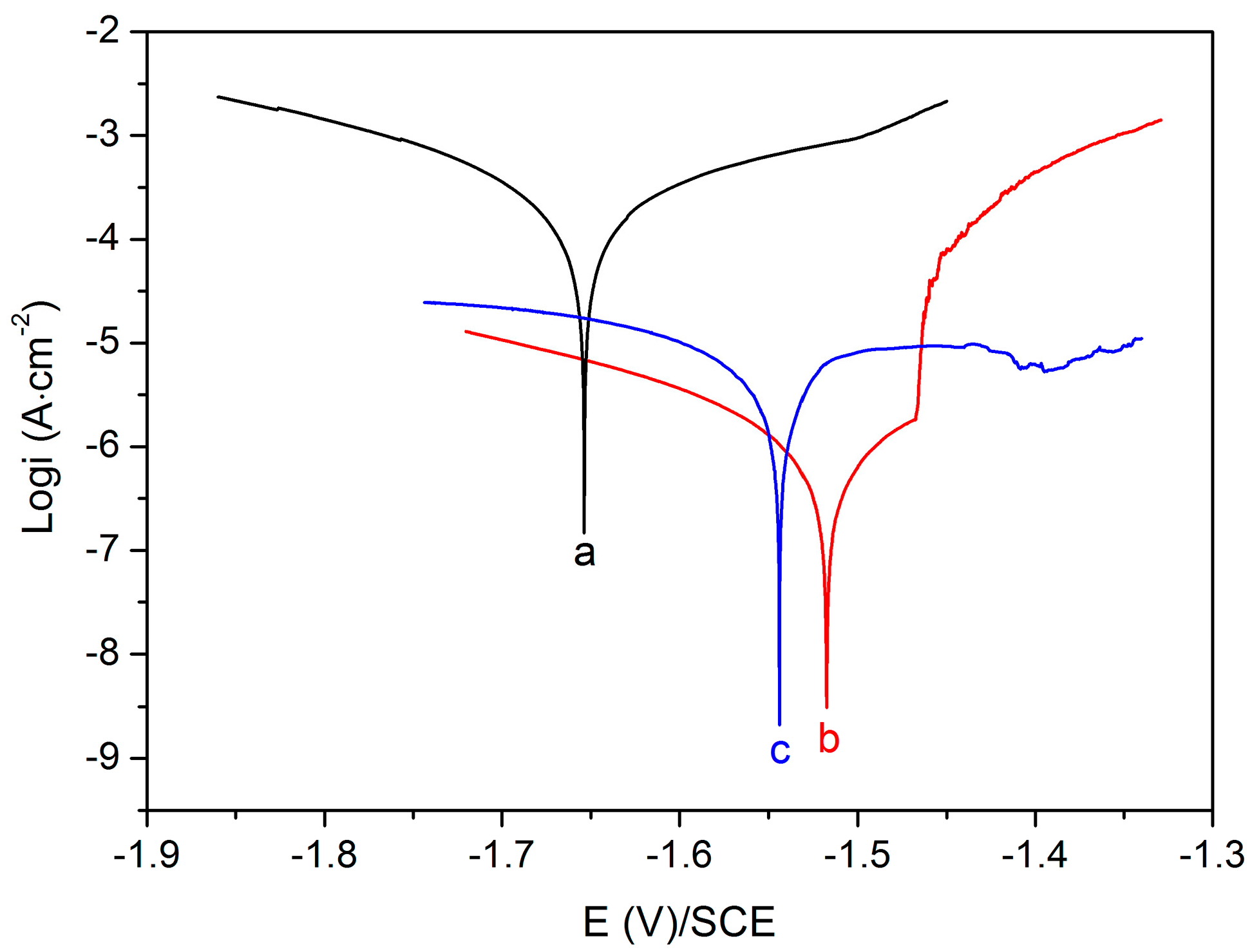
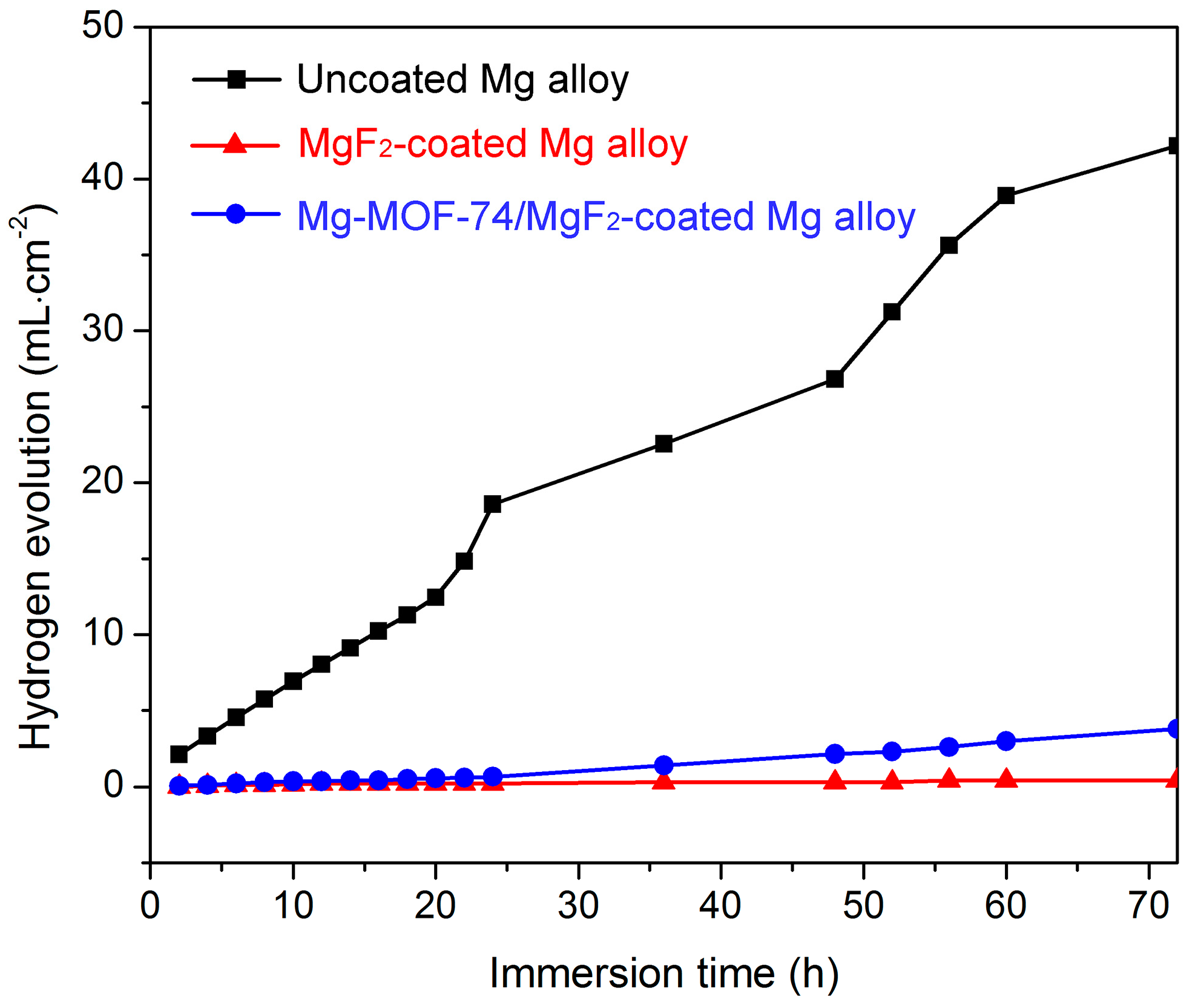
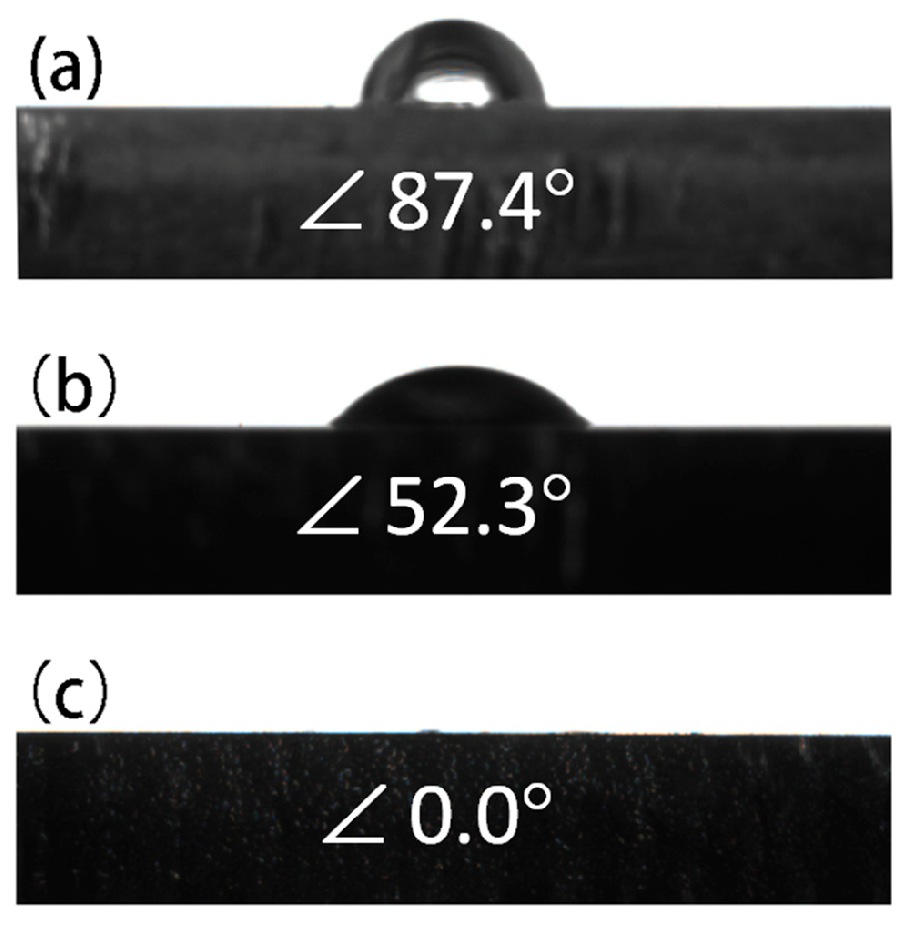
| Sample | Ecorr (V vs. SCE) | icorr (A·cm−2) |
|---|---|---|
| Uncoated Mg alloy | −1.65 | 2.18 × 10−4 |
| MgF2-coated Mg alloy | −1.52 | 1.19 × 10−6 |
| Mg-MOF-74/MgF2-coated Mg alloy | −1.54 | 6.46 × 10−6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Yan, Z.; Ma, X.; Geng, T.; Wu, H.; Li, Z. Mg-MOF-74/MgF2 Composite Coating for Improving the Properties of Magnesium Alloy Implants: Hydrophilicity and Corrosion Resistance. Materials 2018, 11, 396. https://doi.org/10.3390/ma11030396
Liu W, Yan Z, Ma X, Geng T, Wu H, Li Z. Mg-MOF-74/MgF2 Composite Coating for Improving the Properties of Magnesium Alloy Implants: Hydrophilicity and Corrosion Resistance. Materials. 2018; 11(3):396. https://doi.org/10.3390/ma11030396
Chicago/Turabian StyleLiu, Wei, Zhijie Yan, Xiaolu Ma, Tie Geng, Haihong Wu, and Zhongyue Li. 2018. "Mg-MOF-74/MgF2 Composite Coating for Improving the Properties of Magnesium Alloy Implants: Hydrophilicity and Corrosion Resistance" Materials 11, no. 3: 396. https://doi.org/10.3390/ma11030396