Experimental Investigation of the Effect of Hydrogen on Fracture Toughness of 2.25Cr-1Mo-0.25V Steel and Welds after Annealing
Abstract
:1. Introduction
2. Experimental Details
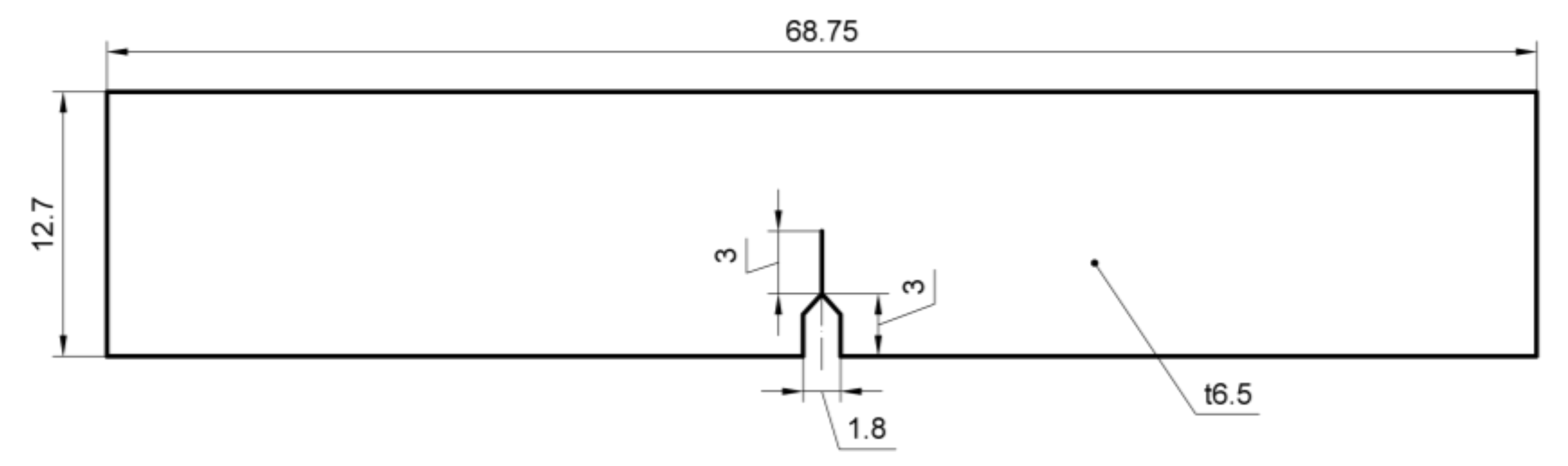
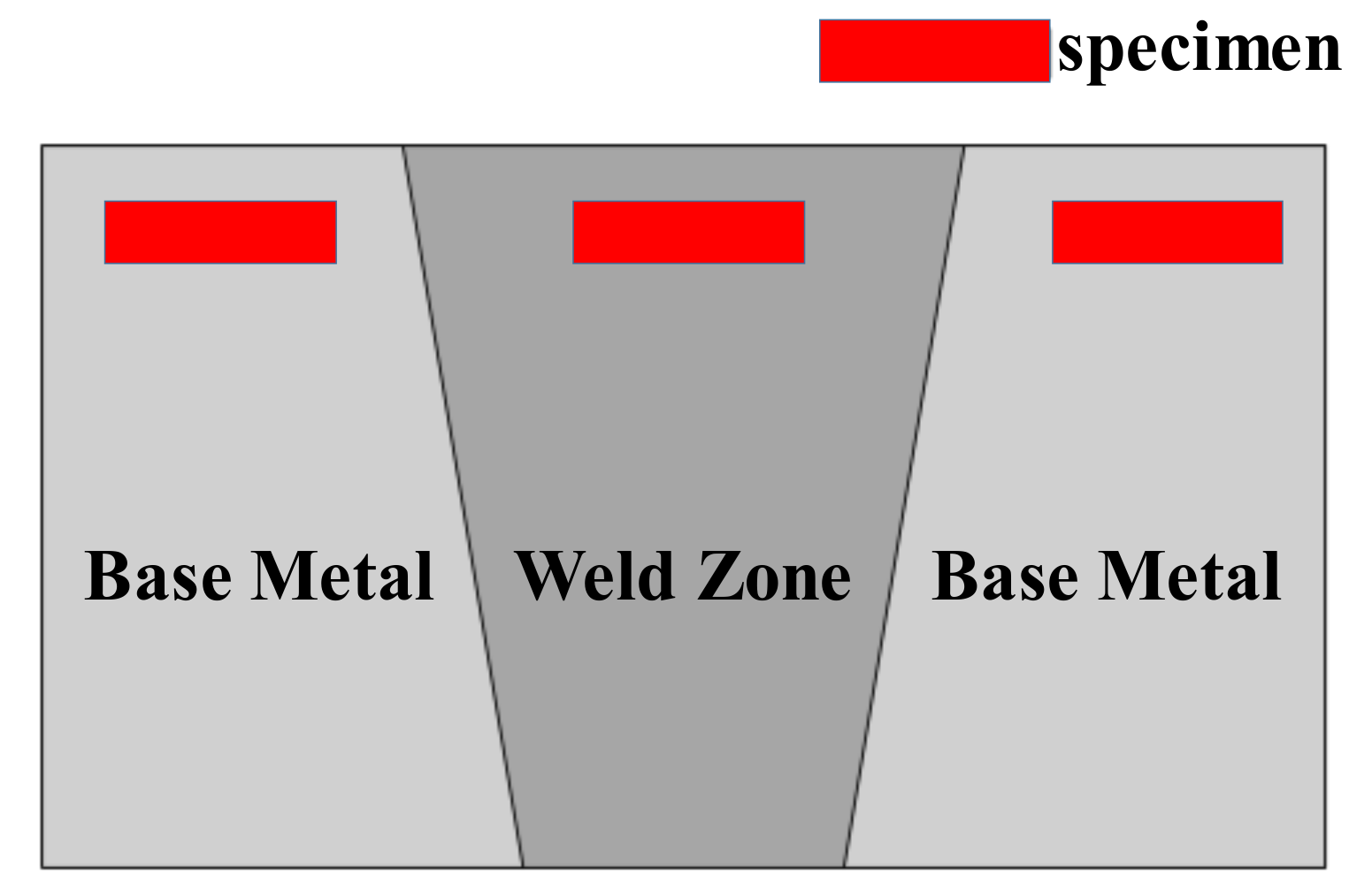
2.1. Materials and Specimens
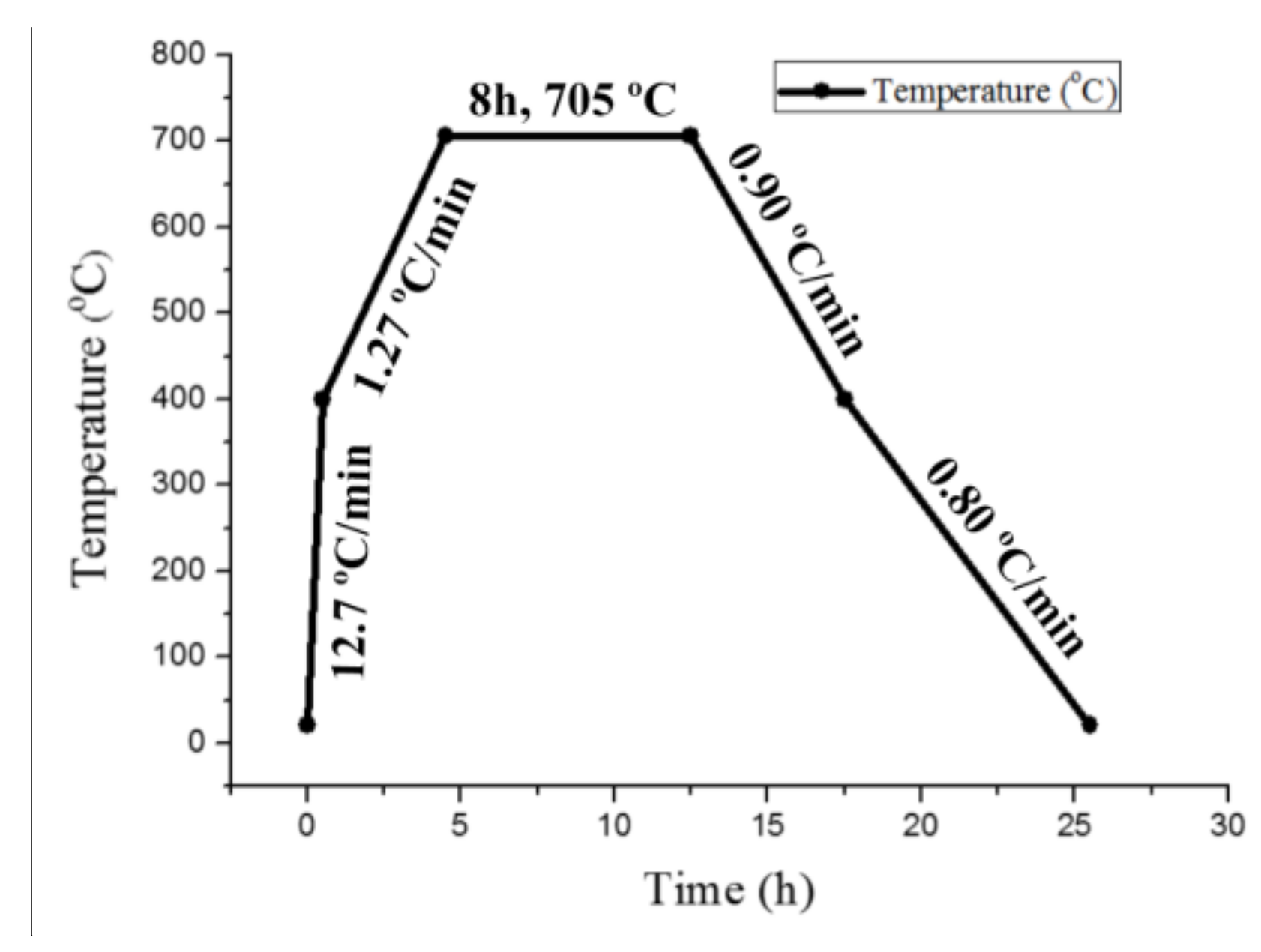
2.2. Hydrogen Charging Process
2.3. Fracture Toughness Test
3. Results
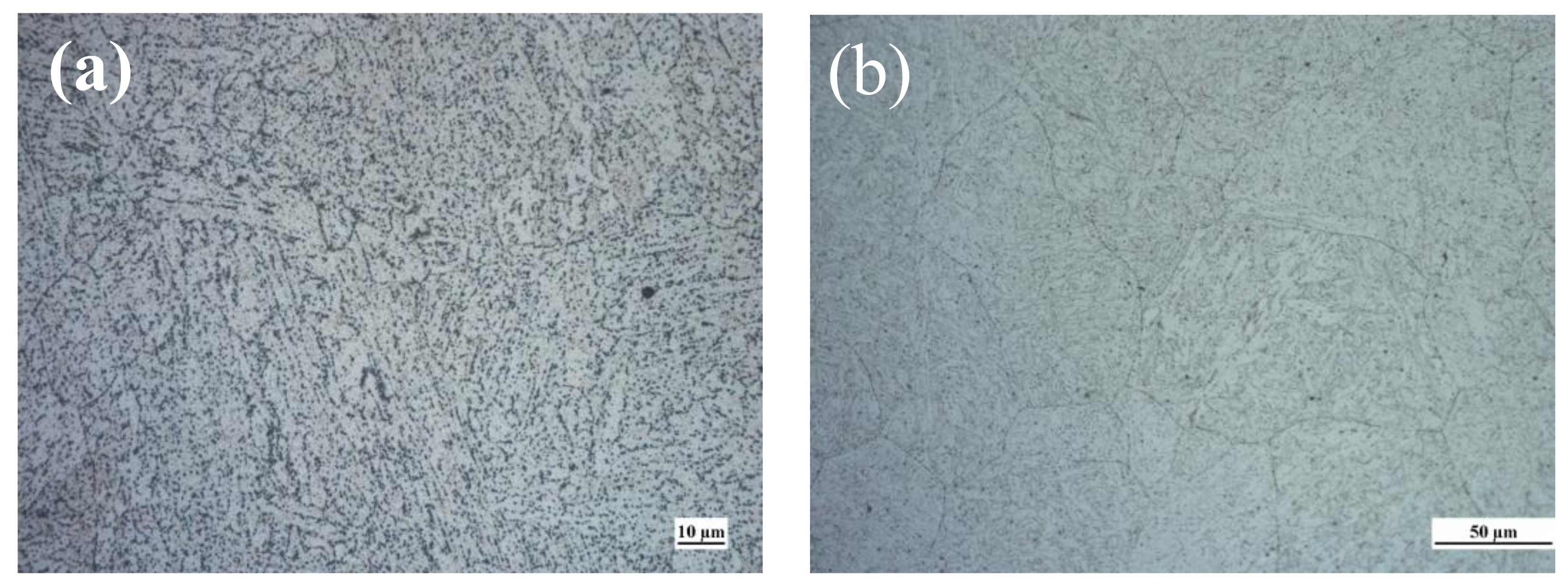
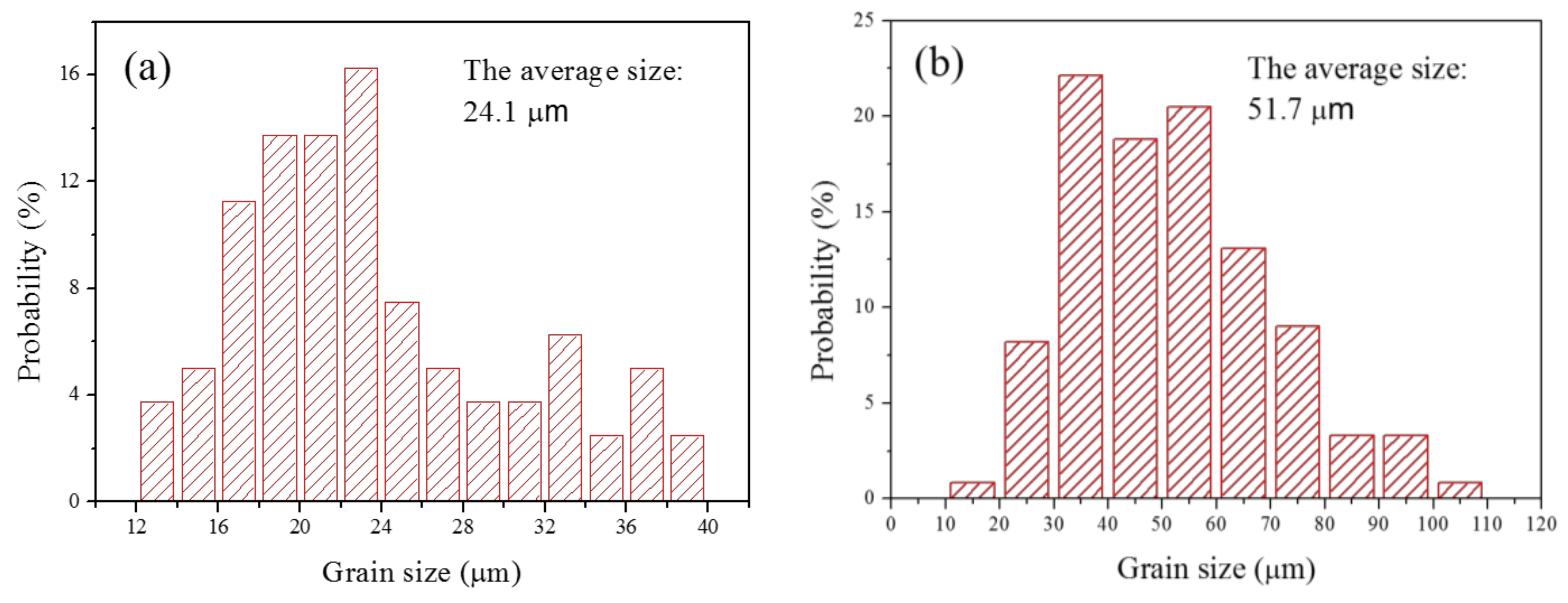
3.1. Microstructure and Grain Size
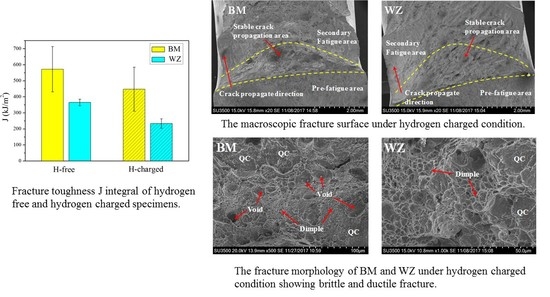
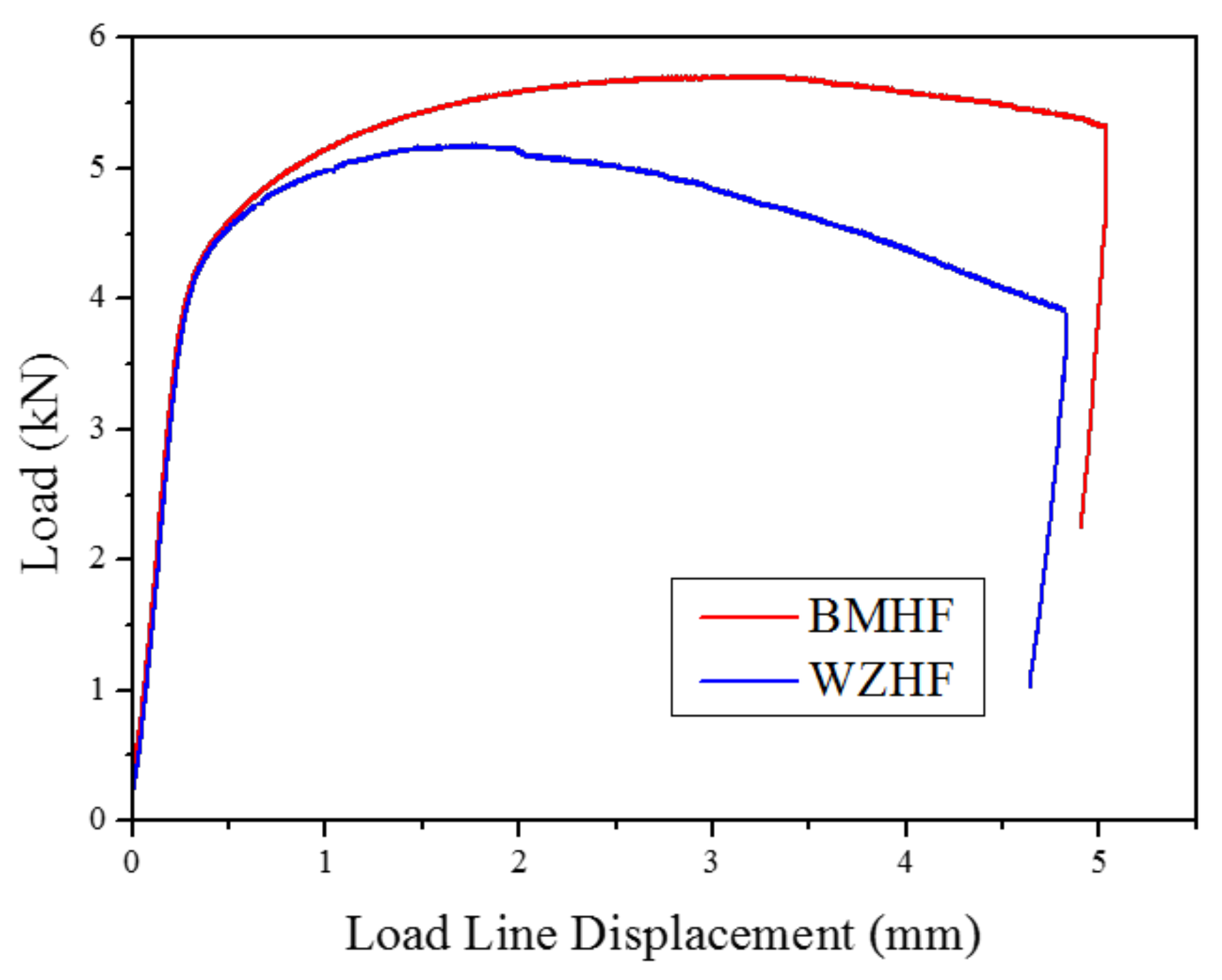
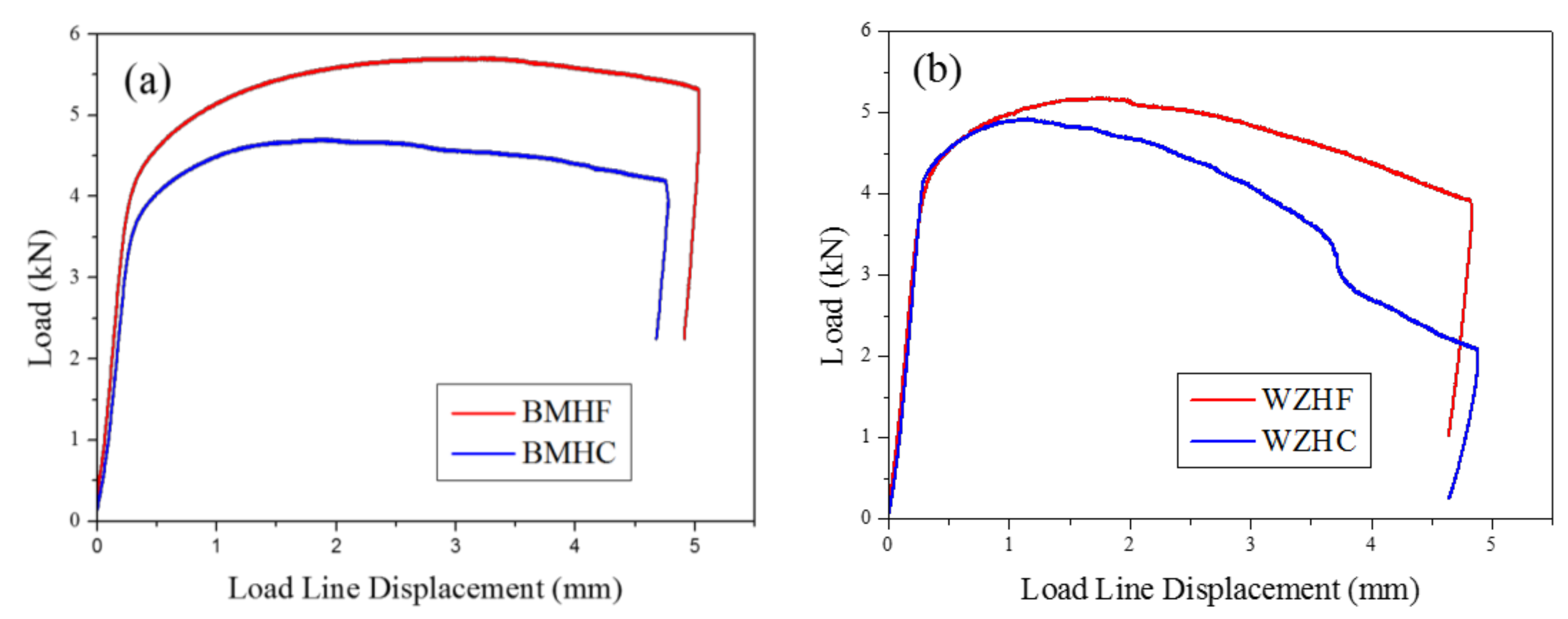
3.2. Fracture Toughness
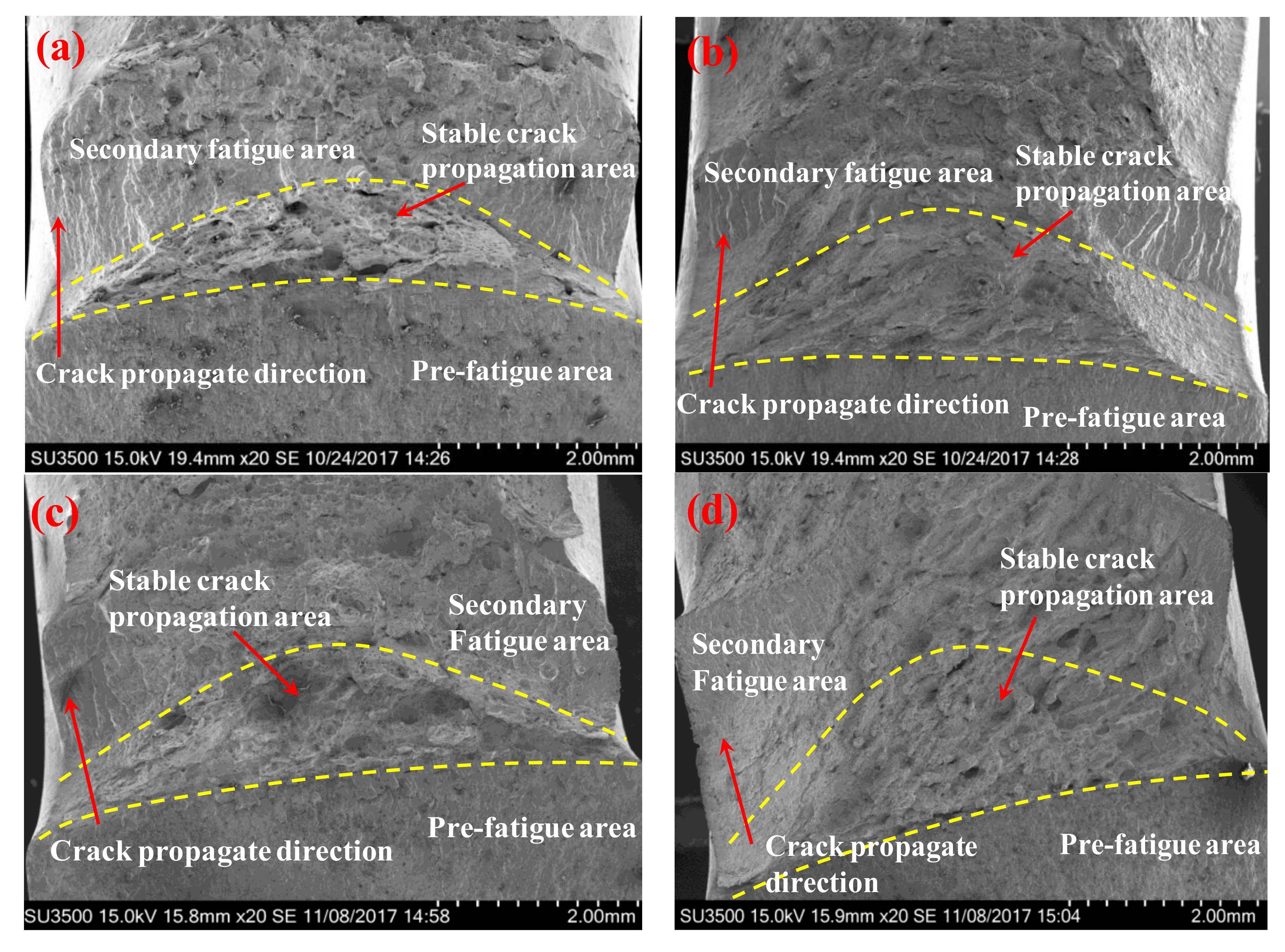
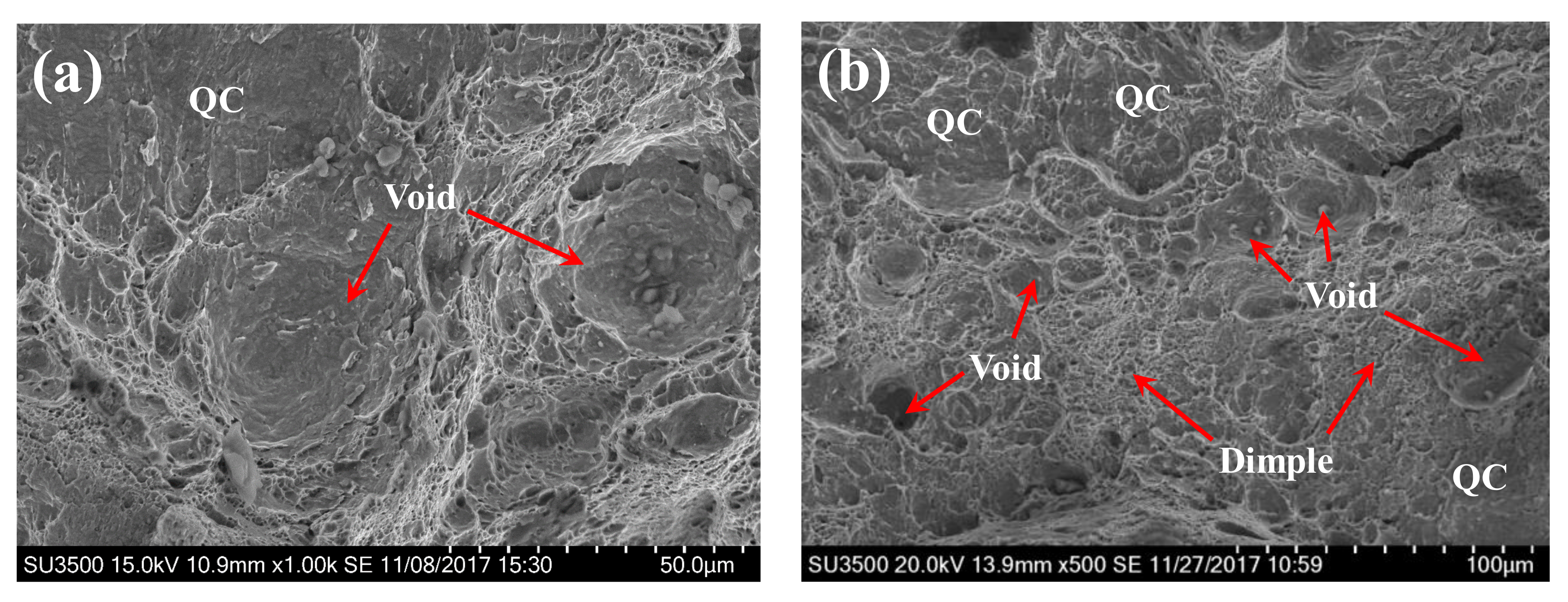
3.3. Fracture Morphology
4. Discussion
4.1. Mechanisms of Hydrogen Effects
4.2. Resistance to HE
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, C.Y.; Fu, R.D.; Zhou, W.H.; Zhang, W.H.; Zheng, Y.Z. Effect of reheating processes on grain boundary heritance for 2.25Cr-1Mo-0.25V steel. Mater. Sci. Eng. A 2006, 24, 1135–1138. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, P.; Li, D.; Li, Y. The evolutions of microstructure and mechanical properties of 2.25Cr-1Mo-0.25 V steel with different initial microstructures during tempering. Mater. Sci. Eng. A 2017, 699, 165–175. [Google Scholar] [CrossRef]
- Pillot, S.; Coudreuse, L. Hydrogen-induced disbonding and embrittlement of steels used in petrochemical refining. In Gaseous Hydrogen Embrittlement of Materials in Energy Technologies: The Problem, its Characterisation and Effects on Particular Alloy Classes; Woodhead Publishing: Sawston, UK, 2012; pp. 51–93. [Google Scholar] [CrossRef]
- Tavares, S.S.M.; Bastos, I.N.; Pardal, J.M.; Montenegro, T.R.; Silva, M.R.D. Slow strain rate tensile test results of new multiphase 17% Cr stainless steel under hydrogen cathodic charging. Int. J. Hydrogen Energy 2015, 40, 16992–16999. [Google Scholar] [CrossRef]
- Kamilyan, M.; Silverstein, R.; Eliezer, D. Hydrogen trapping and hydrogen embrittlement of Mg alloys. J. Mater. Sci. 2017, 52, 11091–11100. [Google Scholar] [CrossRef]
- Huang, F.; Li, X.G.; Liu, J.; Qu, Y.M.; Jia, J.; Du, C.W. Hydrogen-induced cracking susceptibility and hydrogen trapping efficiency of different microstructure X80 pipeline steel. J. Mater. Sci. 2011, 46, 715–722. [Google Scholar] [CrossRef]
- Briottet, L.; Moro, I.; Escot, M.; Furtado, J.; Bortot, P.; Tamponi, G.M.; Solin, J.; Odemer, G.; Blanc, C.; Andrieu, E. Fatigue crack initiation and growth in a CrMo steel under hydrogen pressure. Int. J. Hydrogen Energy 2015, 40, 17021–17030. [Google Scholar] [CrossRef]
- García, T.E.; Rodríguez, C.; Belzunce, F.J.; Cuesta, I.I. Effect of hydrogen embrittlement on the tensile properties of CrMoV steels by means of the small punch test. Mater. Sci. Eng. A 2016, 664, 165–176. [Google Scholar] [CrossRef]
- Colombo, C.; Fumagalli, G.; Bolzoni, F.; Gobbi, G.; Vergani, L. Fatigue behavior of hydrogen pre-charged low alloy Cr–Mo steel. Int. J. Fatigue 2016, 83, 2–9. [Google Scholar] [CrossRef]
- Pillot, S.; Chauvy, C.; Corre, S.; Coudreuse, L.; Gingell, A.; Héritier, D.; Toussaint, P. Effect of temper and hydrogen embrittlement on mechanical properties of 2,25Cr–1Mo steel grades—Application to Minimum Pressurizing Temperature (MPT) issues. Part I: General considerations & materials’ properties. Int. J. Press. Vessel. Pip. 2013, 110, 17–23. [Google Scholar] [CrossRef]
- Pillot, S.; Chauvy, C.; Corre, S.; Coudreuse, L.; Gingell, A.; Héritier, D.; Toussaint, P. Effect of temper and hydrogen embrittlement on mechanical properties of 2,25Cr–1Mo steel grades—Application to Minimum Pressurizing Temperature (MPT) issues. Part II: Vintage reactors & MPT determination. Int. J. Press. Vessel. Pip. 2013, 110, 24–31. [Google Scholar] [CrossRef]
- Astafurova, E.G.; Moskvina, V.A.; Maier, G.G.; Melnikov, E.V.; Zakharov, G.N.; Astafurov, S.V.; Galchenko, N.K. Effect of hydrogenation on mechanical properties and tensile fracture mechanism of a high-nitrogen austenitic steel. J. Mater. Sci. 2017, 52, 4224–4233. [Google Scholar] [CrossRef]
- Seita, M.; Hanson, J.P.; Gradečak, S.; Demkowicz, M.J. Probabilistic failure criteria for individual microstructural elements: An application to hydrogen-assisted crack initiation in alloy 725. J. Mater. Sci. 2017, 52, 2763–2779. [Google Scholar] [CrossRef]
- Shekhter, A.; Kim, S.; Carr, D.G.; Croker, A.B.L.; Ringer, S.P. Assessment of temper embrittlement in an ex-service 1Cr–1Mo–0.25V power generating rotor by Charpy V-Notch testing, KIC fracture toughness and small punch test. Int. J. Press. Vessel. Pip. 2002, 79, 611–615. [Google Scholar] [CrossRef]
- Guan, K.; Hua, L.; Wang, Q.; Zou, X.; Song, M. Assessment of toughness in long term service CrMo low alloy steel by fracture toughness and small punch test. Nucl. Eng. Des. 2011, 241, 1407–1413. [Google Scholar] [CrossRef]
- Tanaka, K.; Amita, T.; Satou, T.; Koba, K.; Kusumoto, J.; Kanaya, A. Evaluation on high temperature fracture toughness of CrMoV cast steel by small punch testing. Int. J. Press. Vessel. Pip. 2009, 86, 643–648. [Google Scholar] [CrossRef]
- Guo, Q.; Lu, F.; Liu, X.; Yang, R.; Cui, H.; Gao, Y. Correlation of microstructure and fracture toughness of advanced 9Cr/CrMoV dissimilarly welded joint. Mater. Sci. Eng. A 2015, 638, 240–250. [Google Scholar] [CrossRef]
- Metallic Materials—Unified Method of Test for the Determination of Quasistatic Fracture Toughness; ISO 12135:2016; International Organization for Standardization: Geneva, Switzerland, 2016; Available online: https://iso.org/standard/60891.html (accessed on 22 March 2018).
- Standard Test Method for Measurement of Fracture Toughness; ASTM E1820-17a; ASTM International: West Conshohocken, PA, USA, 2017; Available online: https://doi.org/10.1520/E1820-17A (accessed on 22 March 2018).
- Matsuoka, S.; Tanaka, H.; Homma, N.; Murakami, Y. Influence of hydrogen and frequency on fatigue crack growth behavior of Cr-Mo steel. Int. J. Fract. 2011, 168, 101–112. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, G.; Qin, M.; Li, Q.; Zhang, Z.; Chen, K.; Li, Y.; Hu, H.; Wu, W.; Zhang, J. Effect of high temperature deformation on the microstructure, mechanical properties and hydrogen embrittlement of 2.25Cr–1Mo-0.25V steel. Int. J. Hydrogen Energy 2017, 42, 24549–24559. [Google Scholar] [CrossRef]
- Martínez-Pañeda, E.; García, T.E.; Rodríguez, C. Fracture toughness characterization through notched small punch test specimens. Mater. Sci. Eng. A 2016, 657, 422–430. [Google Scholar] [CrossRef]
- García, T.E.; Rodríguez, C.; Belzunce, F.J.; Peñuelas, I.; Cuesta, I.I. Estimation of the Fracture Toughness of Structural Steels by Means of the CTOD Evaluation on Notched Small Punch Specimens. Procedia Mater. Sci. 2014, 3, 861–866. [Google Scholar] [CrossRef]
- Gao, W.; Chen, K.; Guo, X.; Zhang, L. Fracture Toughness of type 316LN Stainless Steel Welded Joints. Mater. Sci. Eng. A 2016, 685, 107–114. [Google Scholar] [CrossRef]
- Chai, M.; Duan, Q.; Hou, X.; Zhang, Z.; Li, L. Fracture Toughness Evaluation of 316LN Stainless Steel and Weld Using Acoustic Emission Technique. ISIJ Int. 2016, 56, 875–882. [Google Scholar] [CrossRef]
- Wang, R. Effects of hydrogen on the fracture toughness of a X70 pipeline steel. Corros. Sci. 2009, 51, 2803–2810. [Google Scholar] [CrossRef]
- Hui, W.; Xu, Z.; Zhang, Y.; Zhao, X.; Shao, C.; Weng, Y. Hydrogen embrittlement behavior of high strength rail steels: A comparison between pearlitic and bainitic microstructures. Mater. Sci. Eng. A 2017, 704, 199–206. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Wang, Y.; Shen, S.; Song, X. Effect of hydrogen on tensile properties and fracture behavior of PH 13-8 Mo steel. Mater. Des. 2016, 108, 608–617. [Google Scholar] [CrossRef]
- Djukic, M.B.; Zeravcic, V.S.; Bakic, G.M.; Sedmak, A.; Rajicic, B. Hydrogen damage of steels: A case study and hydrogen embrittlement model. Eng. Fail Anal. 2015, 58, 485–498. [Google Scholar] [CrossRef]
- Wang, J.S. The thermodynamics aspects of hydrogen induced embrittlement. Eng. Fract. Mech. 2001, 68, 647–669. [Google Scholar] [CrossRef]
- Troiano, A.R. The Role of Hydrogen and Other Interstitials in the Mechanical Behavior of Metals. Metallogr. Microstruct. Anal. 2016, 5, 557–569. [Google Scholar] [CrossRef]
- Martin, M.L.; Robertson, I.M.; Sofronis, P. Interpreting hydrogen-induced fracture surfaces in terms of deformation processes: A new approach. Acta Mater. 2011, 59, 3680–3687. [Google Scholar] [CrossRef]
- Birnbaum, H.K.; Sofronis, P. Hydrogen-enhanced localized plasticity—A mechanism for hydrogen-related fracture. Mater. Sci. Eng. A 1994, 176, 191–202. [Google Scholar] [CrossRef]
- Chandler, M.Q.; Horstemeyer, M.F.; Baskes, M.I.; Gullett, P.M.; Wagner, G.J.; Jelinek, B. Hydrogen effects on nanovoid nucleation in face-centered cubic single-crystals. Acta Mater. 2008, 56, 95–104. [Google Scholar] [CrossRef]
- Phaniraj, M.P.; Kim, H.J.; Suh, J.Y.; Shim, J.H.; Park, S.J.; Lee, T.H. Hydrogen embrittlement in high interstitial alloyed 18Cr10Mn austenitic stainless steels. Int. J. Hydrogen Energy 2015, 40, 13635–13642. [Google Scholar] [CrossRef]
- Zan, N.; Ding, H.; Guo, X.F.; Tang, Z.Y.; Bleck, W. Effects of grain size on hydrogen embrittlement in a Fe-22Mn-0.6C TWIP steel. Int. J. Hydrogen Energy 2015, 40, 10687–10696. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, M.; Rong, L. Effect of grain size on the hydrogen embrittlement sensitivity of a precipitation strengthened Fe–Ni based alloy. Mater. Sci. Eng. A 2014, 594, 98–102. [Google Scholar] [CrossRef]
- Mine, Y.; Tachibana, K.; Horita, Z. Effect of Hydrogen on Tensile Properties of Ultrafine-Grained Type 310S Austenitic Stainless Steel Processed by High-Pressure Torsion. Metall. Mater. Trans. A 2011, 42, 1619–1629. [Google Scholar] [CrossRef]
- Malitckii, E.; Yagodzinskyy, Y.; Lehto, P.; Remes, H.; Romu, J.; Hänninen, H. Hydrogen effects on mechanical properties of 18% Cr ferritic stainless steel. Mater. Sci. Eng. A 2017, 700, 331–337. [Google Scholar] [CrossRef]


| Element | C | Si | Mn | P | S | Cr | Mo | V | Al |
|---|---|---|---|---|---|---|---|---|---|
| Percentage | 0.15 | 0.1 | 0.54 | 0.009 | 0.01 | 2.3 | 0.98 | 0.3 | 0.05 |
| Element | C | S | P | Si | Mn | Cr | Ni | Mo | Cu | V |
|---|---|---|---|---|---|---|---|---|---|---|
| Percentage | 0.12 | 0.004 | 0.004 | 0.22 | 1.07 | 2.45 | 0.03 | 1.03 | 0.11 | 0.42 |
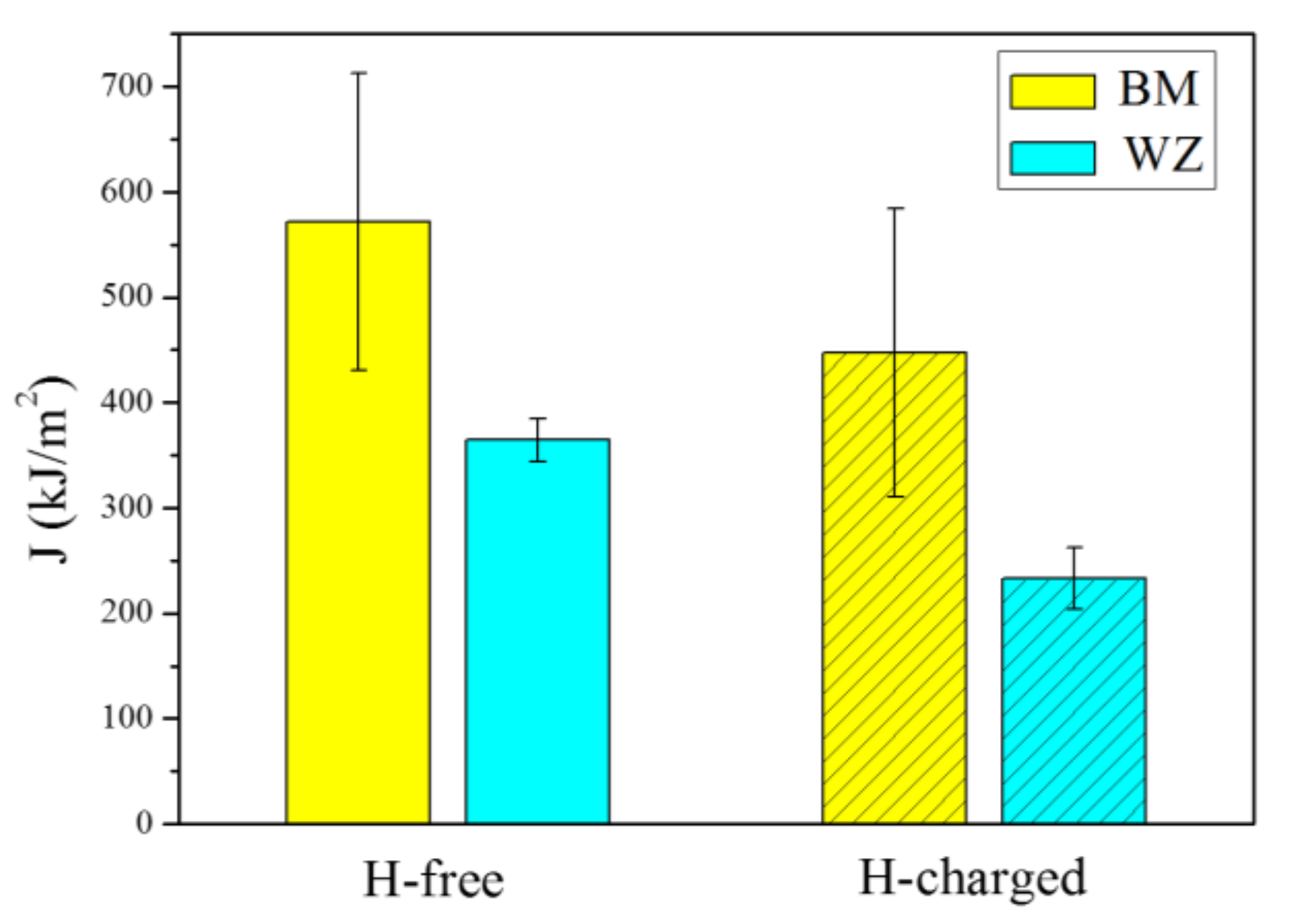
| Specimen | Average Grain Size (μm) | Hydrogen Condition | Average Fracture Toughness (kJ/m2) | SEM Features | Fracture Mechanisms |
|---|---|---|---|---|---|
| BM | 24.1 | H-free | 571.4 | voids and dimples | ductile fracture |
| BM | 24.1 | H-charged | 447.5 | voids, dimples and quasi-cleavage facets | ductile and brittle fracture |
| WZ | 51.7 | H-free | 364.3 | dimples | ductile fracture |
| WZ | 51.7 | H-charged | 232.8 | dimples and quasi-cleavage facets | ductile and brittle fracture |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Chai, M.; Wu, W.; Liu, Y.; Qin, M.; Cheng, G. Experimental Investigation of the Effect of Hydrogen on Fracture Toughness of 2.25Cr-1Mo-0.25V Steel and Welds after Annealing. Materials 2018, 11, 499. https://doi.org/10.3390/ma11040499
Song Y, Chai M, Wu W, Liu Y, Qin M, Cheng G. Experimental Investigation of the Effect of Hydrogen on Fracture Toughness of 2.25Cr-1Mo-0.25V Steel and Welds after Annealing. Materials. 2018; 11(4):499. https://doi.org/10.3390/ma11040499
Chicago/Turabian StyleSong, Yan, Mengyu Chai, Weijie Wu, Yilun Liu, Mu Qin, and Guangxu Cheng. 2018. "Experimental Investigation of the Effect of Hydrogen on Fracture Toughness of 2.25Cr-1Mo-0.25V Steel and Welds after Annealing" Materials 11, no. 4: 499. https://doi.org/10.3390/ma11040499