Bismuth Oxysulfide and Its Polymer Nanocomposites for Efficient Purification
Abstract
:1. Introduction
2. Results
2.1. Morphological and Structural Characterization
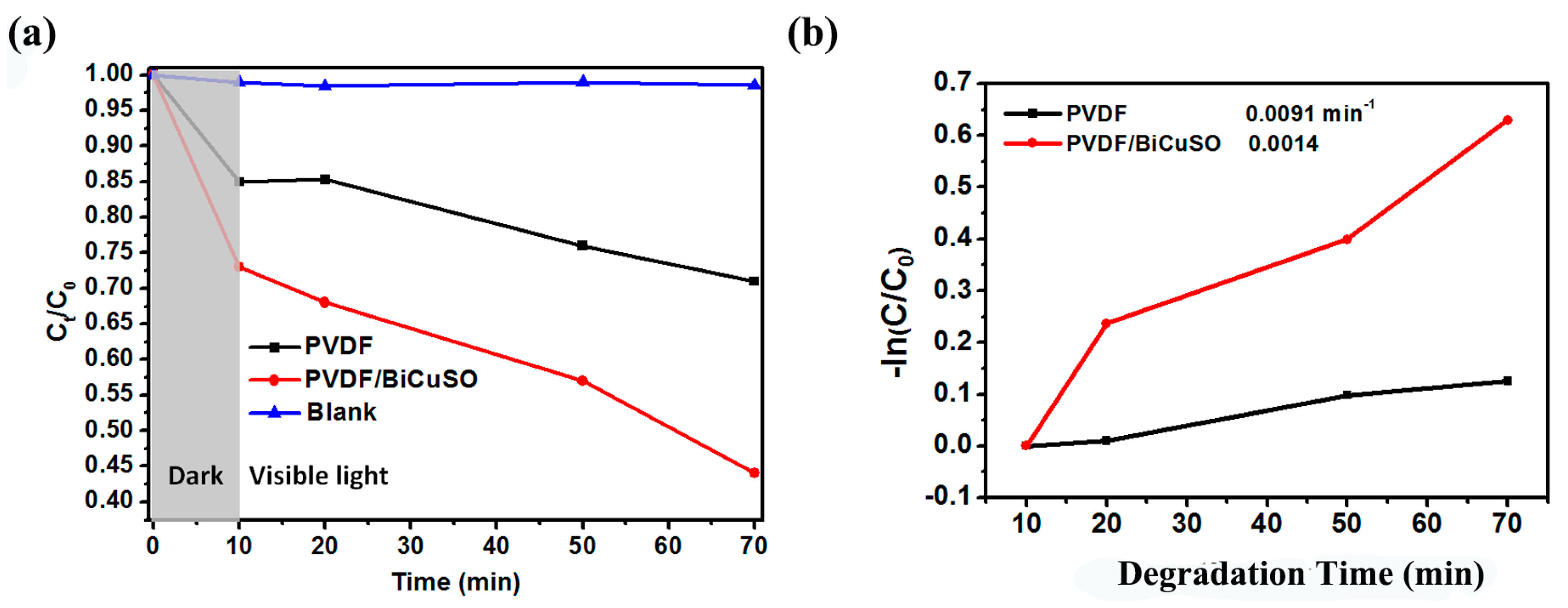
2.2. Photocatalytic Activities
3. Materials and Methods
3.1. Preparation of Photocatalysts
3.2. Fabrication of PVDF@BiCuSO_Nanofibers via Electronspinning
3.3. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- He, M.; Ichinose, T.; Kobayashi, M.; Arashidani, K.; Yoshida, S.; Nishikawa, M.; Takano, H.; Sun, G.; Shibamoto, T. Differences in allergic inflammatory responses between urban PM2.5 and fine particle derived from desert-dust in murine lungs. Toxicol. Appl. Pharmacol. 2016, 297, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Mishra, Y.K.; Adelung, R. ZnO tetrapod materials for functional applications. Mater. Today 2017. [Google Scholar] [CrossRef]
- Yamashita, H.; Harada, M.; Misaka, J.; Takeuchi, M.; Neppolian, B.; Anpo, M. Photocatalytic degradation of organic compounds diluted in water using visible light-responsive metal ion-implanted TiO2 catalysts: Fe ion-implanted TiO2. Catal. Today 2003, 84, 191–196. [Google Scholar] [CrossRef]
- Yamashita, H.; Harada, M.; Misaka, J.; Takeuchi, M.; Ikeue, K.; Anpo, M. Degradation of propanol diluted in water under visible light irradiation using metal ion-implanted titanium dioxide photocatalysts. J. Photochem. Photobiol. A Chem. 2002, 148, 257–261. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Brunekreef, B.; Holgate, S.T. Air pollution and health. Lancet 2002, 360, 1233–1242. [Google Scholar] [CrossRef]
- Liu, C.; Hsu, P.C.; Lee, H.W.; Ye, M.; Zheng, G.; Liu, N.; Li, W.; Cui, Y. Transparent air filter for high-efficiency PM2.5 capture. Nat. Commun. 2015, 6, 6205. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Raza, A.; Si, Y.; Yu, J.; Sun, G.; Ding, B. Tortuously structured polyvinyl chloride/polyurethane fibrous membranes for high-efficiency fine particulate filtration. J. Colloid Interface Sci. 2013, 398, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, S.; Zhao, X.; Yu, J.; Ding, B. Sandwich structured polyamide-6/polyacrylonitrile nanonets/bead-on-string composite membrane for effective air filtration. Sep. Purif. Technol. 2015, 152, 14–22. [Google Scholar] [CrossRef]
- Das, D.; Das, S.; Ishtiaque, S.M. Optimal design of nonwoven air filter media: Effect of fibre shape. Fibers Polym. 2014, 15, 1456–1461. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, S.; Yin, X.; Yu, J.; Ding, B. Slip-effect functional air filter for efficient purification of PM2.5. Sci. Rep. 2016, 6, 35472. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, Y.; Hua, T.; Jiang, P.; Yin, X.; Yu, J.; Ding, B. Low-resistance dual-purpose air filter releasing negative ions and effectively capturing pm2.5. ACS Appl. Mater. Interfaces 2017, 9, 12054–12063. [Google Scholar] [CrossRef] [PubMed]
- Yeom, B.Y.; Shim, E.; Pourdeyhimi, B. Boehmite nanoparticles incorporated electrospun nylon-6 nanofiber web for new electret filter media. Macromol. Res. 2010, 18, 884–890. [Google Scholar] [CrossRef]
- Ye, L.; Yang, C.; Tian, L.; Zhan, L.; Peng, T. Tunable photocatalytic selectivity of fluoropolymer PVDF modified TiO2. Appl. Surf. Sci. 2011, 257, 8072–8077. [Google Scholar] [CrossRef]
- Li, X.; Wang, N.; Fan, G.; Yu, J.; Gao, J.; Sun, G.; Ding, B. Electreted polyetherimide-silica fibrous membranes for enhanced filtration of fine particles. J. Colloid Interface Sci. 2015, 439, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Sodano, H.A. Ultra high energy density nanocomposite capacitors with fast discharge using Ba0.2Sr0.8TiO3 nanowires. Nano Lett. 2013, 13, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, S.; Liu, Y.; Ding, J.; Lin, Y.H.; Xu, H.; Xu, B.; Nan, C.W. Bi(1−x)la(x)CuSeO as new tunable full solar light active photocatalysts. Sci. Rep. 2016, 6, 24620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, W.; Jiang, D.; Gao, E.; Sun, S. Photoreduction of CO2 on BiOCl nanoplates with the assistance of photoinduced oxygen vacancies. Nano Res. 2014, 8, 821–831. [Google Scholar] [CrossRef]
- Wang, H.; Xu, H.; Zeng, C.; Shen, Y.; Lin, Y.-H.; Nan, C.-W.; McKittrick, J. Visible light photocatalytic activity of bismuth ferrites tuned by Bi/Fe ratio. J. Am. Ceram. Soc. 2016, 99, 1133–1136. [Google Scholar] [CrossRef]
- Guan, M.; Xiao, C.; Zhang, J.; Fan, S.; An, R.; Cheng, Q.; Xie, J.; Zhou, M.; Ye, B.; Xie, Y. Vacancy associates promoting solar-driven photocatalytic activity of ultrathin bismuth oxychloride nanosheets. J. Am. Chem. Soc. 2013, 135, 10411–10417. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zan, L.; Tian, L.; Peng, T.; Zhang, J. The {001} facets-dependent high photoactivity of BiOCl nanosheets. Chem. Commun. 2011, 47, 6951–6953. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhao, K.; Xiao, X.; Zhang, L. Synthesis and facet-dependent photoreactivity of BiOCl single-crystalline nanosheets. J. Am. Chem. Soc. 2012, 134, 4473–4476. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, V.A.; Arabatzis, I.M.; Konstantinou, I.K.; Dimou, A.D.; Albanis, T.A.; Falaras, P. Metolachlor photocatalytic degradation using TiO2 photocatalysts. Appl. Catal. B: Environ. 2004, 49, 195–205. [Google Scholar] [CrossRef]
- Lei, F.; Sun, Y.F.; Liu, K.T.; Gao, S.; Liang, L.; Pan, B.C.; Xie, Y. Oxygen Vacancies Confined in Ultrathin Indium Oxide Porous Sheets for Promoted Visible-Light Water Splitting. J. Am. Chem. Soc. 2014, 136, 6826–6829. [Google Scholar] [CrossRef] [PubMed]
- Lardhi, S.; Curutchet, A.; Cavallo, L.; Harb, M.; Le Bahers, T. Ab initio assessment of Bi1−xRexCuOS (Re = La, Gd, Y, Lu) solid solutions as a semiconductor for photochemical water splitting. Phys. Chem. Chem. Phys. 2017, 19, 12321–12330. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, W.; Liu, D.; Wei, Z.; Zhu, Y. Photocatalytic performance enhanced via surface bismuth vacancy of Bi6S2O15 core/shell nanowires. Appl. Catal. B Environ. 2015, 176–177, 306–314. [Google Scholar] [CrossRef]





| Samples | Pure BiCuSO | Vacant BiCuSO |
|---|---|---|
| BET (m2/g) | 26.40 | 31.64 |
| Samples | Pure BiCuSO | Vacant BiCuSO |
|---|---|---|
| K (10−3 m−2 min−1) | 0.7 | 6.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Qiao, L.; Wang, H.; Lan, S.; Shen, Y.; Lin, Y.; Nan, C. Bismuth Oxysulfide and Its Polymer Nanocomposites for Efficient Purification. Materials 2018, 11, 447. https://doi.org/10.3390/ma11030447
Luo Y, Qiao L, Wang H, Lan S, Shen Y, Lin Y, Nan C. Bismuth Oxysulfide and Its Polymer Nanocomposites for Efficient Purification. Materials. 2018; 11(3):447. https://doi.org/10.3390/ma11030447
Chicago/Turabian StyleLuo, Yidong, Lina Qiao, Huanchun Wang, Shun Lan, Yang Shen, Yuanhua Lin, and Cewen Nan. 2018. "Bismuth Oxysulfide and Its Polymer Nanocomposites for Efficient Purification" Materials 11, no. 3: 447. https://doi.org/10.3390/ma11030447




