Study of Hyperbranched Poly(ethyleneimine) Polymers of Different Molecular Weight and Their Interaction with Epoxy Resin
Abstract
:1. Introduction
1.1. Hyperbranched Polymers
1.2. Dielectric Relaxation Spectroscopy of Epoxy-HBP Systems
2. Results and Discussion
2.1. Preliminary Experiments
2.2. Differential Scanning Calorimetry (DSC) Results
2.3. Dielectric Relaxation Spectroscopy (DRS)
2.3.1. PEI800 and PEI25000 Systems
2.3.2. Epoxy Resin
2.3.3. ELP800 and ELP25000 Uncured Systems
2.3.4. ELP800 and ELP25000 Fully Cured Systems
2.4. Dynamic Mechanical Analysis (DMA)
2.4.1. HBPEIs and Uncured ELP Mixtures
2.4.2. ELP800 and ELP25000 Fully Cured Systems
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Samples
3.3. Experimental Techniques
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gao, C.; Yan, D. Hyperbranched polymers: From synthesis to applications. Prog. Polym. Sci. 2004, 29, 183–275. [Google Scholar] [CrossRef]
- Voit, B. New developments in hyperbranched polymers. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 2505–2525. [Google Scholar] [CrossRef]
- Malmström, E.; Hult, A. Hyperbranched polymers: A review. J. Macromol. Sci. Rev. Macromol. Chem. Phys. 1997, C37, 555–579. [Google Scholar] [CrossRef]
- Yates, C.R.; Hayes, W. Synthesis and applications of hyperbranched polymers. Eur. Polym. J. 2004, 40, 1257–1281. [Google Scholar] [CrossRef]
- Hawker, C.J.; Lee, R.; Fréchet, J.M.J. One-step synthesis of hyperbranched dendritic polyesters. J. Am. Chem. Soc. 1991, 113, 4583–4588. [Google Scholar] [CrossRef]
- Holter, D.; Burgath, A.; Frey, H. Degree of branching in hyperbranched polymers. Acta Polym. 1997, 48, 30–35. [Google Scholar] [CrossRef]
- Jikei, M.; Kakimoto, M. Hyperbranched polymers: A promising new class of materials. Prog. Polym. Sci. 2001, 26, 1233–1285. [Google Scholar] [CrossRef]
- Scholl, M.; Kadlecova, Z.; Klok, H.A. Dendritic and hyperbranched polyamides. Prog. Polym. Sci. 2009, 34, 24–61. [Google Scholar] [CrossRef]
- Massa, D.J.; Shriner, K.A.; Turner, S.R.; Voit, B. Novel blends of hyperbranched polyesters and linear polymers. Macromolecules 1995, 28, 3214–3220. [Google Scholar] [CrossRef]
- Mecking, S.; Thomann, R.; Frey, H.; Sunder, A. Preparation of catalytically active palladium nanoclusters in compartments of amphiphilic hyperbranched polyglycerol. Macromolecules 2000, 33, 3958–3960. [Google Scholar] [CrossRef]
- Stiriba, S.E.; Kautz, H.; Frey, H. Hyperbranched molecular nanocapsules: Comparison of the hyperbranched architecture with the perfect linear analogue. J. Am. Chem. Soc. 2002, 124, 9698–9699. [Google Scholar] [CrossRef] [PubMed]
- Slagt, M.Q.; Stiriba, S.E.; Gebbink, R.J.M.K.; Kautz, H.; Frey, H.; van Koten, G. Encapsulation of hydrophilic pincer-platinum (II) complexes in amphiphilic hyperbranched polyglycerol nanocapsules. Macromolecules 2002, 35, 5734–5737. [Google Scholar] [CrossRef]
- Hedrick, J.L.; Hawker, C.J.; Miller, R.D.; Twieg, R.; Srinivasan, S.A.; Trollsas, M. Structure control in organic–inorganic hybrids using hyperbranched high-temperature polymers. Macromolecules 1997, 30, 7607–7610. [Google Scholar] [CrossRef]
- Sun, Q.H.; Xu, K.T.; Lam, J.W.Y.; Cha, J.A.K.; Zhang, X.X.; Tang, B.Z. Nanostructured magnetoceramics from hyperbranched polymer precursors. Mater. Sci. Eng. C 2001, 16, 107–112. [Google Scholar] [CrossRef]
- Cosulich, M.E.; Russo, S.; Pasquale, S.; Mariani, A. Performance evaluation of hyperbranched aramids as potential supports for protein immobilization. Polymer 2000, 41, 4951–4956. [Google Scholar] [CrossRef]
- Lim, Y.B.; Kim, S.M.; Lee, Y.; Lee, W.K.; Yang, T.G.; Lee, M.J.; Suh, H.; Park, J.S. Cationic hyperbranched poly(amino ester): A novel class of DNA condensing molecule with cationic surface, biodegradable three-dimensional structure, and tertiary amine groups in the interior. J. Am. Chem. Soc. 2001, 123, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Frey, H.; Haag, R. Dendritic polyglycerol: A new versatile biocompatible material. J. Biotechnol. 2002, 90, 257–267. [Google Scholar] [CrossRef]
- Kim, Y.H.; Webster, O.W. Hyperbranched polyphenylenes. Macromolecules 1992, 25, 5561–5572. [Google Scholar] [CrossRef]
- Hong, Y.; Cooper-White, J.J.; Mackay, M.E.; Hawker, C.J.; Malmström, E.; Rehnberg, N. A novel processing aid for polymer extrusion: Rheology and processing of polyethylene and hyperbranched polymer blends. J. Rheol. 1999, 43, 781–793. [Google Scholar] [CrossRef]
- Hong, Y.; Coombs, S.J.; Cooper-White, J.J.; Mackay, M.E.; Hawker, C.J.; Malmström, E.; Rehnberg, N. Film blowing of linear low-density polyethylene blended with a novel hyperbranched polymer processing aid. Polymer 2000, 41, 7705–7713. [Google Scholar] [CrossRef]
- Mulkern, T.J.; Tan, N.C.B. Processing and characterization of reactive polystyrene/hyperbranched polyester blends. Polymer 2000, 41, 3193–3203. [Google Scholar] [CrossRef]
- Jang, J.; Oh, J.H.; Moon, S.I. Crystallization behavior of poly(ethylene terephthalate) blended with hyperbranched polymers: The effect of terminal groups and composition of hyperbranched polymers. Macromolecules 2000, 33, 1864–1870. [Google Scholar] [CrossRef]
- Ratna, D.; Simon, G.P. Thermomechanical properties and morphology of blends of a hydroxy-functionalized hyperbranched polymer and epoxy resin. Polymer 2001, 42, 8833–8839. [Google Scholar] [CrossRef]
- Tang, L.M.; Qu, T.; Tuo, X.L.; Zhang, X.L.; Liu, D.S. Synthesis, morphology and application of alkylaryl hyperbranched polyesters. Polym. J. 2002, 34, 112–116. [Google Scholar] [CrossRef]
- Johansson, M.; Hult, A. Synthesis, characterization and UV curing of acrylate functional hyperbranched polyester resins. J. Coat. Technol. 1995, 67, 35–39. [Google Scholar]
- Emrick, T.; Chang, H.T.; Frechet, J.M.J.; Woods, J.; Baccei, L. Hyperbranched aromatic epoxies in the design of adhesive materials. Polym. Bull. 2000, 45, 1–7. [Google Scholar] [CrossRef]
- Liu, H.; Wilen, C.E.; Skrifvars, M. Reaction of epoxy resin and hyperbranched polyacids. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 4457–4465. [Google Scholar] [CrossRef]
- Schmaljohann, D.; Pötschke, P.; Hässler, R.; Voit, B.I.; Froehling, P.E.; Mostert, B.; Loontjens, J.A. Blends of amphiphilic hyperbranched polyesters and different polyolefins. Macromolecules 1999, 32, 6333–6339. [Google Scholar] [CrossRef]
- Burkinshaw, S.M.; Froehling, P.E.; Mignanelli, M. The effect of hyperbranched polymers on the dyeing of polypropylene fibres. Dyes Pigments 2002, 53, 229–235. [Google Scholar] [CrossRef]
- Xu, J.; Wu, H.; Mills, O.P.; Heiden, P.A. A morphological investigation of thermosets toughened with novel thermoplastics. I. Bismaleimide modified with hyperbranched polyester. J. Appl. Polym. Sci. 1999, 72, 1065–1076. [Google Scholar] [CrossRef]
- Gopala, A.; Wu, H.; Xu, J.; Heiden, P. Investigation of readily processable thermoplastic-toughened thermosets. IV. BMIs toughened with hyperbranched polyester. J. Appl. Polym. Sci. 1999, 71, 1809–1817. [Google Scholar] [CrossRef]
- Wu, H.; Xu, J.; Liu, Y.; Heiden, P. Investigation of readily processable thermoplastic toughened thermosets. V. Epoxy resin toughened with hyperbranched polyester. J. Appl. Polym. Sci. 1999, 72, 151–163. [Google Scholar] [CrossRef]
- Boogh, L.; Pettersson, B.; Manson, J.A.E. Dendritic hyperbranched polymers as tougheners for epoxy resins. Polymer 1999, 40, 2249–2261. [Google Scholar] [CrossRef]
- Mezzenga, R.; Boogh, L.; Pettersson, B.; Manson, J.A.E. Chemically induced phase separated morphologies in epoxy resin hyperbranched polymer blends. Macromol. Symp. 2000, 149, 17–22. [Google Scholar] [CrossRef]
- Mezzenga, R.; Boogh, L.; Manson, J.A.E. A review of dendritic hyperbranched polymer as modifiers in epoxy composites. Compos. Sci. Technol. 2001, 61, 787–795. [Google Scholar] [CrossRef]
- Mezzenga, R.; Plummer, C.J.G.; Boogh, L.; Manson, J.A.E. Morphology build-up in dendritic hyperbranched polymer modified epoxy resins: Modelling and characterization. Polymer 2001, 42, 305–317. [Google Scholar] [CrossRef]
- Gryshchuk, O.; Jost, N.; Karger-Kocsis, J. Toughening of vinylester–urethane hybrid resins by functional liquid nitrile rubbers and hyperbranched polymers. Polymer 2002, 43, 4763–4768. [Google Scholar] [CrossRef]
- Gryshchuk, O.; Jost, N.; Karger-Kocsis, J. Toughening of vinylester-urethane hybrid resins through functionalized polymers. J. Appl. Polym. Sci. 2002, 84, 672–680. [Google Scholar] [CrossRef]
- Jannerfeldt, G.; Boogh, L.; Manson, J.A.E. The morphology of hyperbranched polymer compatibilized polypropylene/polyamide 6 blends. Polym. Eng. Sci. 2001, 41, 293–300. [Google Scholar] [CrossRef]
- Star, A.; Stoddart, J.F. Dispersion and solubilization of single-walled carbon nanotubes with a hyperbranched polymer. Macromolecules 2002, 35, 7516–7520. [Google Scholar] [CrossRef]
- Haag, R. Dendrimers and hyperbranched polymers as high-loading supports for organic synthesis. Chem. Eur. J. 2001, 7, 327–335. [Google Scholar] [CrossRef]
- Klein Gebbink, R.J.M.; Kruithof, C.A.; Van Klink, G.P.M.; Van Koten, G. Dendritic supports in organic synthesis. J. Biotechnol. 2002, 90, 183–193. [Google Scholar] [CrossRef]
- Johansson, M.; Malmström, E.; Jansson, A.; Hult, A. Novel concept for low temperature curing powder coatings based on hyperbranched polyesters. J. Coat. Technol. 2000, 72, 49–54. [Google Scholar] [CrossRef]
- Van Benthem, R.A.T.M. Novel hyperbranched resins for coating applications. Prog. Org. Coat. 2000, 40, 203–214. [Google Scholar] [CrossRef]
- Manczyk, K.; Szewczyk, P. Highly branched high solids alkyd resins. Prog. Org. Coat. 2002, 44, 99–109. [Google Scholar] [CrossRef]
- Zhu, S.W.; Shi, W.F. Flame retardant mechanism of hyperbranched polyurethane acrylates used for UV curable flame retardant coatings. Polym. Degrad. Stab. 2002, 75, 543–547. [Google Scholar] [CrossRef]
- Zhu, S.W.; Shi, W.F. Synthesis and photopolymerization of hyperbranched polyurethane acrylates applied to UV curable flame retardant coatings. Polym. Int. 2002, 51, 223–227. [Google Scholar] [CrossRef]
- Lange, J.; Stenroos, E.; Johansson, M.; Malmström, E. Barrier coatings for flexible packaging based on hyperbranched resins. Polymer 2001, 42, 7403–7410. [Google Scholar] [CrossRef]
- Lackowski, W.M.; Ghosh, P.; Crooks, R.M. Micron-scale patterning of hyperbranched polymer films by micro-contact printing. J. Am. Chem. Soc. 1999, 121, 1419–1420. [Google Scholar] [CrossRef]
- Ghosh, P.; Crooks, R.M. Covalent grafting of a patterned, hyperbranched polymer onto a plastic substrate using microcontact printing. J. Am. Chem. Soc. 1999, 121, 8395–8396. [Google Scholar] [CrossRef]
- Ghosh, P.; Amirpour, M.L.; Lackowski, W.M.; Pishko, M.V.; Crooks, R.M. A simple lithographylic approach for preparing patterned, micron-scale corrals for controlling cell growth. Angew. Chem. Int. Ed. 1999, 38, 1592–1595. [Google Scholar] [CrossRef]
- Aoki, A.; Ghosh, P.; Crooks, R.M. Micrometer-scale patterning of multiple dyes on hyperbranched polymer thin films using photoacid-based lithography. Langmuir 1999, 15, 7418–7421. [Google Scholar] [CrossRef]
- Ghosh, P.; Lackowski, W.M.; Crooks, R.M. Two new approaches for patterning polymer films using templates prepared by microcontact printing. Macromolecules 2001, 34, 1230–1236. [Google Scholar] [CrossRef]
- Crooks, R.M. Patterning of hyperbranched polymer films. Chem. Phys. Chem. 2001, 2, 644–654. [Google Scholar] [CrossRef]
- Ratna, D.; Simon, G.P. Thermal and mechanical properties of a hydroxyl-functional dendritic hyperbranched polymer and trifunctional epoxy resin blends. Polym. Eng. Sci. 2001, 41, 1815–1822. [Google Scholar] [CrossRef]
- Oh, J.H.; Jang, J.S.; Lee, S.H. Curing behavior of tetrafunctional epoxy resin/hyperbranched polymer system. Polymer 2001, 42, 8339–8347. [Google Scholar] [CrossRef]
- Foix, D.; Yu, Y.F.; Serra, A.; Ramis, X.; Salla, J.M. Study on the chemical modification of epoxy/anhydride thermosets using a hydroxyl terminated hyperbranched polymer. Eur. Polym. J. 2009, 45, 1454–1466. [Google Scholar] [CrossRef]
- Fernandez-Francos, X.; Salla, J.M.; Cadenato, A.; Morancho, J.M.; Serra, A.; Mantecón, A.; Ramis, X. A new strategy for controlling shrinkage of DGEBA resins cured by cationic copolymerization with hydroxyl-terminated hyperbranched polymers and ytterbium triflate as an initiator. J. Appl. Polym. Sci. 2009, 111, 2822–2829. [Google Scholar] [CrossRef]
- Sangermano, M.; Priola, A.; Malucelli, G.; Bongiovanni, R.; Quaglia, A.; Voit, B.; Ziemer, A. Phenolic hyperbranched polymers as additives in cationic photopolymerization of epoxy systems. Macromol. Mater. Eng. 2004, 289, 442–446. [Google Scholar] [CrossRef]
- Sangermano, M.; Malucelli, G.; Bongiovanni, R.; Priola, A.; Harden, A. Investigation on the effect of the presence of hyperbranched polymers on thermal and mechanical properties of an epoxy UV-cured system. Polym. Int. 2005, 54, 917–921. [Google Scholar] [CrossRef]
- Johansonn, M.; Glauser, T.; Rospo, G.; Hult, A. Radiation curing of hyperbranched polyester resins. J. Appl. Polym. Sci. 2000, 75, 612–618. [Google Scholar] [CrossRef]
- Ratna, D.; Becker, O.; Krishnamurthy, R.; Simon, G.P.; Varley, R.J. Nanocomposites based on a combination of epoxy resin, hyperbranched epoxy and a layered silicate. Polymer 2003, 44, 7449–7457. [Google Scholar] [CrossRef]
- Cortés, P.; Fraga, I.; Calventus, Y.; Román, F.; Hutchinson, J.M.; Ferrando, F. A new epoxy-based layered silicate nanocomposite using a hyperbranched polymer: Study of the curing reaction and nanostructure development. Materials 2014, 7, 1830–1849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiravand, F.; Fraga, I.; Cortés, P.; Calventus, Y.; Hutchinson, J.M. Thermal analysis of polymer layered silicate nanocomposites: Identification of nanostructure development by DSC. J. Therm. Anal. Calorim. 2014, 118, 723–729. [Google Scholar] [CrossRef]
- Santiago, D.; Fernandez-Francos, X.; Ramis, X.; Salla, J.M.; Sangermano, M. Comparative curing kinetics and thermal-mechanical properties of DGEBA thermosets cured with a hyperbranched poly(ethyleneimine) and an aliphatic triamine. Thermochim. Acta 2011, 526, 9–21. [Google Scholar] [CrossRef]
- Fernandez-Francos, X.; Santiago, D.; Ferrando, F.; Ramis, X.; Salla, J.M.; Serra, A.; Sangermano, M. Network structure and thermomechanical properties of hybrid DGEBA networks cured with 1-methylimidazole and hyperbranched poly(ethyleneimine)s. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 1489–1503. [Google Scholar] [CrossRef]
- Corezzi, S.; Beiner, M.; Huth, H.; Schröter, K.; Capaccioli, S.; Casalini, R.; Fioretto, D.; Donth, E. Two crossover regions in the dynamics of glass forming epoxy resins. J. Chem. Phys. 2002, 117, 2435–2448. [Google Scholar] [CrossRef]
- Beiner, M.; Ngai, K.L. Interrelation between primary and secondary relaxations in polymerizing systems based on epoxy resins. Macromolecules 2005, 38, 7033–7042. [Google Scholar] [CrossRef]
- Roman, F.; Colomer, P.; Calventus, Y.; Hutchinson, J.M. Molecular mobility in hyperbranched polymers and their interaction with an epoxy matrix. Materials 2016, 9, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangion, M.B.M.; Johari, G.P. Relaxations of thermosets. 3. Sub-Tg dielectric relaxations of bisphenol-A based epoxide cured with different cross-linking agents. J. Polym. Sci. Part B Polym. Phys. 1990, 28, 71–83. [Google Scholar] [CrossRef]
- Shimbo, M.; Ochi, M.; Iesako, H. Mechanical relaxation mechanism of epoxide resins cured with acid anhydrides. J. Polym. Sci. Part B Polym. Phys. 1984, 22, 1461–1470. [Google Scholar] [CrossRef]
- Ochi, M.; Yoshizumi, M.; Shimbo, M. Mechanical and dielectric relaxations of epoxide resins containing the spiro-ring structure. 2. Effect of the introduction of methoxy branches on low temperature relaxations of epoxide resins. J. Polym. Sci. Part B Polym. Phys. 1987, 25, 1817–1827. [Google Scholar] [CrossRef]
- Maroulas, P.; Kripotou, S.; Sysel, P.; Hobzova, R.; Kotek, J.; Pissis, P. Molecular dynamics in hyperbranched polyimides cross-linked with ethylene glycol diglycidyl ether. J. Non-Cryst. Sol. 2006, 352, 4800–4803. [Google Scholar] [CrossRef]
- Zhu, P.W.; Zheng, S.; Simon, G. Dielectric relaxations in a hyperbranched polyester with terminal hydroxyl groups: Effects of generation number. Macromol. Chem. Phys. 2001, 202, 3008–3017. [Google Scholar] [CrossRef]
- Fraga, I.; Montserrat, S.; Hutchinson, J.M. TOPEM, a new temperature modulated DSC technique. Application to the glass transition of polymers. J. Therm. Anal. Calorim. 2007, 87, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Van Assche, G.; Van Hemelrijck, A.; Rahier, H.; Van Mele, B. Modulated differential scanning calorimetry: Non-isothermal cure, vitrification, and devitrification of thermosetting systems. Thermochim. Acta 1996, 286, 209–224. [Google Scholar] [CrossRef]
- Fraga, I.; Montserrat, S.; Hutchinson, J.M. Vitrification and devitrification during the non-isothermal cure of a thermoset. Theoretical model and comparison with calorimetric experiments. Macromol. Chem. Phys. 2010, 211, 57–65. [Google Scholar] [CrossRef]
- Fraga, I.; Hutchinson, J.M.; Montserrat, S. Vitrification and devitrification during the non-isothermal cure of a thermoset. J. Therm. Anal. Calorim. 2010, 99, 925–929. [Google Scholar] [CrossRef]
- Malmström, E.; Liu, F.; Boyd, R.H.; Hult, A.; Gedde, U.W. Relaxation processes in hyperbranched polyesters. Polym. Bull. 1994, 32, 679–685. [Google Scholar] [CrossRef]
- Malmström, E.; Hult, A.; Gedde, U.W.; Liu, F.; Boyd, R.H. Relaxation processes in hyperbranched polyesters: Influence of terminal groups. Polymer 1997, 38, 4873–4879. [Google Scholar] [CrossRef]
- Roman, F.; Colomer, P.; Calventus, Y.; Hutchinson, J.M. Study of the molecular dynamics of multiarm star polymers with a poly(ethyleneimine) core and poly(lactide) multiarms. Materials 2017, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Ngai, K.L.; Paluch, M. Classification of secondary relaxation in glass-formers based on dynamic properties. J. Chem. Phys. 2004, 120, 857–873. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bernabé, A.; Diaz-Calleja, R.; Haag, R. Broadband dielectric spectroscopy studies of hyperbranched polyglycerols. Macromol. Chem. Phys. 2006, 207, 970–977. [Google Scholar] [CrossRef]
- Turky, G.; Sangoro, J.R.; Rehim, M.A.; Kremer, F. Secondary relaxations and electrical conductivity in hyperbranched polyester amides. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1651–1657. [Google Scholar] [CrossRef]
- Fraga, I.; Montserrat, S.; Hutchinson, J.M. Vitrification during the isothermal cure of thermosets: Comparison of theoretical simulations with temperature-modulated DSC and dielectric analysis. Macromol. Chem. Phys. 2008, 209, 2003–2011. [Google Scholar] [CrossRef]
- Tombari, E.; Salvetti, G.; Johari, G.P. The temperature and polymerization effects on the relaxation time and conductivity, and the evolution of the localized motions. J. Chem. Phys. 2000, 113, 6957–6965. [Google Scholar] [CrossRef]
- Sunder, A.; Hanselmann, R.; Frey, H.; Mulhaupt, R. Controlled synthesis of hyperbranched polyglycerols by ring-opening multibranching polymerization. Macromolecules 1999, 32, 4240–4246. [Google Scholar] [CrossRef]







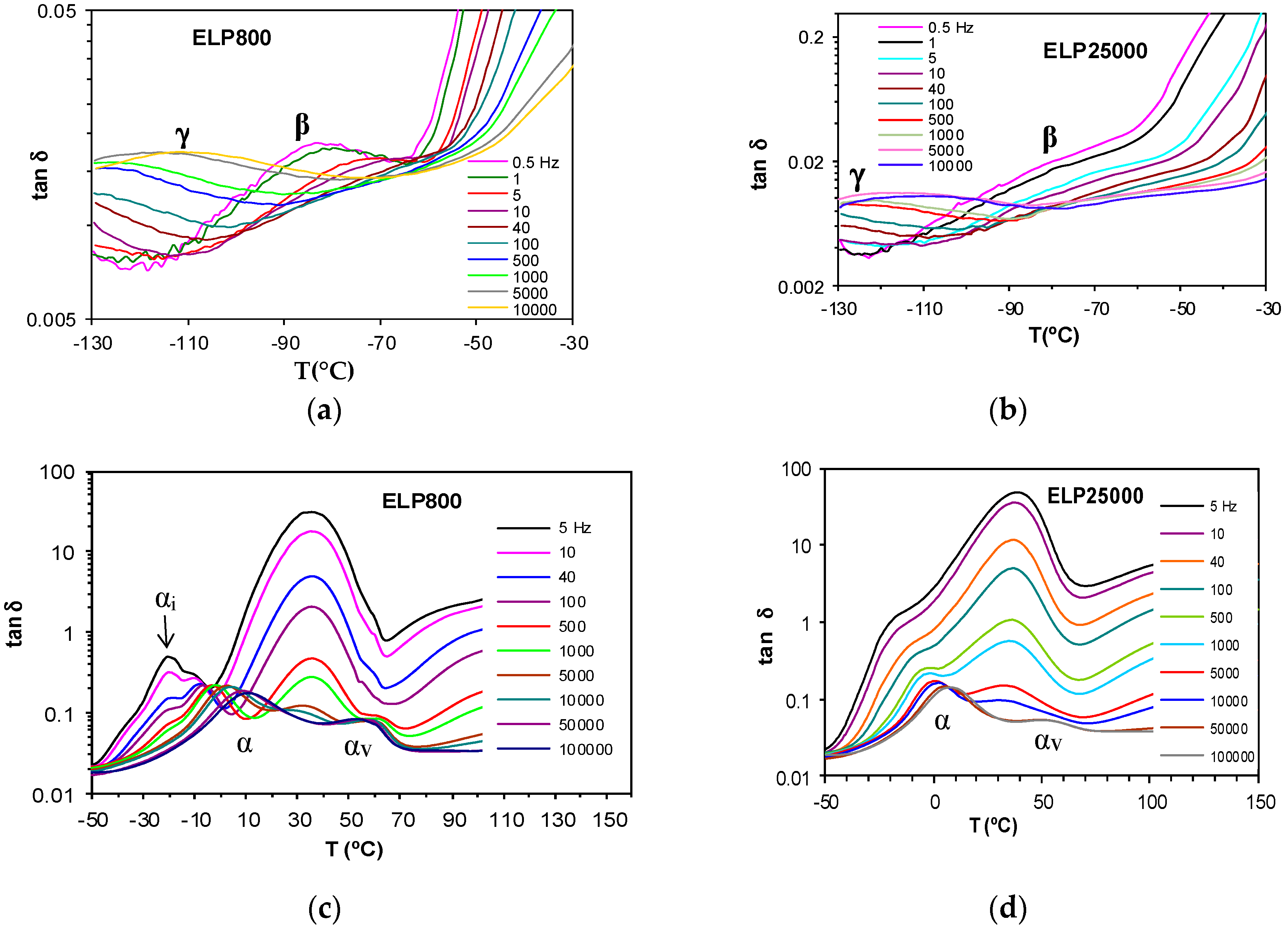



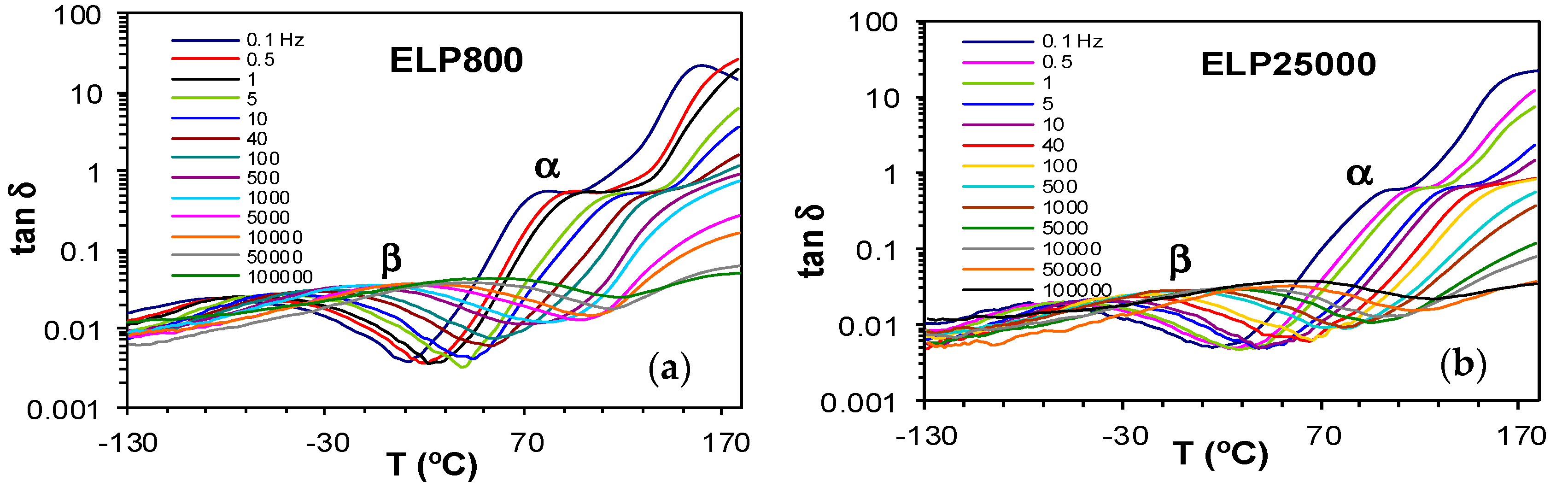
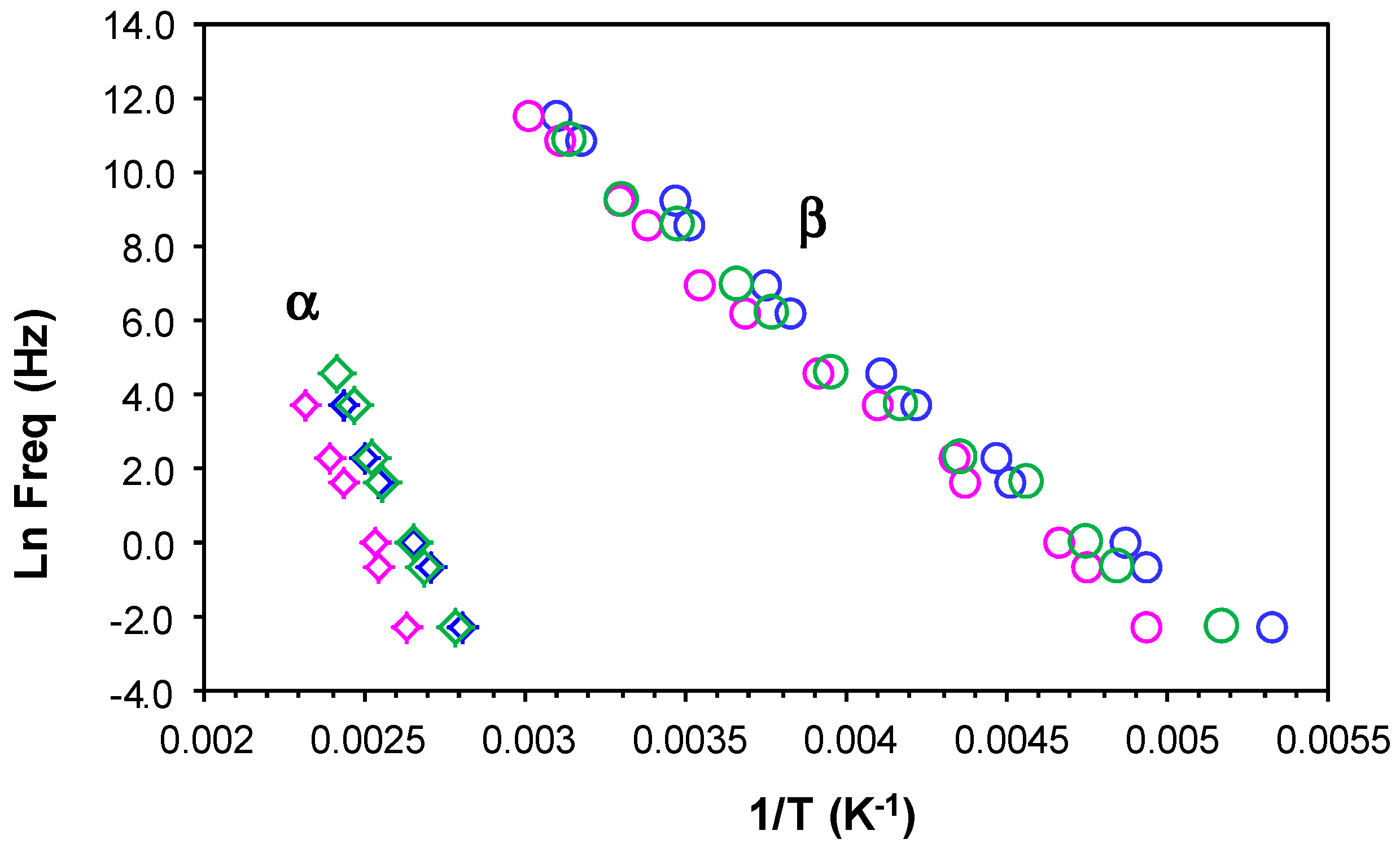




| Sample | Heating Rate (°C/min) | TD5% (°C) | Tonset (°C) | DTGA Peak (°C) |
|---|---|---|---|---|
| PEI800 | 2 | 200 | 322 | 362 |
| 10 | 249 | 365 | 389 | |
| PEI2000 [69] | 2 | 272 | 352 | 369 |
| 10 | 301 | 379 | 399 | |
| PEI25000 | 2 | 283 | 311 | 336 |
| 10 | 309 | 342 | 367 | |
| ELP800 | 2 | 305 | 317 | 343 |
| 10 | 336 | 339 | 365 | |
| ELP2000 [69] | 2 | 303 | 317 | 342 |
| 10 | 305 | 347 | 374 | |
| ELP25000 | 2 | 310 | 314 | 333 |
| 10 | 329 | 346 | 374 |
| Sample | Heating Rate (°C/min) | DSC (Conventional) | DSC (TOPEM) | DSC Cured films 50 °C, 3 h +140 °C, 3 h | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tg0 (°C) | ΔH (J/g) | ΔH (kJ/ee) | Tp (°C) | Tg∞,10 (°C) a | ΔH (J/g) | ΔH (kJ/ee) | Tp (°C) | Tv (°C) | Tg∞,2 (°C) b | Tg∞,10 (°C) c | ||
| PEI800 | 2 | −62 | - | - | - | - | - | - | - | - | - | - |
| 10 | −58 | - | - | - | - | - | - | - | - | - | - | |
| PEI2000 [69] | 2 | −55 | - | - | - | - | - | - | - | - | - | - |
| 10 | −54 | - | - | - | - | - | - | - | - | - | - | |
| PEI25000 | 2 | −53 | - | - | - | - | - | - | - | - | - | - |
| 10 | −52 | - | - | - | - | - | - | - | - | - | - | |
| Epoxy [69] | 2 | −17 | - | - | - | - | - | - | - | - | - | - |
| 10 | −16 | - | - | - | - | - | - | - | - | - | - | |
| ELP800 | 0.5 | −22 | 453 | 99.9 | 55 | 113 | 390 | 86.2 | 54 | 67 | 113 | - |
| 2 | −18 | 432 | 95.0 | 72 | 98 | - | - | - | - | - | 105 | |
| 10 | −20 | 434 | 95.9 | 101 | 101 | - | - | - | - | - | 112 | |
| ELP2000 [69] | 0.5 | −18 | 389 | 87.2 | 57 | 121 | 331 | 74.2 | 54 | 65 | 115 | - |
| 2 | −18 | 409 | 91.7 | 76 | 125 | - | - | - | - | - | 112 | |
| ELP25000 | 0.5 | −20 | 380 | 86.4 | 59 | 116 | 361 | 82.0 | 57 | 68 | 118 | - |
| 2 | −20 | 386 | 87.6 | 79 | 114 | - | - | - | - | - | 112 | |
| 10 | −18 | 354 | 80.6 | 103 | 105 | - | - | - | - | - | 118 | |
| Sample | Heating Rate (°C/min) | Relaxations, DRS Results | ||
|---|---|---|---|---|
| γ | β | α | ||
| Ea (kJ/mol) | Ea (kJ/mol) | Ea app (kJ/mol) | ||
| PEI800 | 0.5 | 46.2 | 181 | 71.3 |
| 2.0 | 45.8 | 155 | 66.1 | |
| PEI2000 [69] | 0.5 | 42.0 | - | 78.1 |
| 2.0 | 41.0 | - | 63.3 | |
| PEI25000 | 0.5 | 45.6 | 171 | 58.8 |
| 2.0 | 41.7 | 169 | 59.4 | |
| EPOXY [69] | 2.0 | 27.7 | 79.9 | 306 |
| ELP800 | 0.5 | 29.6 | 73.5 | 272 |
| 2.0 | 28.5 | 70.3 | 280 | |
| ELP2000 [69] | 0.5 | 36.0 | - | 302 |
| 2.0 | 27.3 | - | 263 | |
| ELP25000 | 0.5 | 35.6 | - | 310 |
| 2.0 | 31.4 | - | 334 | |
| Sample | Heating Rate (°C/min) | DRS Relaxations | |
|---|---|---|---|
| β | α | ||
| Ea (kJ/mol) | Ea app (kJ/mol) | ||
| ELP800 | 2 | 53.1 | 128 |
| ELP2000 | 2 | 53.8 | 158 |
| ELP25000 | 2 | 57.6 | 159 |
| Sample | Heating Rate (°C/min) | Relaxations, DMA Uncured Samples | Relaxations, DMA Fully Cured Samples | ||
|---|---|---|---|---|---|
| β | α | β | α | ||
| Ea (kJ/mol) | Ea app (kJ/mol) | Ea (kJ/mol) | Ea app (kJ/mol) | ||
| PEI800 | 2.0 | 194 | 224 | - | - |
| PEI2000 [69] | 2.0 | - | 209 | - | - |
| PEI25000 | 2.0 | 192 | 234 | - | - |
| EPOXY | 2.0 | 89.0 | 358 | - | - |
| ELP800 | 2.0 | 69.1 | 364 | 62.1 | 319 |
| ELP2000 [69] | 2.0 | 79.0 | 344 | 57.8 | 323 |
| ELP25000 | 2.0 | 84.2 | 367 | 64.5 | 332 |
| Sample | Amine Ratio a | Equivalent H Mass (g/equiv H) b | Equivalent N Mass (g/equiv N) b | Degree of Branching c (DB) |
|---|---|---|---|---|
| PEI800 d | 1:0.82:0.7 | 33.7 | 40.4 | 0.56 |
| PEI25000 d | 1:1.2:0.76 | 38.8 | 42.0 | 0.56 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Román, F.; Colomer, P.; Calventus, Y.; Hutchinson, J.M. Study of Hyperbranched Poly(ethyleneimine) Polymers of Different Molecular Weight and Their Interaction with Epoxy Resin. Materials 2018, 11, 410. https://doi.org/10.3390/ma11030410
Román F, Colomer P, Calventus Y, Hutchinson JM. Study of Hyperbranched Poly(ethyleneimine) Polymers of Different Molecular Weight and Their Interaction with Epoxy Resin. Materials. 2018; 11(3):410. https://doi.org/10.3390/ma11030410
Chicago/Turabian StyleRomán, Frida, Pere Colomer, Yolanda Calventus, and John M. Hutchinson. 2018. "Study of Hyperbranched Poly(ethyleneimine) Polymers of Different Molecular Weight and Their Interaction with Epoxy Resin" Materials 11, no. 3: 410. https://doi.org/10.3390/ma11030410





