Growth and Brilliant Photo-Emission of Crystalline Hexagonal Column of Alq3 Microwires
Abstract
:1. Introduction
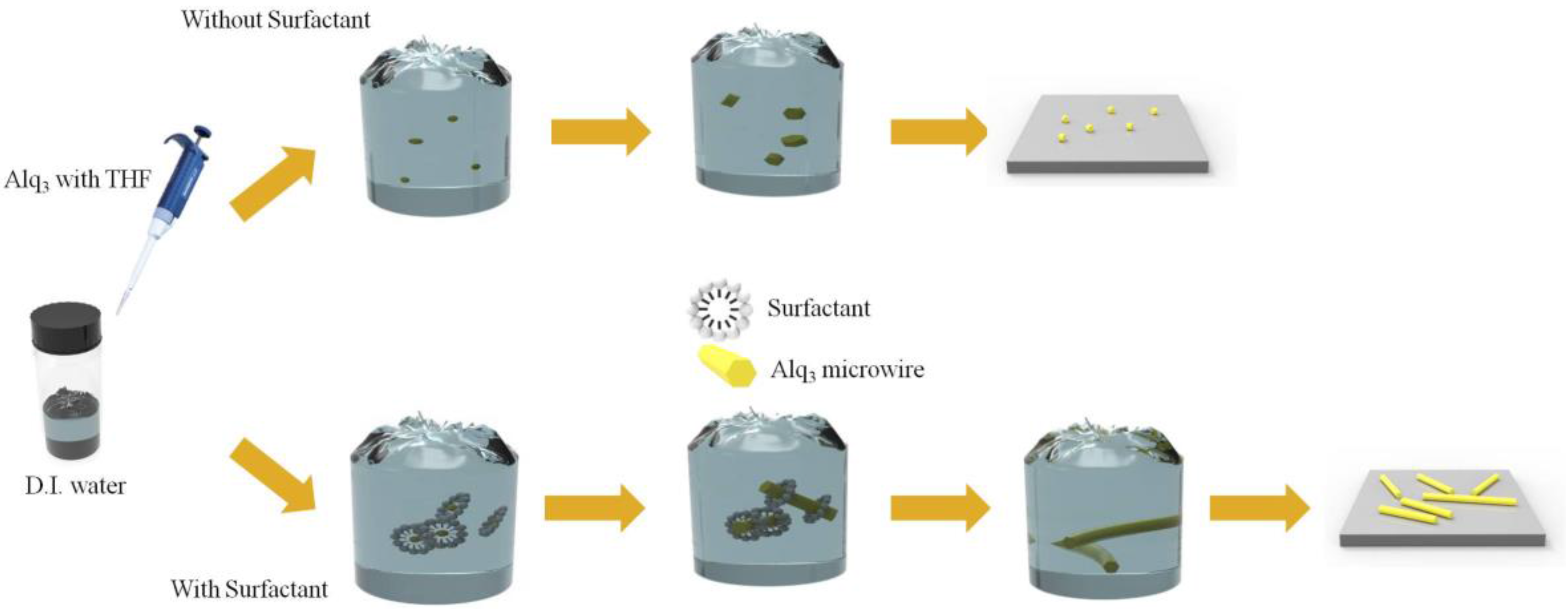
2. Materials and Methods
3. Results
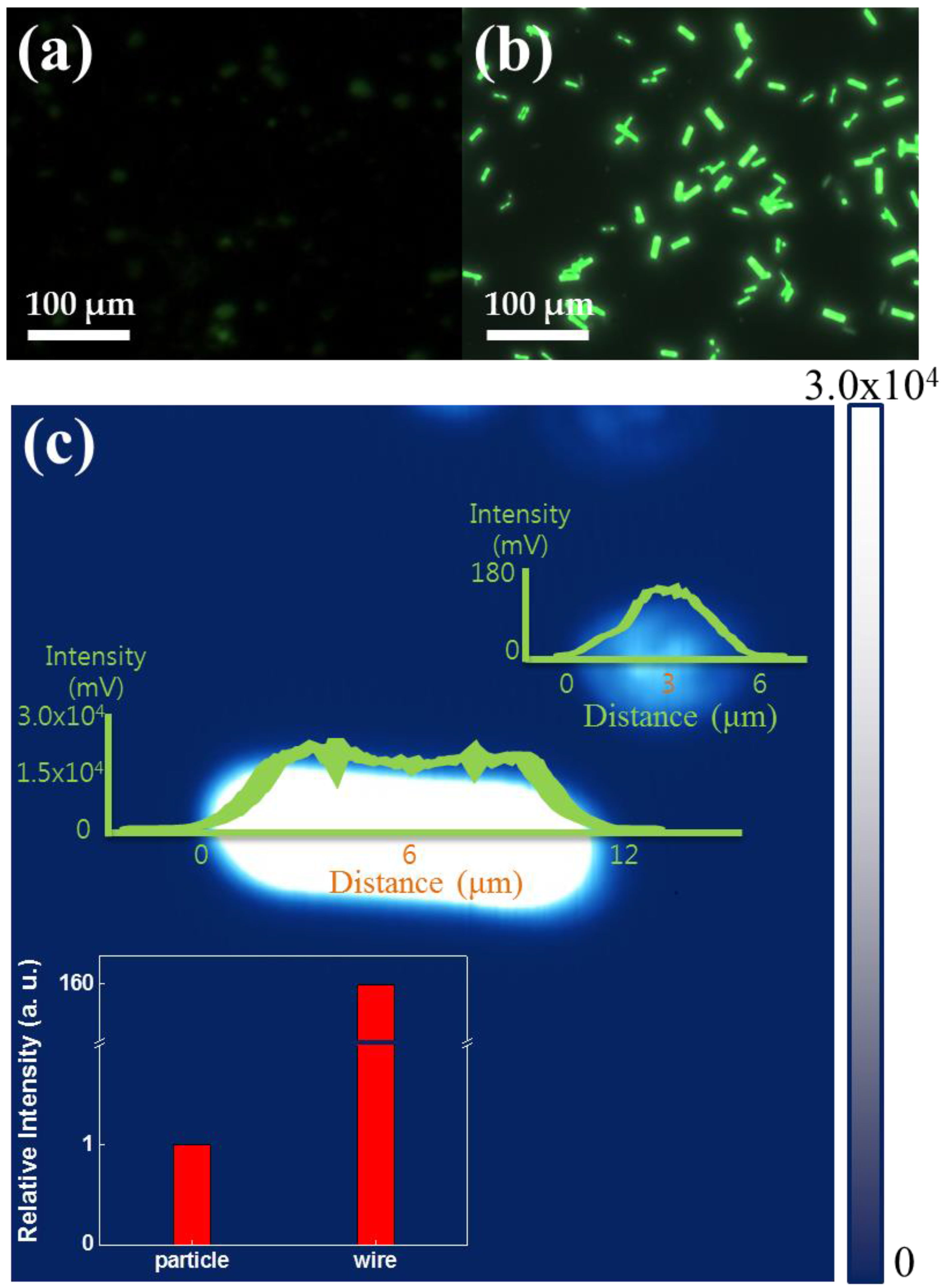
3.1. Morphological Analysis
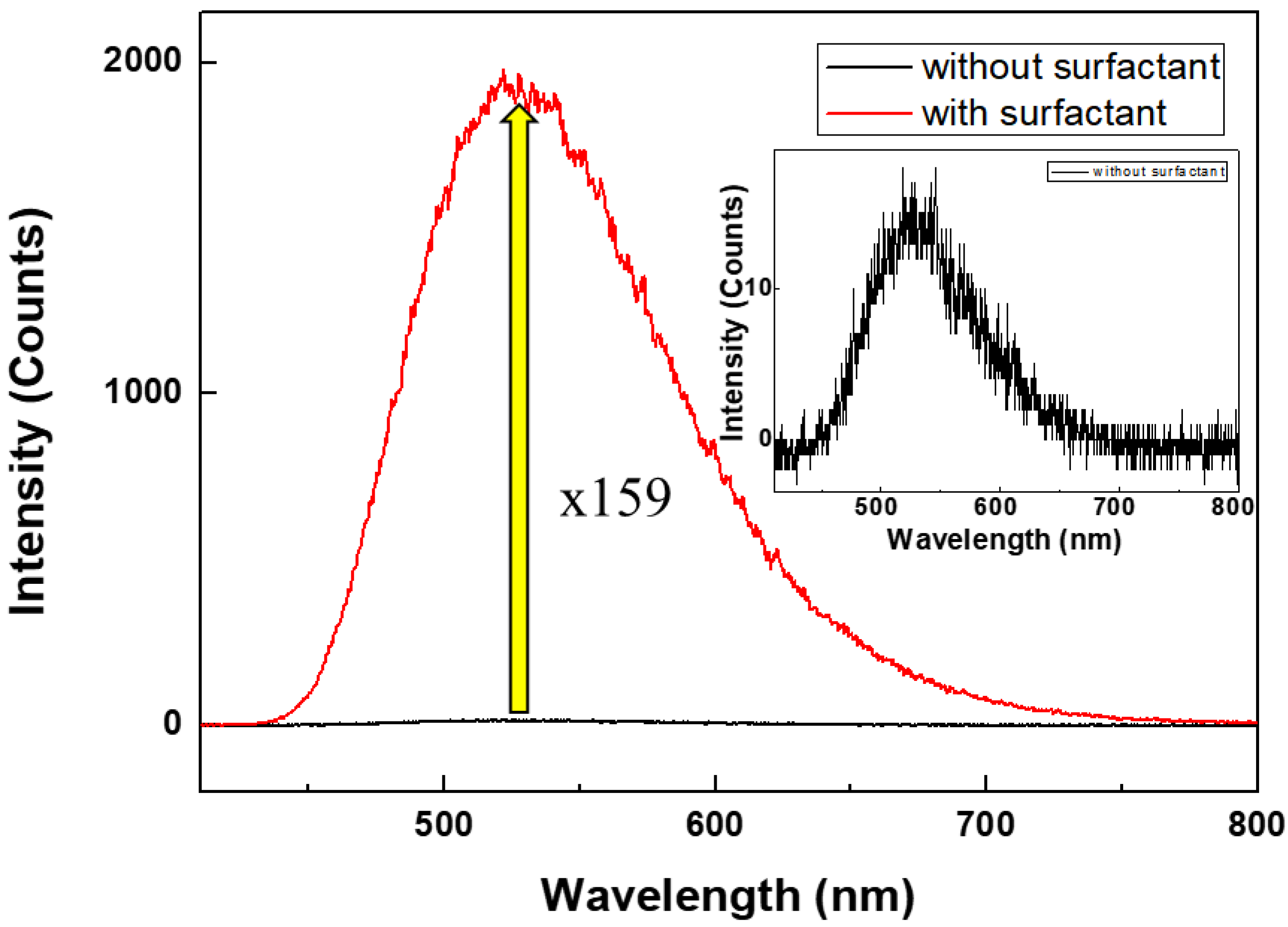
3.2. Optical Properties
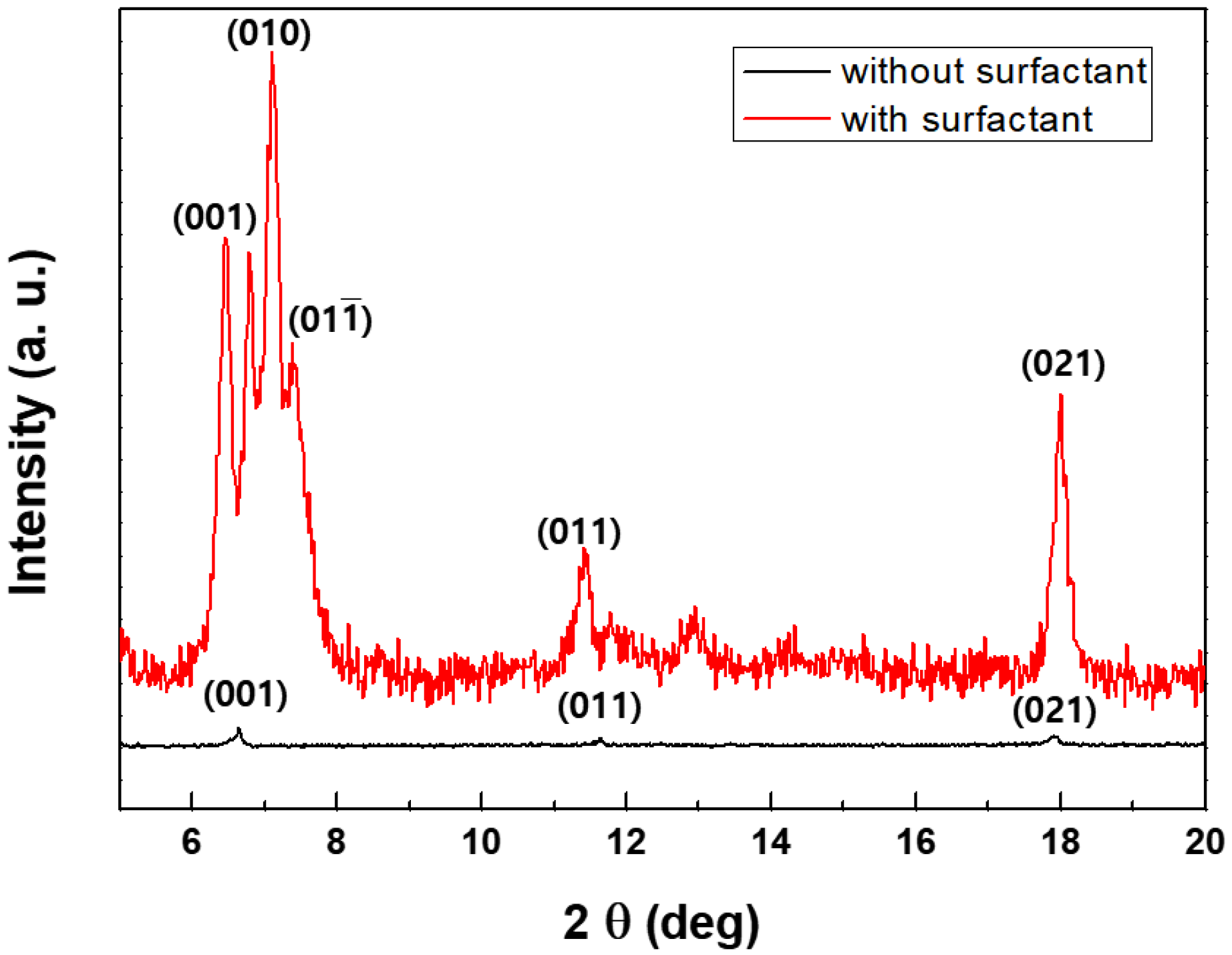
3.3. Structural Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Park, D.H.; Kim, M.S.; Joo, J. Hybrid nanostructures using π-conjugated polymers and nanoscale metals: Synthesis, characteristics, and optoelectronic applications. Chem. Soc. Rev. 2010, 39, 2439. [Google Scholar] [CrossRef] [PubMed]
- Heeger, A.J. Semiconducting polymers: The Third Generation. Chem. Soc. Rev. 2010, 39, 2354. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ma, D. Management of charges and excitons for high-performance white organic light-emitting diodes. Chem. Soc. Rev. 2010, 39, 2387. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.S. Organic crystals: Properties, devices, functionalization and bridges to bio-molecules. Chem. Soc. Rev. 2010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, Y.S. Organic nanophotonic materials: The relationship between excited-state processes and photonic performances. Chem. Commun. 2016, 52, 8906–8917. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Kim, D.C.; Jang, M.H.; Baek, J.; Park, D.; Kang, I.S.; Park, Y.C.; Ahn, S.; Cho, Y.H.; Kim, J.; et al. Extraordinary Strong Fluorescence Evolution in Phosphor on Graphene. Adv. Mater. 2016, 28, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.W.; Vanslyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- D’Andrade, B.W.; Forrest, S.R. White organic light-emitting devices for solid-state lighting. Adv. Mater. 2004, 16, 1585–1595. [Google Scholar] [CrossRef]
- Huang, L.; Liao, Q.; Shi, Q.; Fu, H.; Ma, J.; Yao, J. Rubrene micro-crystals from solution routes: Their crystallography, morphology and optical properties. J. Mater. Chem. 2010, 20, 159–166. [Google Scholar] [CrossRef]
- Garreau, A.; Duvail, J.L. Recent advances in optically active polymer-based nanowires and nanotubes. Adv. Opt. Mater. 2014, 2, 1122–1140. [Google Scholar] [CrossRef]
- Lee, T.; Lin, M.S. Sublimation point depression of tris(8-hydroxyquinoline)aluminum(III) (Alq3) by crystal engineering. Cryst. Growth Des. 2007, 7, 1803–1810. [Google Scholar] [CrossRef]
- Xie, W.; Fan, J.; Song, H.; Jiang, F.; Yuan, H.; Wei, Z.; Ji, Z.; Pang, Z.; Han, S. Controllable synthesis of rice-shape Alq3nanoparticles with single crystal structure. Phys. E Low-Dimens. Syst. Nanostruct. 2016, 84, 519–523. [Google Scholar] [CrossRef]
- Cölle, M.; Brütting, W. Thermal, structural and photophysical properties of the organic semiconductor Alq3. Phys. Status Solidi 2004, 201, 1095–1115. [Google Scholar] [CrossRef]
- Cölle, M.; Dinnebier, R.E.; Brütting, W. The structure of the blue luminescent delta-phase of tris(8-hydroxyquinoline)aluminium(III) (Alq3). Chem. Commun. 2002, 49, 2908–2909. [Google Scholar] [CrossRef]
- Rajeswaran, M.; Blanton, T.N. Single-crystal structure determination of a new polymorph (ε-Alq3) of the electroluminescence OLED (organic light-emitting diode) material, tris(8-hydroxyquinoline)aluminum (Alq3). J. Chem. Crystallogr. 2005, 35, 71–76. [Google Scholar] [CrossRef]
- Bi, H.; Zhang, H.; Zhang, Y.; Gao, H.; Su, Z.; Wang, Y. Fac-Alq3and Mer-Alq3nano/microcrystals with different emission and charge-transporting properties. Adv. Mater. 2010, 22, 1631–1634. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Park, D.H.; Kim, J.; Joo, J.; Ahn, D.J. Oligonucleotide assisted light-emitting Alq3 microrods: Energy transfer effect with fluorescent dyes. Chem. Commun. 2013, 49, 5360. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Kim, H.S.; Jeong, M.Y.; Lee, Y.B.; Kim, H.J.; Kim, D.C.; Kim, J.; Joo, J. Significantly enhanced photoluminescence of doped polymer-metal hybrid nanotubes. Adv. Funct. Mater. 2008, 18, 2526–2534. [Google Scholar] [CrossRef]
- Joo, J.; Park, D.H.; Jeong, M.Y.; Lee, Y.B.; Kim, H.S.; Choi, W.J.; Park, Q.H.; Kim, H.J.; Kim, D.C.; Kim, J. Bright light emission of a single polythiophene nanotube strand with a nanometer-scale metal coating. Adv. Mater. 2007, 19, 2824–2829. [Google Scholar] [CrossRef]
- Park, D.H.; Kim, N.; Cui, C.; Hong, Y.K.; Kim, M.S.; Yang, D.-H.; Kim, D.-C.; Lee, H.; Kim, J.; Ahn, D.J.; et al. DNA detection using a light-emitting polymer single nanowire. Chem. Commun. 2011, 47, 7944–7946. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Kim, B.H.; Jang, M.K.; Bae, K.Y.; Lee, S.J.; Joo, J. Synthesis and characterization of polythiophene and poly (3-methylthiophene) nanotubes and nanowires. Synth. Met. 2005, 153, 341–344. [Google Scholar] [CrossRef]
- Brinkmann, M.; Gadret, G.; Muccini, M.; Taliani, C.; Masciocchi, N.; Sironi, A. Correlation between Molecular Packing and Optical Properties in Different Crystalline Polymorphs and Amorphous Thin Films of mer-Tris(8-hydroxyquinoline)aluminum(III). J. Am. Chem. Soc. 2000, 122, 5147–5157. [Google Scholar] [CrossRef]
- Rajeswaran, M.; Blanton, T.N.; Tang, C.W.; Lenhart, W.C.; Switalski, S.C.; Giesen, D.J.; Antalek, B.J.; Pawlik, T.D.; Kondakov, D.Y.; Zumbulyadis, N.; et al. Structural, thermal, and spectral characterization of the different crystalline forms of Alq3, tris(quinolin-8-olato)aluminum(III), an electroluminescent material in OLED technology. Polyhedron 2009, 28, 835–843. [Google Scholar] [CrossRef]
- Back, S.H.; Park, J.H.; Cui, C.; Ahn, D.J. Bio-recognitive photonics of a DNA-guided organic semiconductor. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Jo, S.G.; Hong, Y.K.; Cui, C.; Lee, H.; Ahn, D.J.; Kim, J.; Joo, J. Highly bright and sharp light emission of a single nanoparticle of crystalline rubrene. J. Mater. Chem. 2011, 21, 8002. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Kim, D.H.; Choi, J.; Lee, H.; Kim, S.-Y.; Park, J.W.; Park, D.H. Growth and Brilliant Photo-Emission of Crystalline Hexagonal Column of Alq3 Microwires. Materials 2018, 11, 472. https://doi.org/10.3390/ma11040472
Kim S, Kim DH, Choi J, Lee H, Kim S-Y, Park JW, Park DH. Growth and Brilliant Photo-Emission of Crystalline Hexagonal Column of Alq3 Microwires. Materials. 2018; 11(4):472. https://doi.org/10.3390/ma11040472
Chicago/Turabian StyleKim, Seokho, Do Hyoung Kim, Jinho Choi, Hojin Lee, Sun-Young Kim, Jung Woon Park, and Dong Hyuk Park. 2018. "Growth and Brilliant Photo-Emission of Crystalline Hexagonal Column of Alq3 Microwires" Materials 11, no. 4: 472. https://doi.org/10.3390/ma11040472



