The Formation Mechanism and Corrosion Resistance of a Composite Phosphate Conversion Film on AM60 Alloy
Abstract
:1. Introduction
2. Experimental
2.1. Fabrication of the Film
2.2. Characterization
3. Results and Discussion
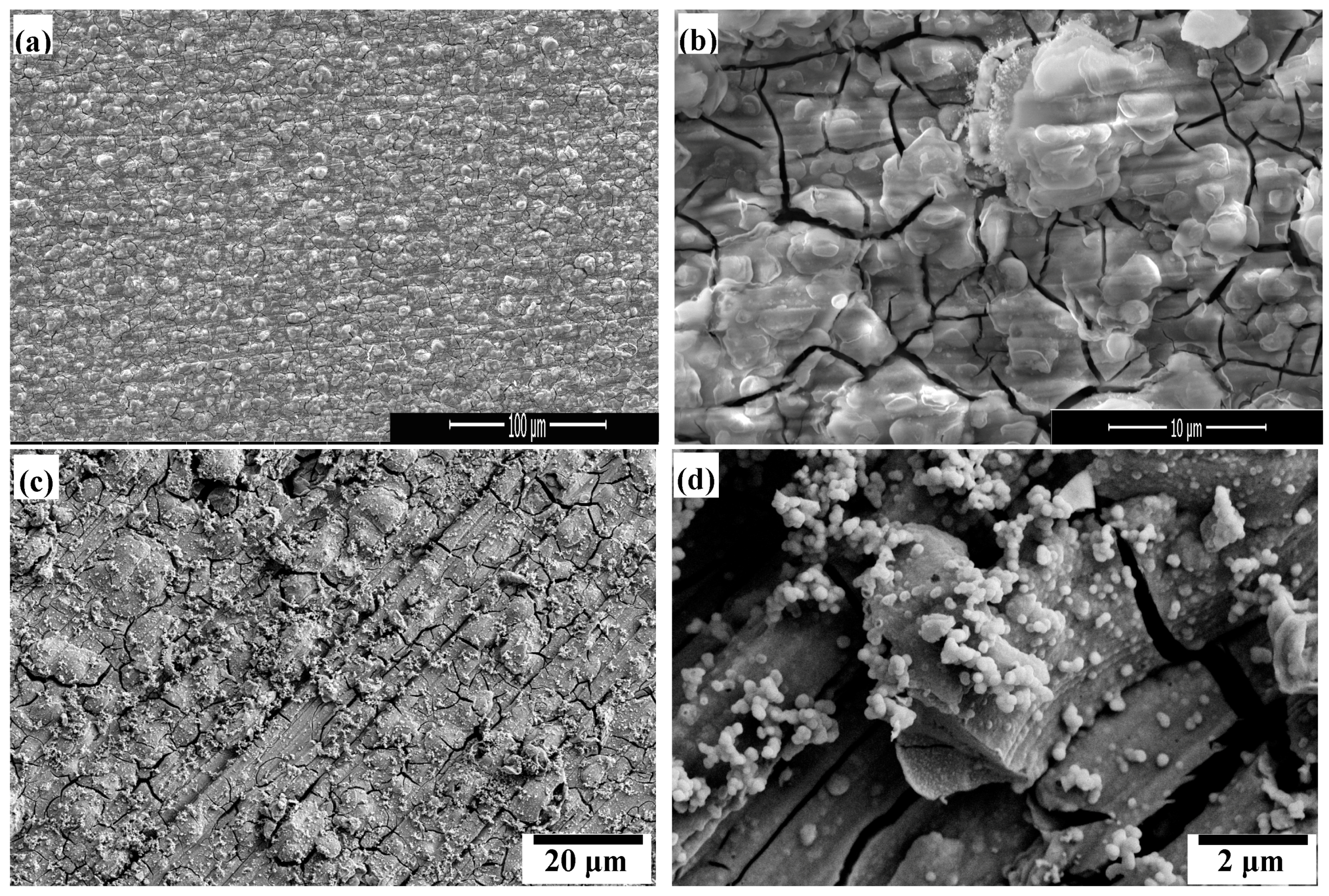
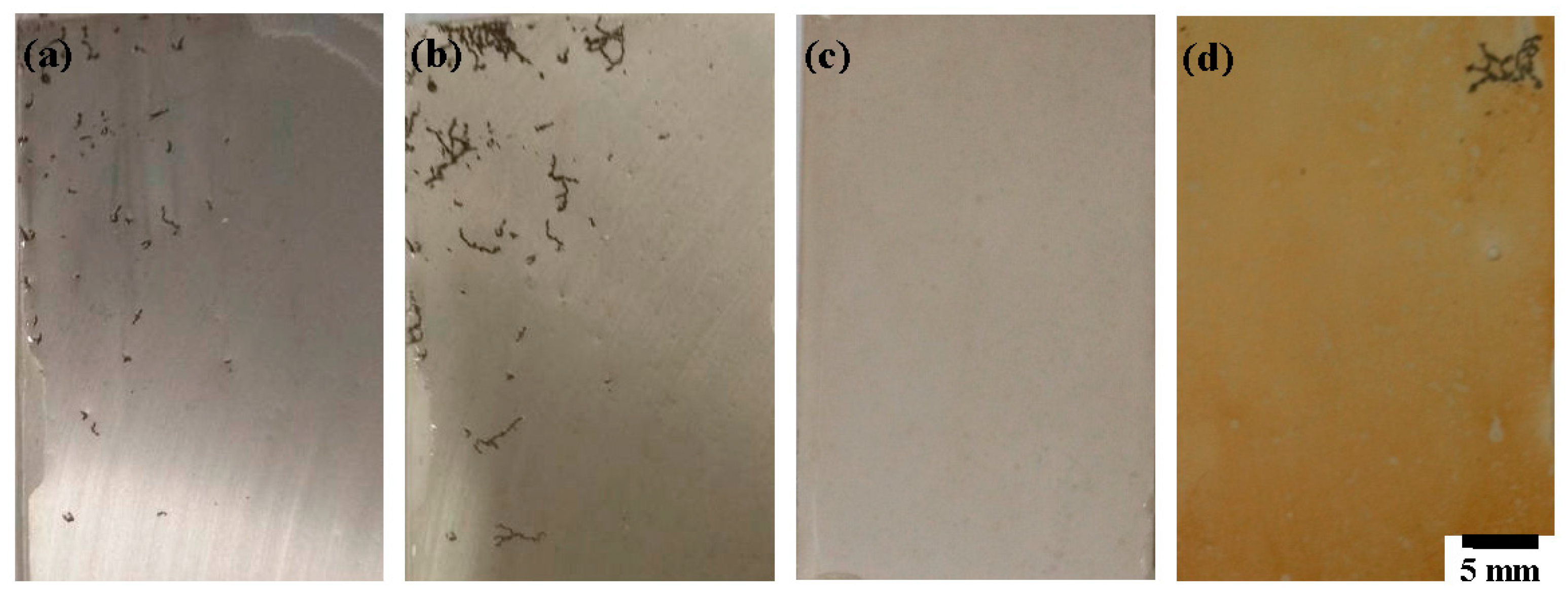
3.1. SEM Morphology and Adhesion of the Films
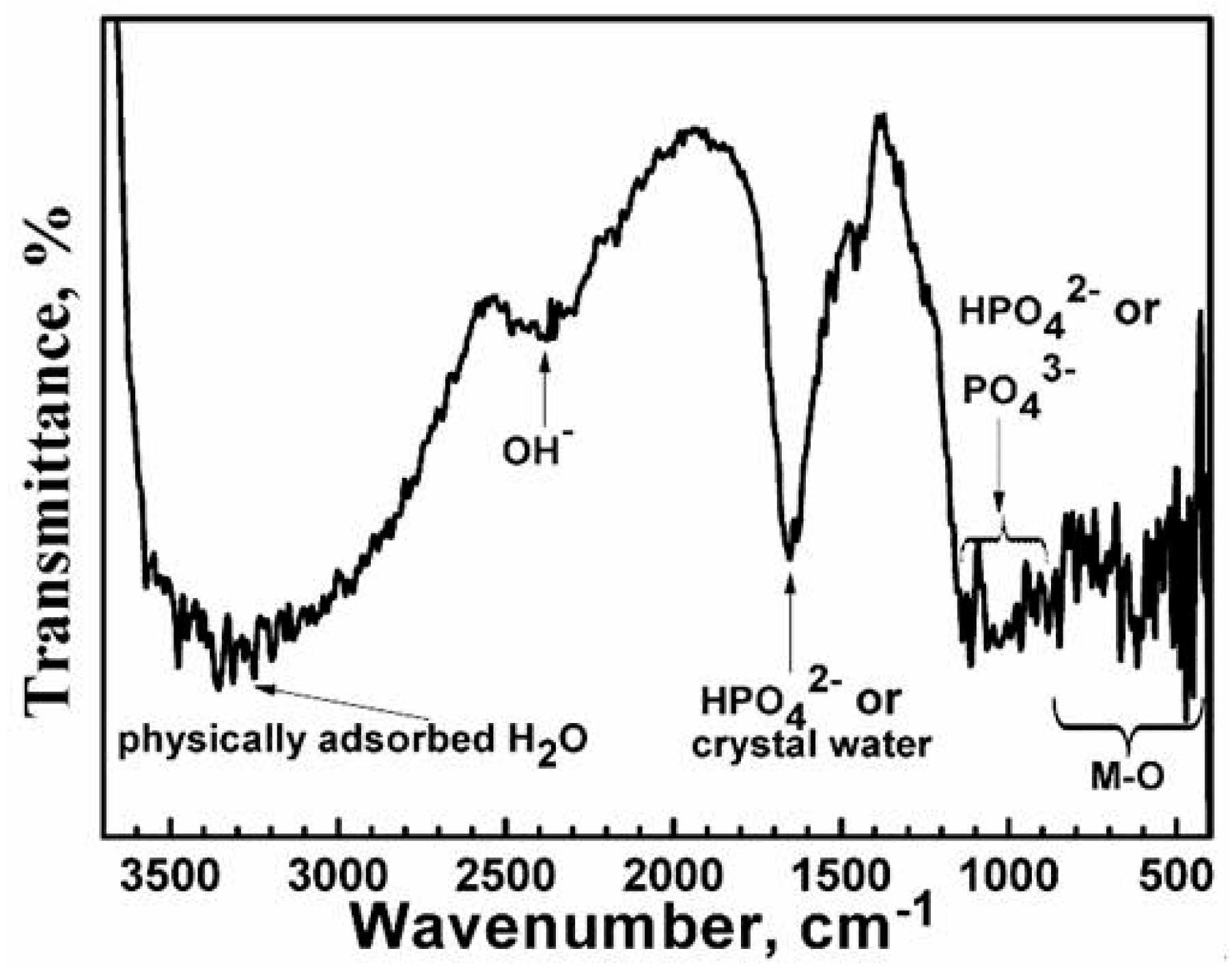
3.2. Composition Analysis of the Film
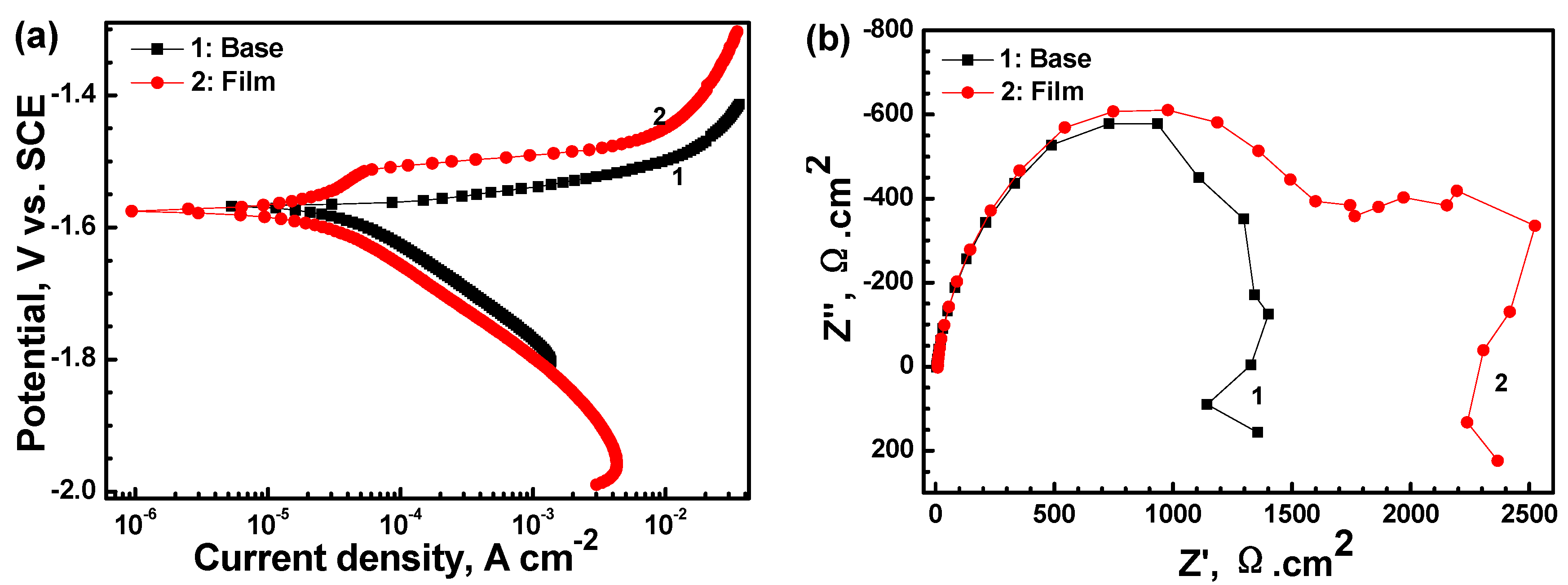
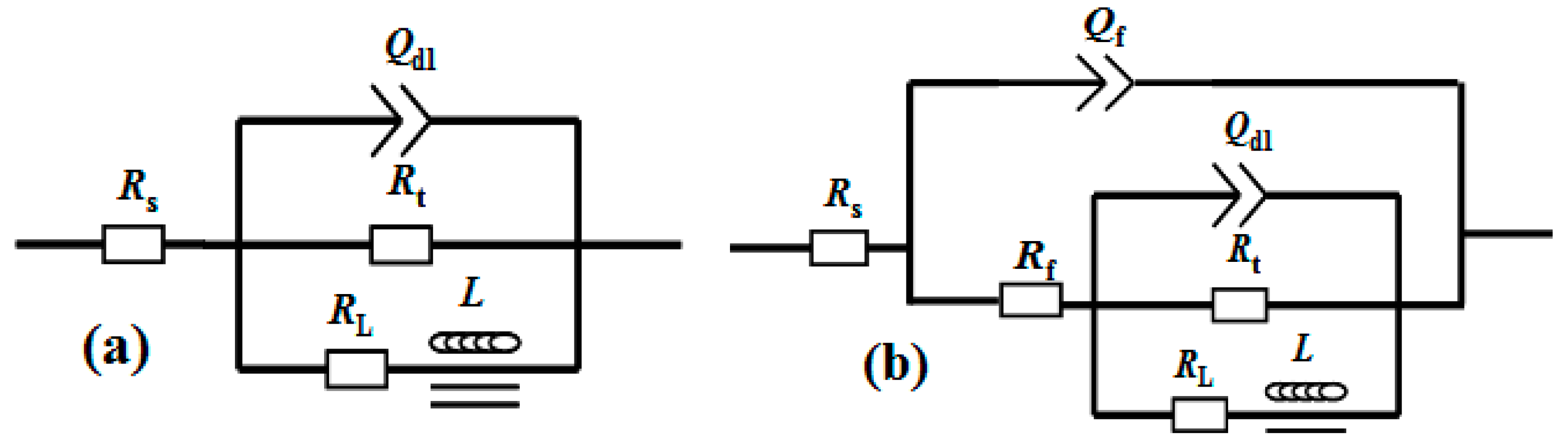
3.3. Corrosion Performance of the Film
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, Y.W.; Wu, P.; Wang, Q.Y.; Wu, H.; Liu, Y.; Deng, Y.W.; Zhou, Y.Z.; Shuai, C.J. The enhancement of Mg corrosion resistance by alloying Mn and laser-melting. Materials 2016, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.B.; Shen, J.; Gao, R.H.; Xie, X.; Luo, X. Enhanced corrosion resistance of magnesium alloy by a silane-based solution treatment after an in-situ formation of the Mg(OH)2 layer. Appl. Surf. Sci. 2016, 365, 268–274. [Google Scholar] [CrossRef]
- Mesíková, Ž.; Šulcová, P.; Trojan, M. Synthesis and characterization of newberyite. J. Therm. Anal. Calorim. 2007, 88, 103–106. [Google Scholar] [CrossRef]
- Zimmermann, D.; Munoz, A.G.; Schultze, J.W. Microscopic local elements in the phosphating process. Electrochim. Acta 2003, 48, 3267–3277. [Google Scholar] [CrossRef]
- Banakh, O.; Journot, T.; Gay, P.A.; Matthey, J.; Csefalvay, C.; Kalinichenko, O.; Sereda, O.; Moussa, M.; Durual, S.; Snizhko, L. Synthesis by anodic–spark deposition of Ca- and P-containing films on pure titanium and their biological response. Appl. Surf. Sci. 2016, 378, 207–215. [Google Scholar] [CrossRef]
- Li, G.Y.; Niu, L.Y.; Lian, J.S.; Jiang, Z.H. A black phosphate coating for C1008 steel. Surf. Coat. Technol. 2004, 176, 215–221. [Google Scholar] [CrossRef]
- Phuong, N.V.; Moon, S.; Chang, D.; Lee, K.H. Effect of microstructure on the zinc phosphate conversion coatings on magnesium alloy AZ91. Appl. Surf. Sci. 2013, 264, 70–78. [Google Scholar] [CrossRef]
- Zhou, W.Q.; Tang, W.; Zhao, Q.; Wu, S.W.; Han, E.H. Influence of additive on structure and corrosion resistance of manganese phosphate film on AZ91 magnesium alloy. Mater. Sci. Forum 2011, 686, 176–180. [Google Scholar] [CrossRef]
- Song, Y.W.; Shan, D.Y.; Han, E.H. A novel biodegradable nicotinic acid/calcium phosphate composite coating on Mg-3Zn alloy. Mater. Sci. Eng. C 2013, 33, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, L.N.; Fan, L.Y.; Xiao, W.J.; Lin, B.P.; Xu, Y.M.; Liang, J.; Cao, B.C. RGDC Peptide-induced biomimetic calcium phosphate coating formed on AZ31 magnesium alloy. Materials 2017, 10, 358. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.X.; Wang, Y.Y.; Wu, S. Preparation and characterization of Zn–Mn phosphate conversion coatings on Mg–Li alloy. Rare Met. Mat. Eng. 2014, 43, 1764–1768. [Google Scholar]
- Chen, X.B.; Zhou, X.; Abbott, T.B.; Easton, M.A.; Birbilis, N. Double–layered manganese phosphate conversion coating on magnesium alloy AZ91D: Insights into coating formation, growth and corrosion resistance. Surf. Coat. Technol. 2013, 217, 147–155. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, X.; Xiao, G.Y.; Lu, Y.P. Phosphate chemical conversion coatings on metallic substrates for biomedical application: A review. Mater. Sci. Eng. C 2015, 47, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Yang, X.; Peng, W.; Guo, H. Effects of magnetic fields on the phosphate conversion coating of AZ91D magnesium alloy. J. Phys. Conf. Ser. 2010, 200, 082010. [Google Scholar] [CrossRef]
- Chen, Y.G.; Luan, B.L.; Song, G.L.; Yang, Q.; Kingston, D.M.; Bensebaa, F. An investigation of new barium phosphate chemical conversion coating on AZ31 magnesium alloy. Surf. Coat. Technol. 2012, 210, 156–165. [Google Scholar] [CrossRef]
- Liu, F.; Shan, D.Y.; Han, E.H.; Liu, C.S. Barium phosphate conversion coating on die–cast AZ91D magnesium alloy. Trans. Nonferrous Met. Soc. China 2008, 18, 344–348. [Google Scholar] [CrossRef]
- Chen, X.B.; Birbilis, N.; Abbott, T.B. Effect of [Ca2+] and [PO43–] levels on the formation of calcium phosphate conversion coatings on die–cast magnesium alloy AZ91D. Corros. Sci. 2012, 55, 226–232. [Google Scholar] [CrossRef]
- Zhou, W.Q.; Wu, S.W.; Sheng, L.; Li, X. Effect of Ca2+ on structure and corrosion resistance of conversion coating formed on cast AZ91D magnesium alloys. Adv. Mater. Res. 2014, 887–888, 1111–1114. [Google Scholar] [CrossRef]
- Cui, X.F.; Li, Q.F.; Li, Y.; Wang, F.H.; Jin, G.; Ding, M.H. Microstructure and corrosion resistance of phytic acid conversion coatings for magnesium alloy. Appl. Surf. Sci. 2008, 255, 2098–2103. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiong, Q.Y.; Xiong, J.P. The study of a phosphate conversion coating on magnesium alloy AZ91D: I. formation, morphology and composition. Int. J. Electrochem. Sci. 2015, 10, 2812–2824. [Google Scholar]
- Ishizaki, T.; Masuda, Y.; Teshima, K. Composite film formed on magnesium alloy AZ31 by chemical conversion from molybdate/phosphate/fluorinate aqueous solution toward corrosion protection. Surf. Coat. Technol. 2013, 217, 76–83. [Google Scholar] [CrossRef]
- Lee, Y.L.; Chu, Y.R.; Li, W.C.; Lin, C.S. Effect of permanganate concentration on the formation and properties of phosphate/permanganate conversion coating on AZ31 magnesium alloy. Corros. Sci. 2013, 70, 74–81. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Shi, L.Q.; Cui, L.Y.; Zhang, C.L.; Li, S.Q.; Zeng, R.C.; Zhang, F.; Wang, Z.L. Corrosion resistance of silane–modified hydroxyapatite films on degradable magnesium alloys. Acta Metall. Sin. (Engl. Lett.) 2018, 31, 180–188. [Google Scholar] [CrossRef]
- Wang, B.J.; Xu, D.K.; Dong, J.H.; Ke, W. Effect of texture on biodegradable behavior of an as–extruded Mg–3%Al–1%Zn alloy in phosphate buffer saline medium. J. Mater. Sci. Technol. 2016, 32, 646–652. [Google Scholar] [CrossRef]
- Stoch, A.; Jastrzębski, W.; Brożek, A.; Stoch, J.; Szaraniec, J.; Trybalska, B.; Kmita, G. FTIR absorption–reflection study of biomimetic growth of phosphates on titanium implants. J. Mol. Struct. 2000, 555, 375–382. [Google Scholar] [CrossRef]
- Ureña-Amate, M.D.; Boutarbouch, N.D.; Socias-Viciana, M.M.; González-Pradas, E. Controlled release of nitrate from hydrotalcite modified formulations. Appl. Clay Sci. 2011, 52, 368–373. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Cai, S.; Xu, G.H.; Zhao, H.; Li, Y.; Wang, X.X.; Huang, K.; Ren, M.G.; Wu, X.D. Crack self-healing of phytic acid conversion coating on AZ31 magnesium alloy by heat treatment and the corrosion resistance. Appl. Surf. Sci. 2014, 313, 896–904. [Google Scholar] [CrossRef]
- Singh, S.S.; Roy, A.; Lee, B.E.; Ohodnicki, J.; Loghmanian, A.; Banerjee, I.; Kumta, P.N. A study of strontium doped calcium phosphate coatings on AZ31. Mater. Sci. Eng. C 2014, 40, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.A.; Kim, T.W.; Paek, M.J.; Ha, H.W.; Choy, J.H.; Hwang, S.J. Phosphate-intercalated Ca–Fe–layered double hydroxides: Crystal structure, bonding character, and release kinetics of phosphate. J. Solid State Chem. 2011, 184, 171–176. [Google Scholar] [CrossRef]
- Liu, G.Y.; Tang, S.W.; Li, D.C.; Hu, J. Self-adjustment of calcium phosphate coating on micro-arc oxidized magnesium and its influence on the corrosion behaviour in simulated body fluids. Corros. Sci. 2014, 79, 206–214. [Google Scholar] [CrossRef]
- Ayala, A.; Fetter, G.; Palomares, E.; Bosch, P. CuNi/Al hydrotalcites synthesized in presence of microwave irradiation. Mater. Lett. 2011, 65, 1663–1665. [Google Scholar] [CrossRef]
- Abdelkader, N.B.H.; Bentouami, A.; Derriche, Z.; Bettahar, N.; de Menorval, L.C. Synthesis and characterization of Mg–Fe layer double hydroxides and its application on adsorption of Orange G from aqueous solution. Chem. Eng. J. 2011, 169, 231–238. [Google Scholar] [CrossRef]
- Esquivel, D.; Cruz-Cabeza, A.J.; Jiménez-Sanchidrián, C.; Romero-Salguero, F.J. Local environment and acidity in alkaline and alkaline-earth exchanged β zeolite: Structural analysis and catalytic properties. Microporous Mesoporous Mater. 2011, 142, 672–679. [Google Scholar] [CrossRef]
- Song, Y.W.; Shan, D.Y.; Chen, R.S.; Zhang, F.; Han, E.H. A novel phosphate conversion film on Mg–8.8Li alloy. Surf. Coat. Technol. 2009, 203, 1107–1113. [Google Scholar] [CrossRef]
- Wu, L.P.; Zhao, L.; Dong, J.H.; Ke, W.; Chen, N. Potentiostatic conversion of phosphate mineral coating on AZ31 magnesium alloy in 0.1 M K2HPO4 solution. Electrochim. Acta 2014, 145, 71–80. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.W.; Shan, D.Y.; Han, E.H. In situ growth of Mg–Al hydrotalcite conversion film on AZ31 magnesium alloy. Corros. Sci. 2011, 53, 3281–3288. [Google Scholar] [CrossRef]
- Wan, T.T.; Liu, Z.X.; Bu, M.Z.; Wang, P.C. Effect of surface pretreatment on corrosion resistance and bond strength of magnesium AZ31 alloy. Corros. Sci. 2013, 66, 33–42. [Google Scholar] [CrossRef]
- Zhang, W.; Tian, B.; Du, K.Q.; Zhang, H.X.; Wang, F.H. Preparation and corrosion performance of PEO coating with low porosity on magnesium alloy AZ91D in acidic KF system. Int. J. Electrochem. Sci. 2011, 6, 5228–5248. [Google Scholar]
- Nabiyouni, M.; Ren, Y.F.; Bhaduri, S.B. Magnesium substitution in the structure of orthopedic nanoparticles: A comparison between amorphous magnesium phosphates, calcium magnesium phosphates, and hydroxyapatites. Mater. Sci. Eng. C 2015, 52, 11–17. [Google Scholar] [CrossRef] [PubMed]


| Element | C | O | Mg | P | Ca | Mn | Ba | Al |
|---|---|---|---|---|---|---|---|---|
| Content (at %) | 3.31 | 58.81 | 13.93 | 11.47 | 0.97 | 4.96 | 4.58 | 20.7 |
| Sample | Rs (Ω·cm2) | Y0 (μΩ−1·cm−2·s−1) | n | Rf (Ω·cm2) | Y0’ (μΩ−1·cm−2·s−1) | n’ | Rt (Ω·cm2) | L (kH·cm2) | RL (Ω·cm2) |
|---|---|---|---|---|---|---|---|---|---|
| Base | 15.9 | - | - | - | 19.08 | 0.87 | 870 | 557 | 645 |
| Film | 19.04 | 10.46 | 0.82 | 1162 | 7.48 | 0.88 | 582 | 219 | 2519 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Lan, X.; Wang, C.; Zhang, Q. The Formation Mechanism and Corrosion Resistance of a Composite Phosphate Conversion Film on AM60 Alloy. Materials 2018, 11, 402. https://doi.org/10.3390/ma11030402
Chen J, Lan X, Wang C, Zhang Q. The Formation Mechanism and Corrosion Resistance of a Composite Phosphate Conversion Film on AM60 Alloy. Materials. 2018; 11(3):402. https://doi.org/10.3390/ma11030402
Chicago/Turabian StyleChen, Jun, Xiangna Lan, Chao Wang, and Qinyong Zhang. 2018. "The Formation Mechanism and Corrosion Resistance of a Composite Phosphate Conversion Film on AM60 Alloy" Materials 11, no. 3: 402. https://doi.org/10.3390/ma11030402






