Preparation of Flame Retardant Polyacrylonitrile Fabric Based on Sol-Gel and Layer-by-Layer Assembly
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
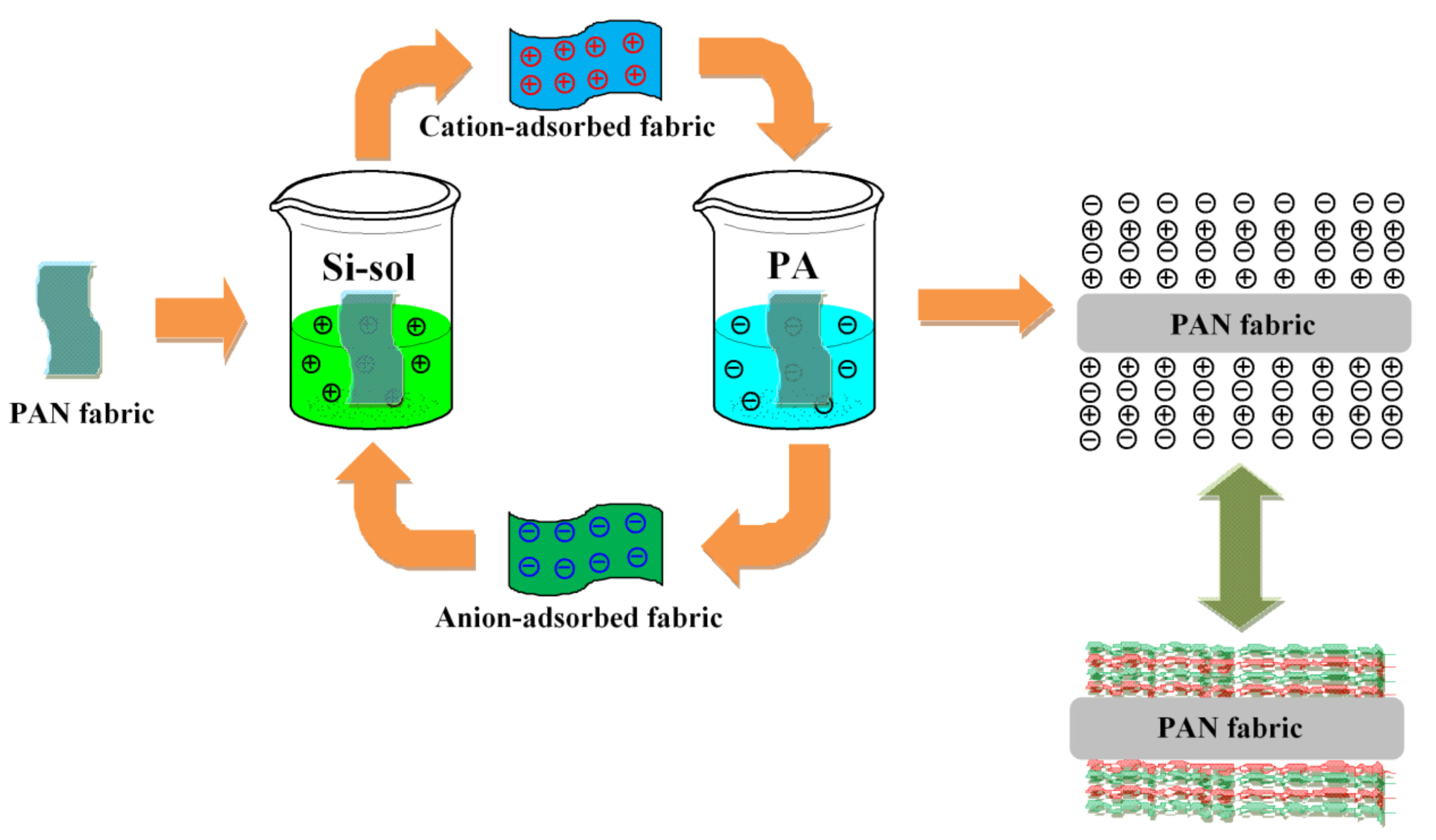
2.2. Preparation of Cationic Solution Via Sol-Gel Process
2.3. Preparation of Anionic Solution
2.4. Preparation of Flame Retardant PAN Fabrics through Layer-by-Layer Assembly
2.5. Characterizations
3. Results and Discussion
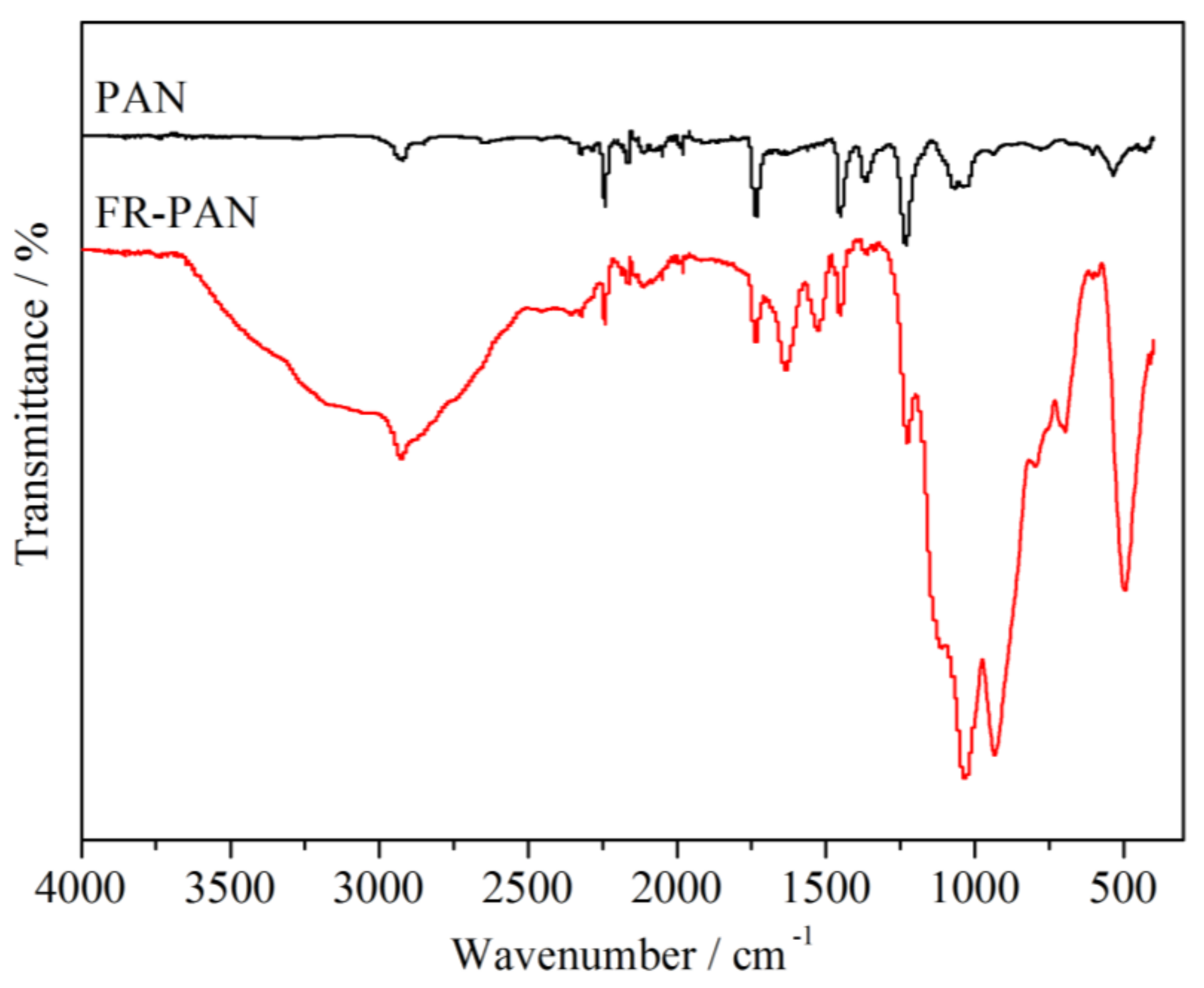
3.1. FTIR Analysis
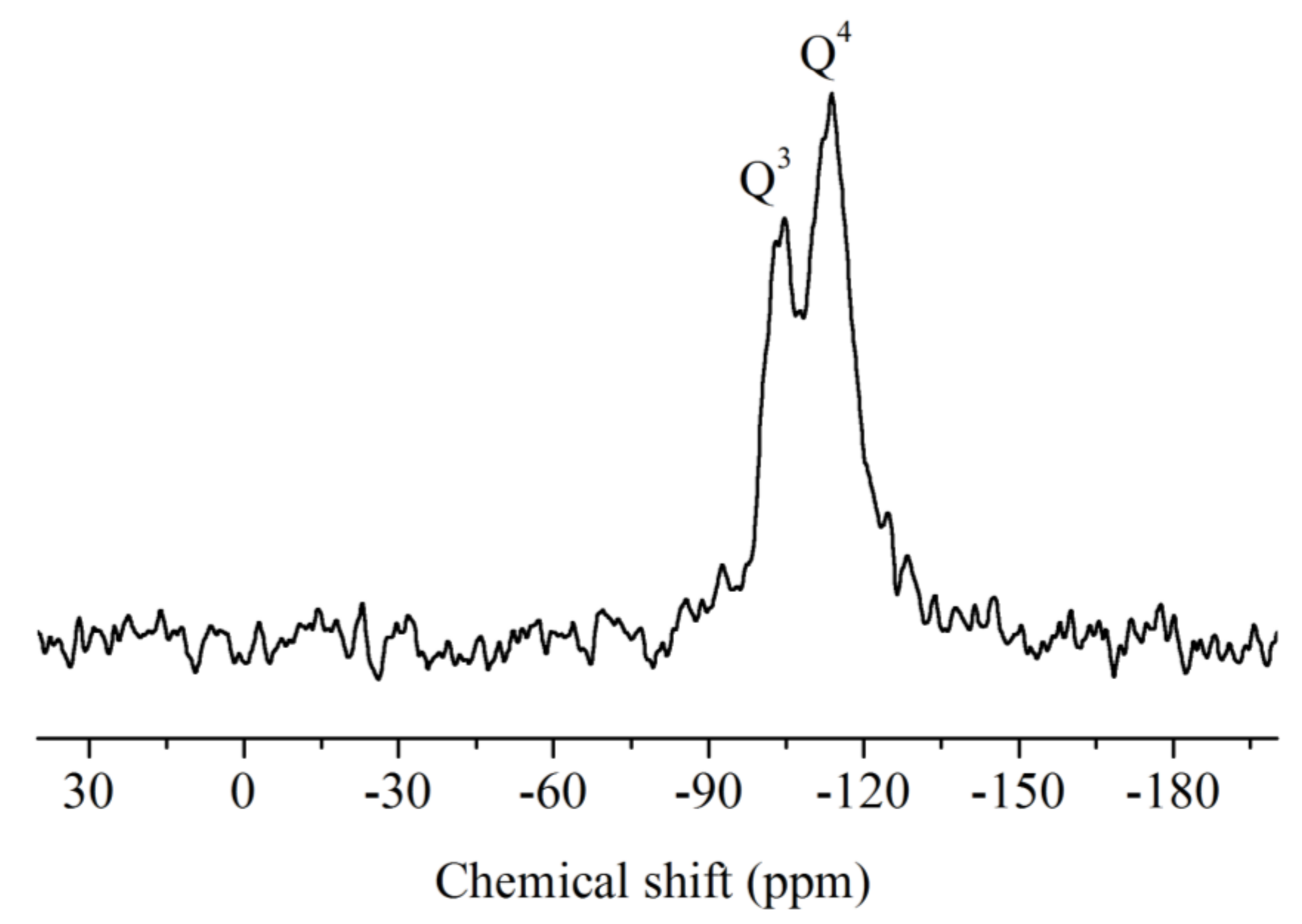
3.2. 29Si NMR Analysis
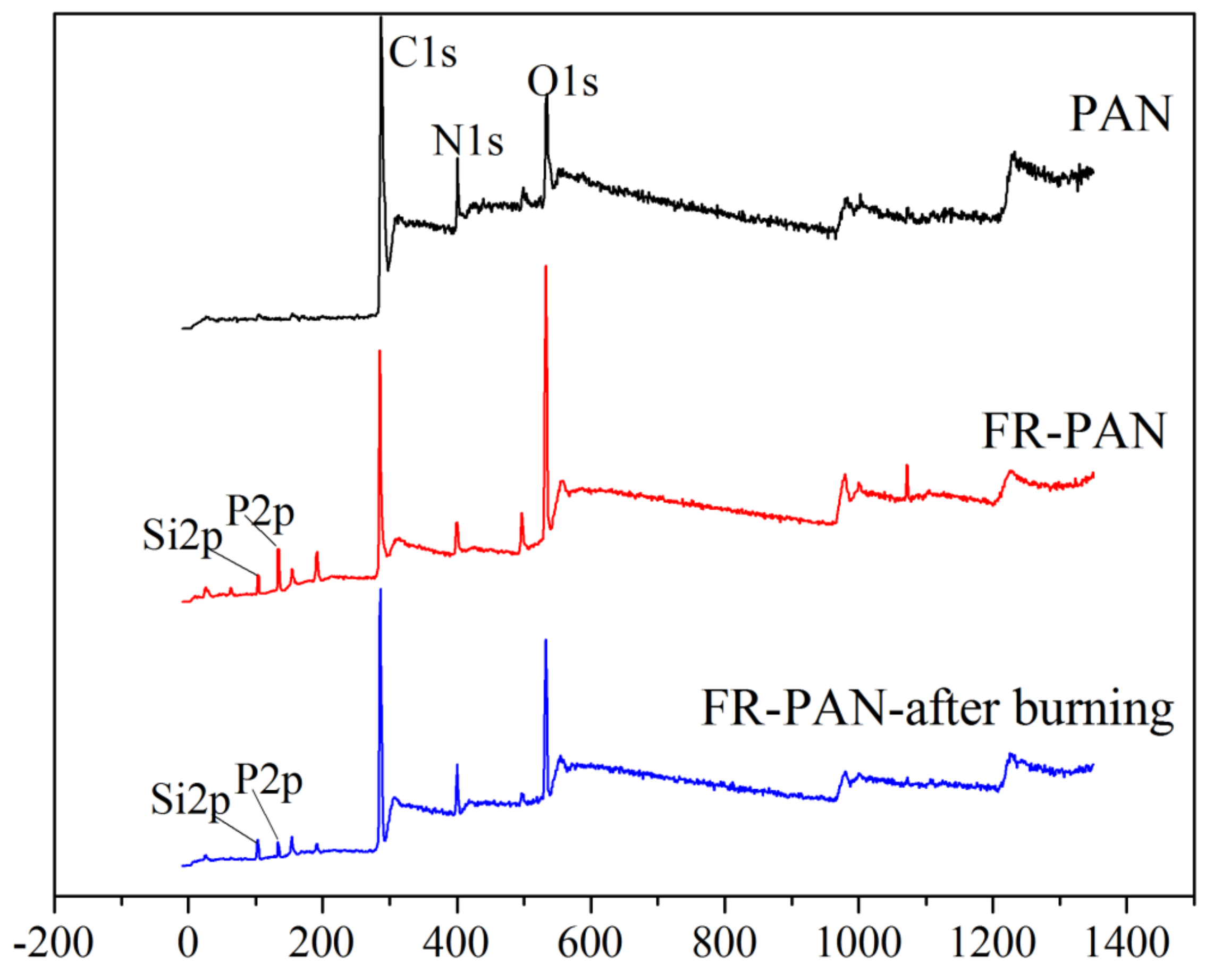
3.3. XPS Analysis
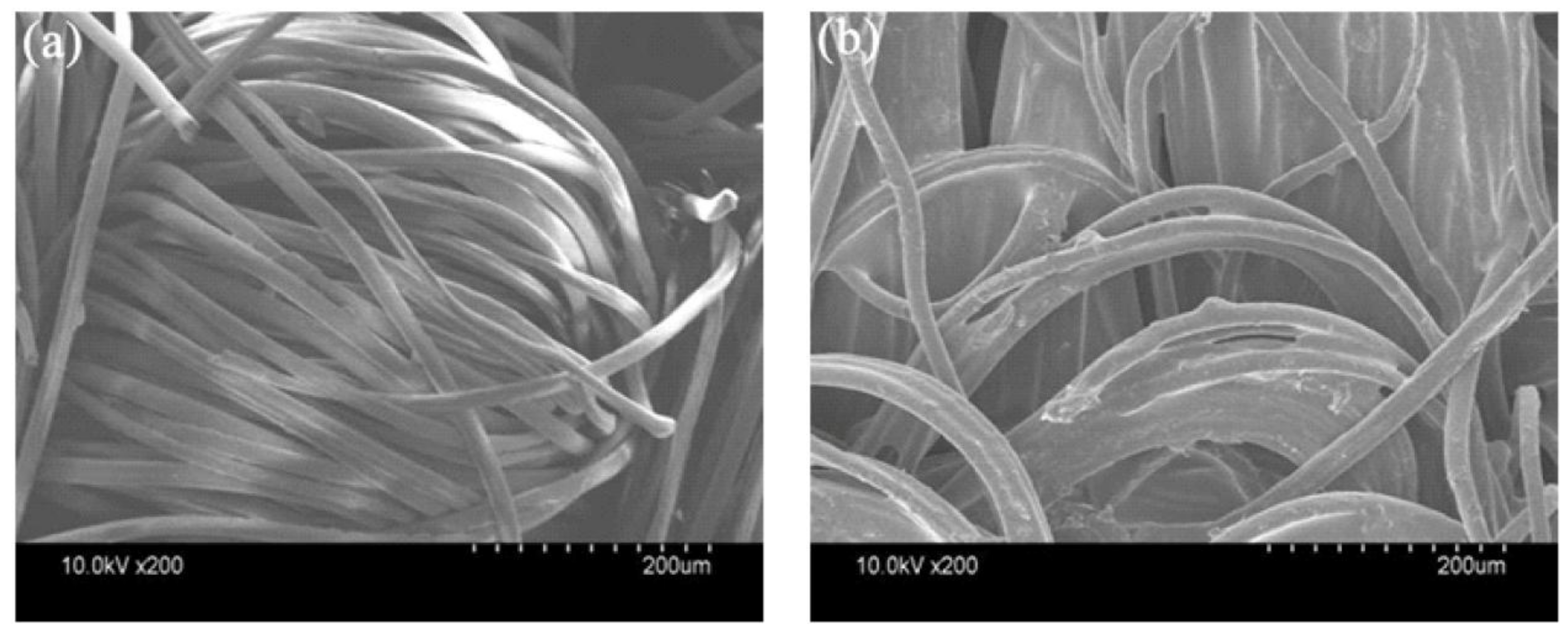
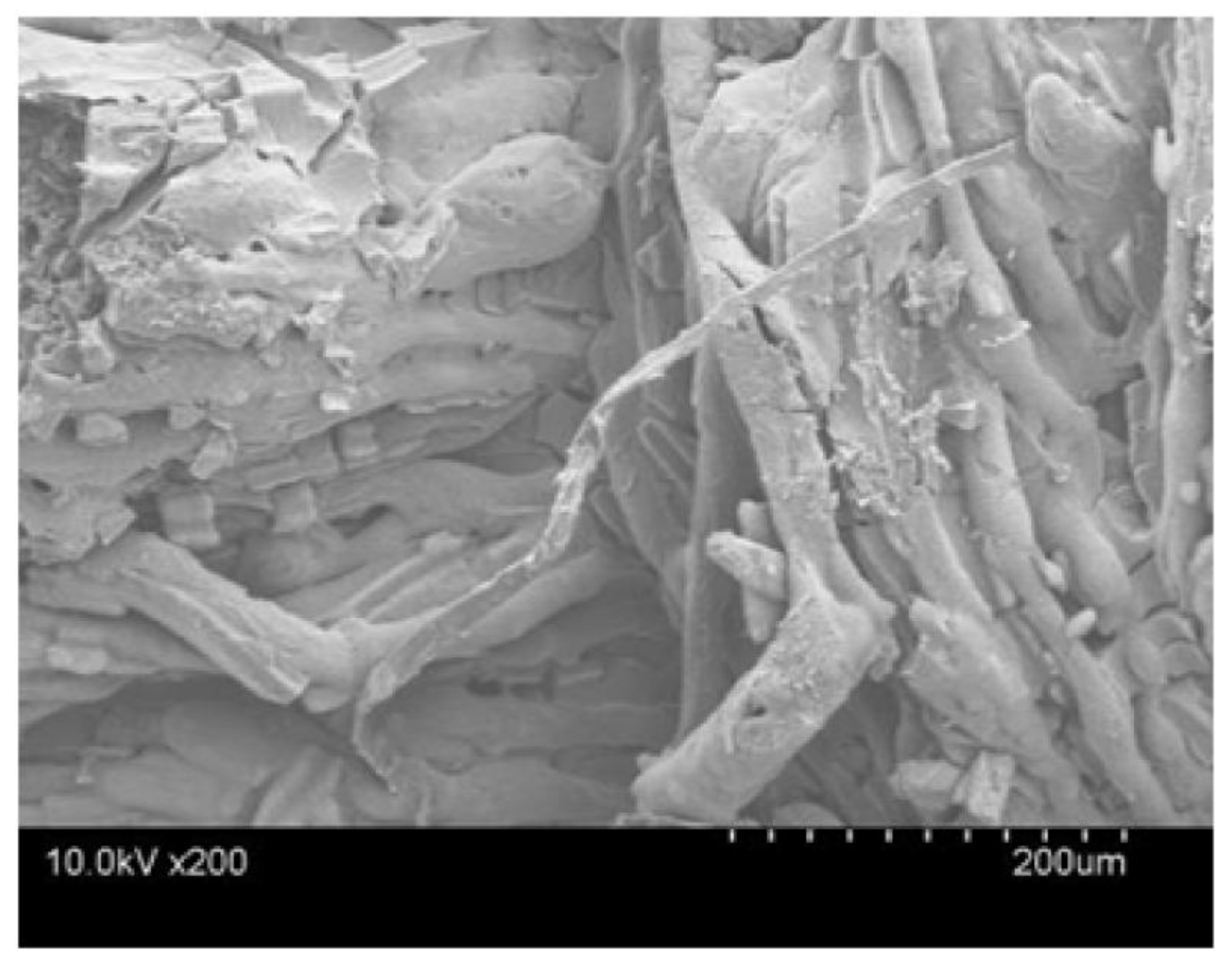
3.4. SEM Analysis
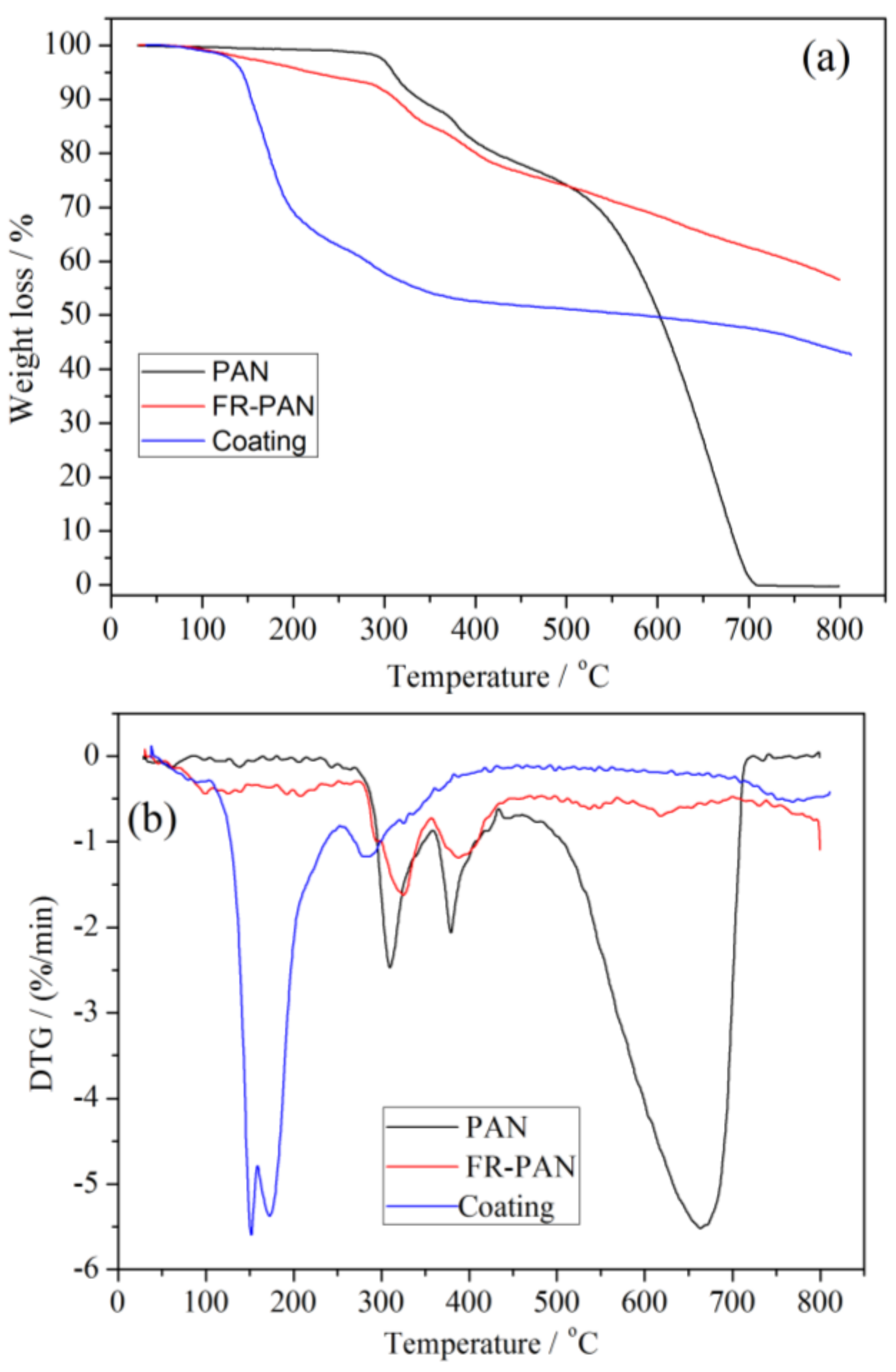
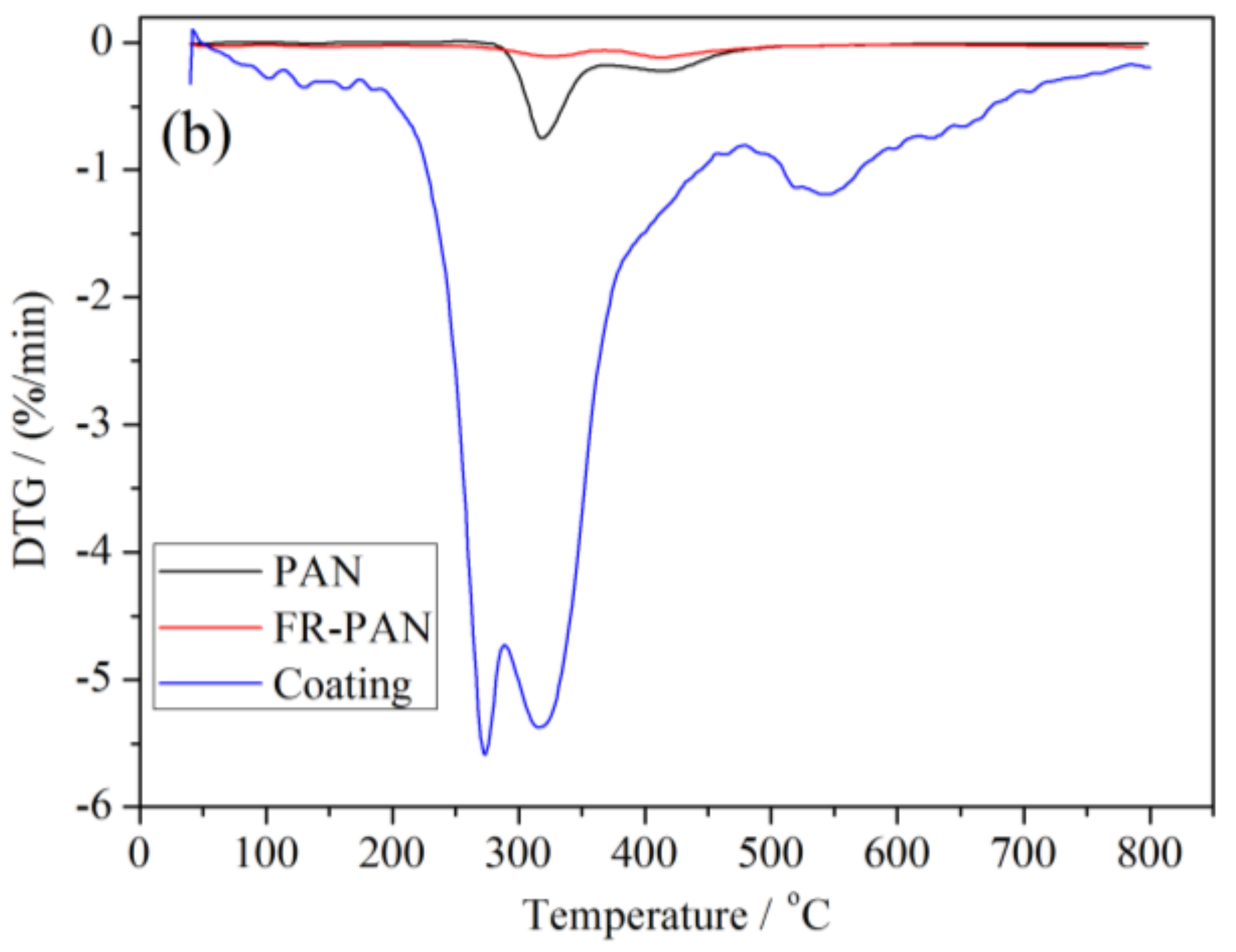
3.5. Thermal Stability Analysis
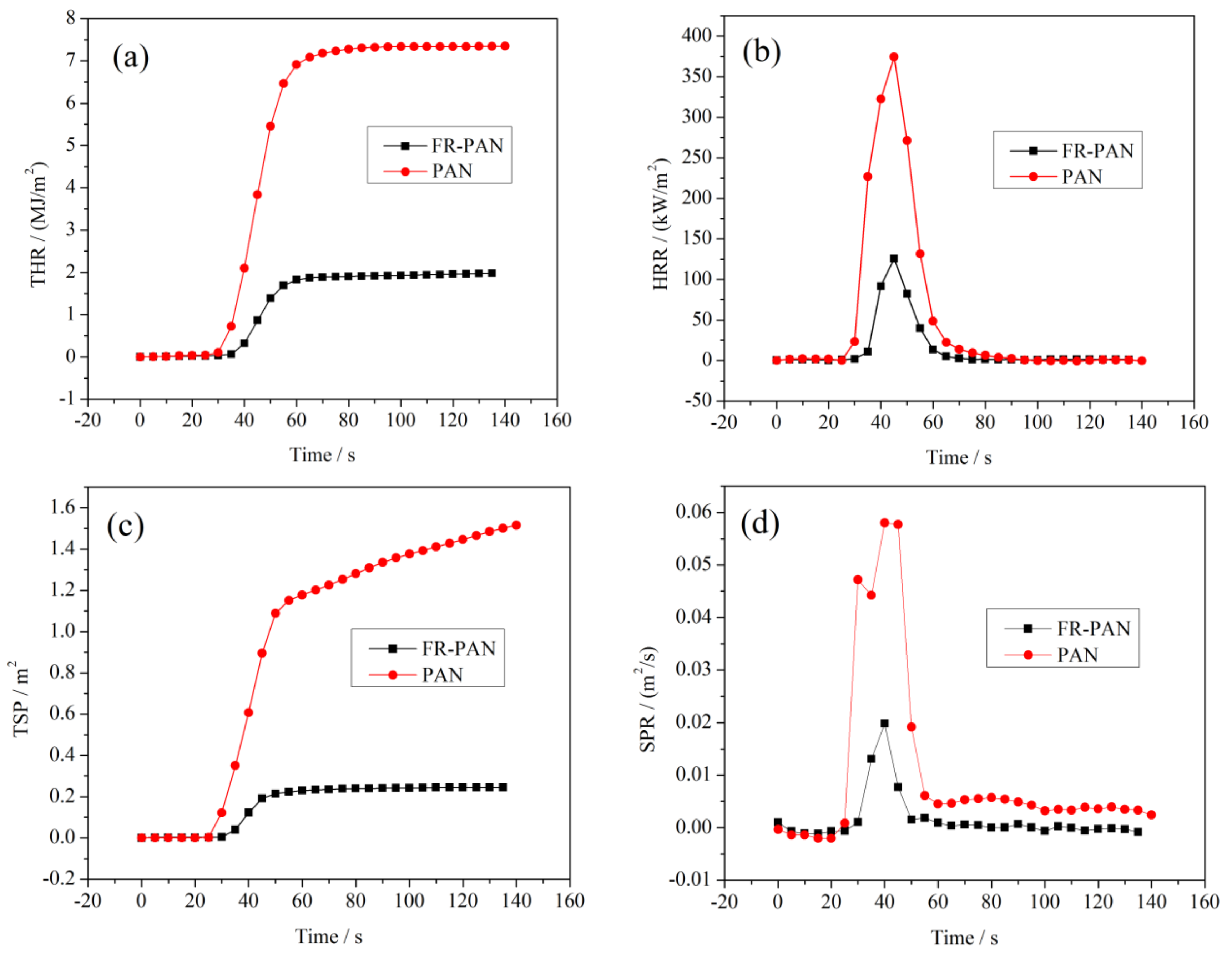
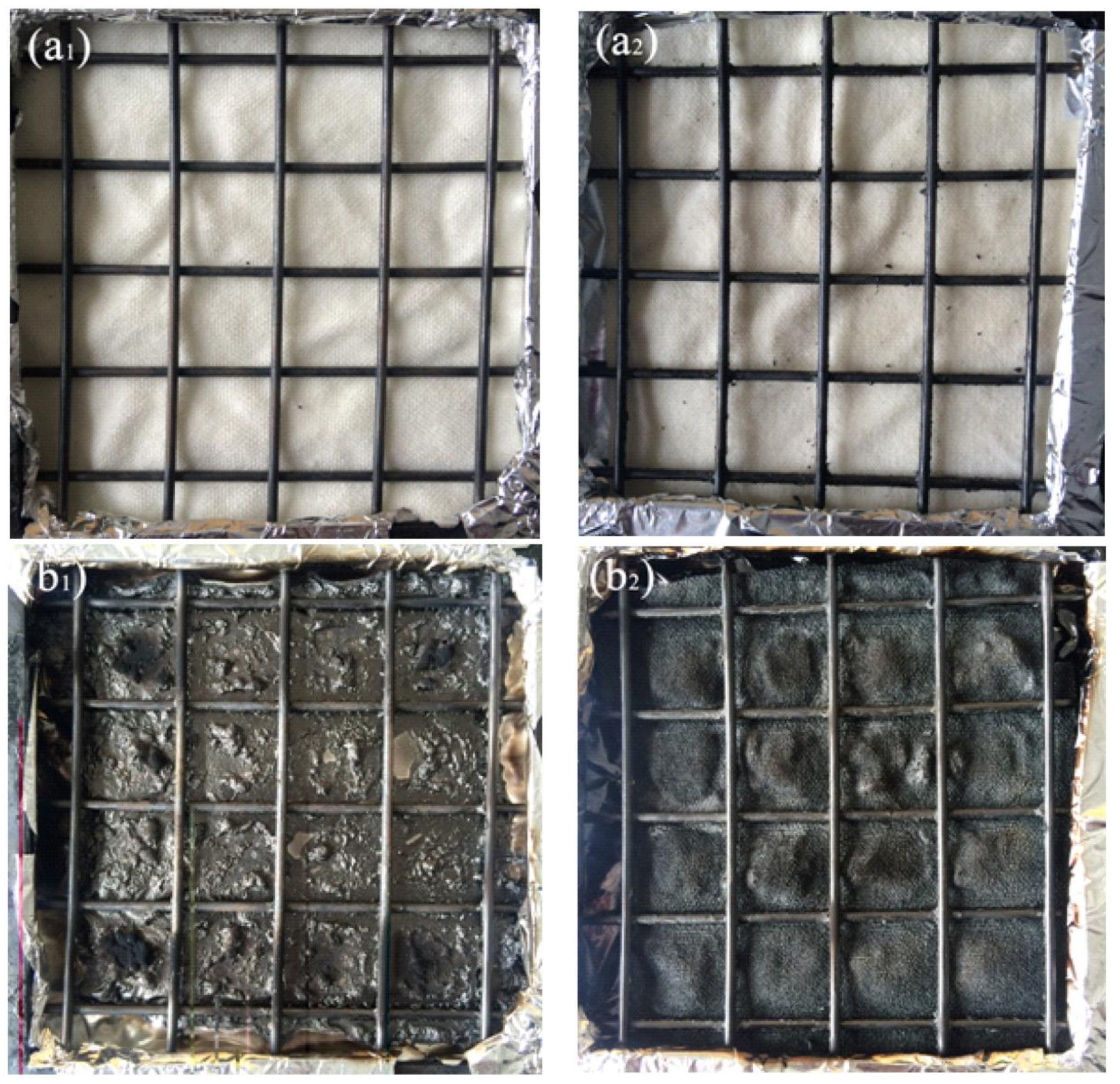
3.6. Flame Retardancy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xue, T.J.; Mckinney, M.A.; Wilkie, C.A. The thermal degradation of polyacrylonitrile. Polym. Degrad. Stab. 1997, 58, 193–202. [Google Scholar] [CrossRef]
- Yaman, N. Preparation and flammability properties of hybrid materials containing phosphorous compounds via sol-gel process. Fire Mater. 2009, 10, 413–418. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, B.W.; Ren, Y.L.; Lu, Y.C. Research progress of flame retardant polyacrylonitrile and its fiber. J. Tex. Res. 2010, 31, 146–152. [Google Scholar]
- Ren, Y.L.; Cheng, B.W.; Xu, L.; Jiang, A.B.; Lu, Y.C. Fire-retardant copolymer of acrylonitrile with O,O-diethyl-O-allyl thiophosphate. J. Appl. Polym. Sci. 2009, 115, 1489–1494. [Google Scholar] [CrossRef]
- Ren, Y.L.; Wang, L.J.; Liu, T.T. Preparation and performance of halogen-free fire retardant polyacrylonitrile fiber. Polym. Mater. Sci. Eng. 2016, 32, 130–133. [Google Scholar]
- Wyman, P.; Crook, V.; Ebdon, J.; Hunt, B.; Joseph, P. Flame-retarding effects of dialkyl-p-vinylbenzyl phosphonates in copolymers with acrylonitrile. Polym. Int. 2006, 55, 764–771. [Google Scholar] [CrossRef]
- Zhang, J.; Horrocks, A.R.; Hall, M.E. Flammability of polyacrylonitrile and its copolymers. IV. The flame retardant mechanism of ammonium polyphosphate. Fire Mater. 2010, 18, 307–312. [Google Scholar] [CrossRef]
- Sahiner, N.; Pekel, N.; Guven, O. Radiation synthesis, characterization and amidoximation of N-vinyl-2-pyrrolidone/acrylonitrile interpenetrating polymer networks. React. Funct. Polym. 1999, 39, 139–146. [Google Scholar] [CrossRef]
- Tsafack, M.J.; Levalois-Grützmacher, J. Plasma-induced graft-polymerization of flame retardant monomers onto PAN fabrics. Surf. Coat. Technol. 2006, 200, 3503–3510. [Google Scholar] [CrossRef]
- Yan, X.; Zhou, W.; Zhao, X.; Xu, J.; Liu, P. Preparation, flame retardancy and thermal degradation behaviors of polyacrylonitrile fibers modified with diethylenetriamine and zinc ions. J. Therm. Anal. Calorim. 2015, 124, 719–728. [Google Scholar] [CrossRef]
- Alongi, J.; Carosio, F.; Malucelli, G. Current emerging techniques to impart flame retardancy to fabrics: An overview. Polym. Degrad. Stab. 2014, 106, 138–149. [Google Scholar] [CrossRef]
- Liang, S.Y.; Neisius, N.M.; Gaan, S. Recent developments in flame retardant polymeric coatings. Prog. Org. Coat. 2013, 76, 1642–1665. [Google Scholar] [CrossRef]
- Wang, X.; Romero, M.Q.; Zhang, X.Q.; Wang, R.; Wang, D.Y. Intumescent multilayer hybrid coating for flame retardant cotton fabrics based on layer-by-layer assembly and sol–gel process. RSC Adv. 2015, 5, 10647–10655. [Google Scholar] [CrossRef] [Green Version]
- Fang, F.; Xiao, D.; Zhang, X.; Meng, Y.; Cheng, C.; Bao, C.; Ding, X.; Cao, H.; Tian, X. Construction of intumescent flame retardant and antimicrobial coating on cotton fabric via layer-by-layer assembly technology. Surf. Coat. Technol. 2015, 276, 726–734. [Google Scholar] [CrossRef]
- Fang, F.; Zhang, X.; Meng, Y.; Gu, Z.; Bao, C.; Ding, X.; Li, S.; Chen, X.; Tian, X. I ntumescent flame retardant coatings on cotton fabric of chitosan and ammonium polyphosphate via layer-by-layer assembly. Surf. Coat. Technol. 2015, 262, 9–14. [Google Scholar] [CrossRef]
- Carosio, F.; Alongi, J. Influence of layer by layer coatings containing octapropylammonium polyhedral oligomeric silsesquioxane and ammonium polyphosphate on the thermal stability and flammability of acrylic fabrics. J. Anal. Appl. Pyrolysis 2016, 119, 114–123. [Google Scholar] [CrossRef]
- Brancatelli, G.; Colleoni, C.; Massafra, M.R.; Rosace, G. Effect of hybrid phosphorus-doped silica thin films produced by sol-gel method on the thermal behavior of cotton fabrics. Polym. Degrad. Stab. 2011, 96, 483–490. [Google Scholar] [CrossRef]
- Colleoni, C.; Donelli, I.; Freddi, G.; Guido, E.; Migani, V.; Rosace, G. A novel sol-gel multi-layer approach for cotton fabric finishing by tetraethoxysilane precursor. Surf. Coat. Technol. 2013, 235, 192–203. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, W.; Huang, J.; Lai, Y.; Xing, T.; Chen, G.; Jin, W.; Liu, H.; Sun, B. Flame retardance and thermal stability of wool fabric treated by boron containing silica sols. Mater. Des. 2015, 85, 796–799. [Google Scholar] [CrossRef]
- Aksit, A.; Onar, N.; Kutlu, B.; Sergin, E.; Yakin, I. Synergistic effect of phosphorus, nitrogen and silicon on flame retardancy properties of cotton fabric treated by sol-gel process. Int. J. Cloth. Sci. Technol. 2016, 28, 319–327. [Google Scholar] [CrossRef]
- Ren, Y.L.; Zhang, Y.; Zhao, J.Y.; Wang, X.L.; Zeng, Q.; Gu, Y.T. Phosphorus-doped organic–inorganic hybrid silicon coating for improving fire retardancy of polyacrylonitrile fabric. J. Sol-Gel Sci. Technol. 2016, 82, 280–288. [Google Scholar] [CrossRef]
- Ren, Y.L.; Zhang, Y.; Gu, Y.T.; Zeng, Q. Flame retardant polyacrylonitrile fabrics prepared by organic-inorganic hybrid silica coating via sol-gel technique. Prog. Org. Coat. 2017, 112, 225–233. [Google Scholar] [CrossRef]
- Alongi, J.; Colleoni, C.; Malucelli, G.; Rosace, G. Hybrid phosphorus-doped silica architectures derived from a multistep sol–gel process for improving thermal stability and flame retardancy of cotton fabrics. Polym. Degrad. Stab. 2012, 97, 1334–1344. [Google Scholar] [CrossRef]
- Alongi, J.; Colleoni, C.; Rosace, G.; Malucelli, G. Phosphorus- and nitrogen-doped silica coatings for enhancing the flame retardancy of cotton: Synergisms or additive effects? Polym. Degrad. Stab. 2013, 98, 579–589. [Google Scholar] [CrossRef]
- Alongi, J.; Colleoni, C.; Rosace, G.; Malucelli, G. Sol–gel derived architectures for enhancing cotton flame retardancy: Effect of pure and phosphorus-doped silica phases. Polym. Degrad. Stab. 2014, 99, 92–98. [Google Scholar] [CrossRef]
- Nazare, S.; Kandola, B.; Horrocks, A.R. Use of cone calorimetry to quantify the burning hazard of apparel fabrics. Fire Mater. 2002, 26, 191–199. [Google Scholar] [CrossRef]
- Wang, D.Y.; Liu, X.Q.; Wang, J.S.; Wang, Y.Z.; Stec, A.A.; Hull, T.R. Preparation and characterisation of a novel fire retardant PET/α-zirconium phosphate naocomposite. Polym. Degrad. Stab. 2009, 94, 544–549. [Google Scholar] [CrossRef]
- Kandare, E.; Luangtriratana, P.; Kandola, B.K. Fire reaction properties of flax/epoxy laminates and their balsa-core sandwich composites with or without fire protection. Composites B 2014, 56, 602–610. [Google Scholar] [CrossRef]
- Grancaric, A.M.; Botteri, L.; Alongi, J.; Tarbuk, A. Silica precursor as synergist for cotton flame retardancy. Int. J. Cloth. Sci. Technol. 2016, 28, 378–386. [Google Scholar] [CrossRef]
- Ren, Y.L.; Cheng, B.W.; Jiang, A.B.; Lu, Y.C.; Xu, L. Thermal degradation kinetics of poly(O,O-diethyl-O-allylthiophosphate-co-acrylonitrile) in nitrogen. J. Appl. Polym. Sci. 2010, 115, 3705–3709. [Google Scholar] [CrossRef]
- Penczek, S.; Pretula, J.; Kubisa, P.; Kaluzynski, K.; Szymanski, R. Reactions of H3PO4 forming polymers. Apparently simple reactions leading to sophisticated structures and applications. Prog. Polym. Sci. 2015, 45, 44–70. [Google Scholar] [CrossRef]
- Wang, L.H.; Ren, Y.L.; Wang, X.L.; Zhao, J.Y.; Zhang, Y.; Zeng, Q.; Gu, Y.T. Fire retardant viscose fiber fabric produced by graft polymerization of phosphorus and nitrogen-containing monomer. Cellulose 2016, 23, 2689–2700. [Google Scholar] [CrossRef]
- Sinko, K.; Meiszterics, A.; Rohonczy, J.; Kobzi, B.; Kubuki, S. Effect of phosphorus precursors on the structure of bioactive calcium phosphate silicate systems. Mater. Sci. Eng. C 2017, 73, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Irwin, A.D.; Holmgren, J.S.; Jonas, J. Solid-state 29Si NMR study of polycondensation during heat treatment of sol-gel derived silicas. Mater. Lett. 1987, 6, 25–30. [Google Scholar] [CrossRef]
- Karatas, S.; Hosgor, Z.; Kayaman-Apohan, N.; Gungor, A. Preparation and characterization of phosphine oxide containing organosilica hybrid coatings by photopolymerization and sol-gel process. Prog. Org. Coat. 2009, 65, 49–55. [Google Scholar] [CrossRef]
- Chen, X.W.; Liang, C.X.; Guan, J.P.; Yang, X.H.; Tang, R.C. Flame retardant and hydrophobic properties of novel sol-gel derived phytic acid/silica hybrid organic-inorganic coatings for silk fabric. Appl. Surf. Sci. 2018, 427, 69–80. [Google Scholar] [CrossRef]
- Surianarayanan, M.; Vijayaraghavan, R.; Raghavan, K.V. Spectroscopic investigations of polyacrylonitrile thermal degradation. J. Polym. Sci. Part A Polym. Chem. 1998, 36, 2503–2512. [Google Scholar] [CrossRef]
- Martin, S.C.; Liggat, J.J.; Snape, C.E. In situ NMR investigation into the thermal degradation and stabilisation of PAN. Polym. Degrad. Stab. 2001, 74, 407–412. [Google Scholar] [CrossRef]
- Qin, O.; Cheng, L.; Wang, H.; Li, K. Mechanism and kinetics of the stabilization reactions of itaconic acid-modified polyacrylonitrile. Polym. Degrad. Stab. 2008, 93, 1415–1421. [Google Scholar]
- Liu, Y.; Pan, Y.T.; Wang, X.; Acuna, P.; Zhu, P.; Wagenknecht, U.; Heinrich, G.; Zhang, X.Q.; Wang, R.; Wang, D.Y. Effect of phosphorus-containing inorganic–organic hybrid coating on the flammability of cotton fabrics: Synthesis, characterization and flammability. Chem. Eng. J. 2016, 294, 167–175. [Google Scholar] [CrossRef]

| Samples | C/wt % | O/wt % | N/wt % | P/wt % | Si/wt % |
|---|---|---|---|---|---|
| PAN | 66.53 | 21.31 | 9.12 | - | - |
| FR-PAN | 54.21 | 25.77 | 4.68 | 7.99 | 5.36 |
| FR-PAN after LOI test | 67.12 | 16.25 | 7.70 | 3.12 | 5.80 |
| Samples | Tonset, 5% (°C) | Tmax1 (°C) | Tmax2 (°C) | Tmax3 (°C) | Residue (wt %) | |
|---|---|---|---|---|---|---|
| PAN | air | 297 | 308 | 381 | 664 | 0 |
| N2 | 310 | 320 | 420 | - | 44 | |
| FR-PAN | air | 281 | 324 | 392 | - | 57 |
| N2 | 201 | 330 | 405 | - | 68 | |
| Coating | air | 150 | 154 | 175 | 286 | 43 |
| N2 | 260 | 270 | 330 | 550 | 52 | |
| Samples | TTI (s) | PHRR (kW/m2) | Time to PHRR (s) | THR (MJ/m2) | PSPR (m2/s) | TSP (m2) | aMLR (g/s) | FIGRA ((kW/m2)/s) | Residue (wt %) |
|---|---|---|---|---|---|---|---|---|---|
| PAN | 25 | 374 | 45 | 7.3 | 0.06 | 1.5 | 0.02 | 8.3 | 38 |
| FR-PAN | 28 | 126 | 45 | 2.0 | 0.02 | 0.2 | 0.01 | 2.8 | 51 |
| Si/P-coated fabric [21] | 27 | 168 | 45 | 3.1 | 0.03 | 0.3 | 0.02 | 3.7 | 47 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Huo, T.; Qin, Y.; Liu, X. Preparation of Flame Retardant Polyacrylonitrile Fabric Based on Sol-Gel and Layer-by-Layer Assembly. Materials 2018, 11, 483. https://doi.org/10.3390/ma11040483
Ren Y, Huo T, Qin Y, Liu X. Preparation of Flame Retardant Polyacrylonitrile Fabric Based on Sol-Gel and Layer-by-Layer Assembly. Materials. 2018; 11(4):483. https://doi.org/10.3390/ma11040483
Chicago/Turabian StyleRen, Yuanlin, Tongguo Huo, Yiwen Qin, and Xiaohui Liu. 2018. "Preparation of Flame Retardant Polyacrylonitrile Fabric Based on Sol-Gel and Layer-by-Layer Assembly" Materials 11, no. 4: 483. https://doi.org/10.3390/ma11040483




