Synthesis of Controlled-Size Silica Nanoparticles from Sodium Metasilicate and the Effect of the Addition of PEG in the Size Distribution
Abstract
:1. Introduction
2. Materials and Methods
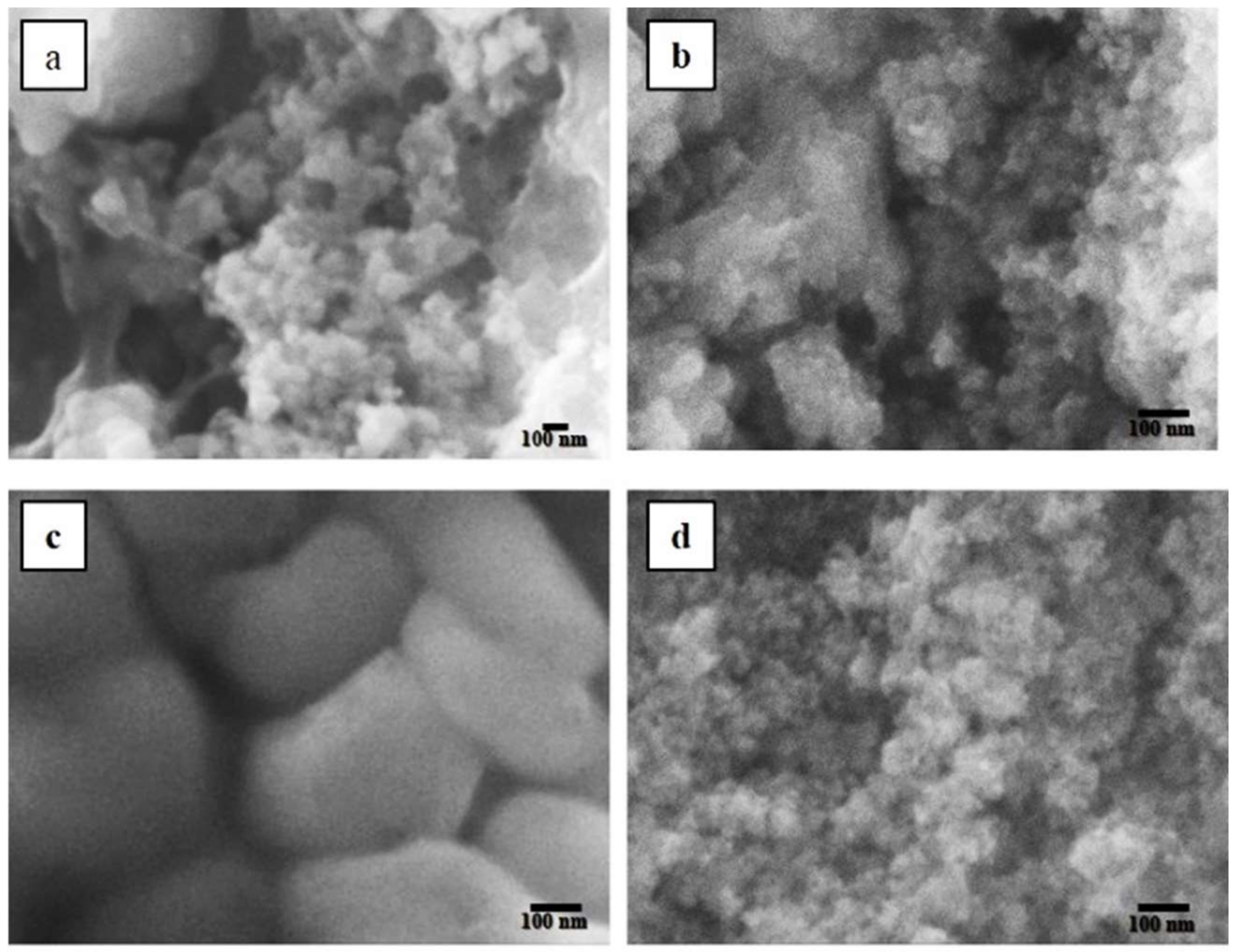
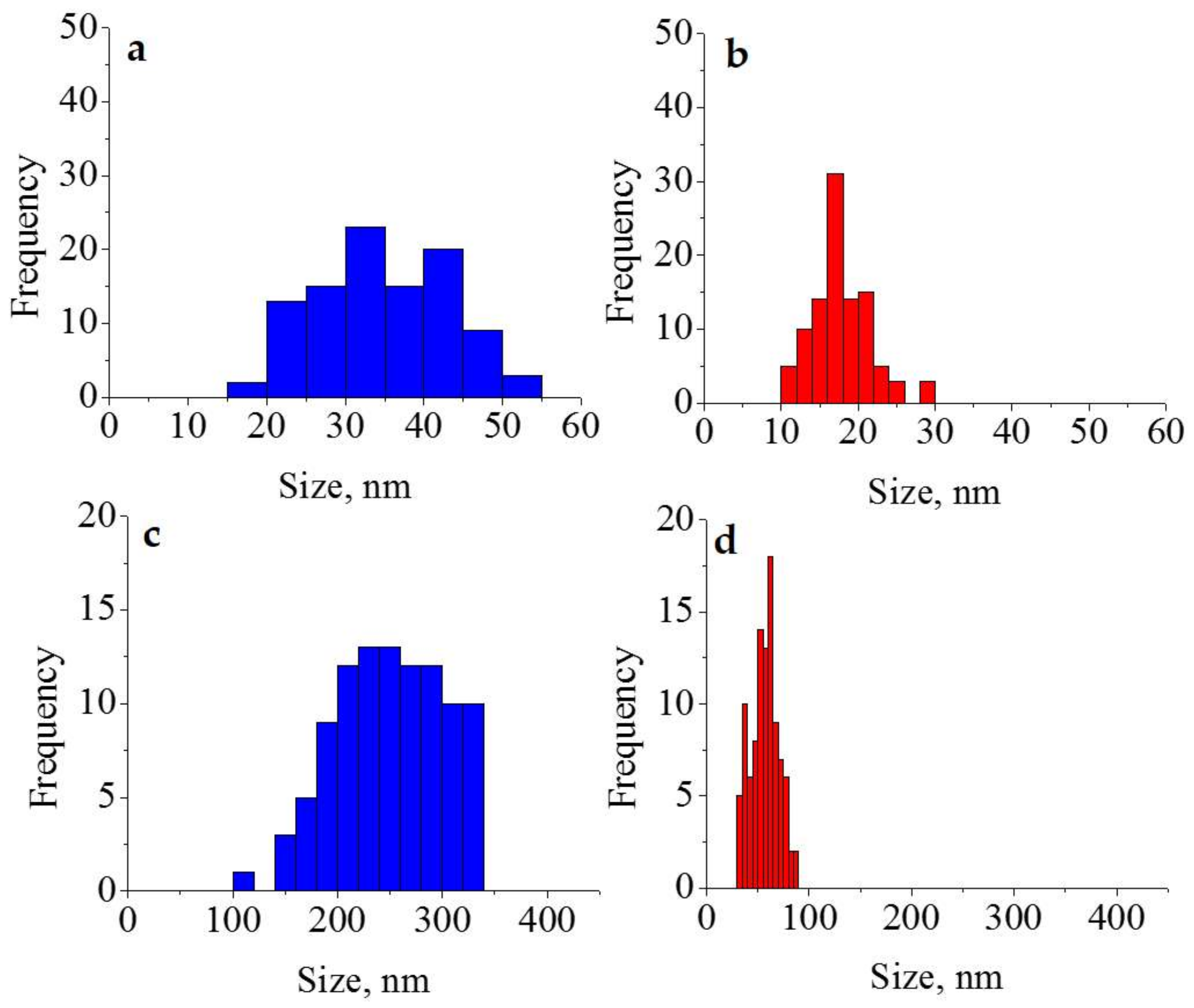
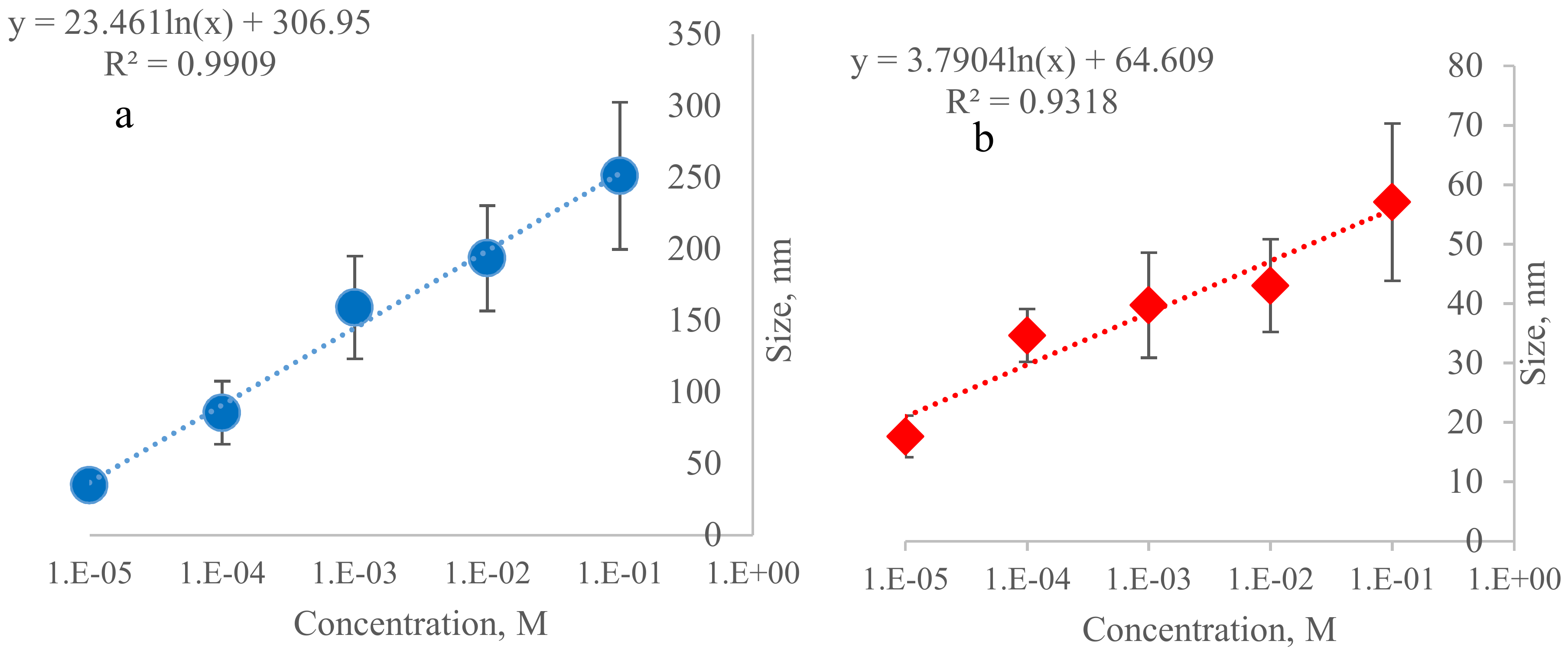
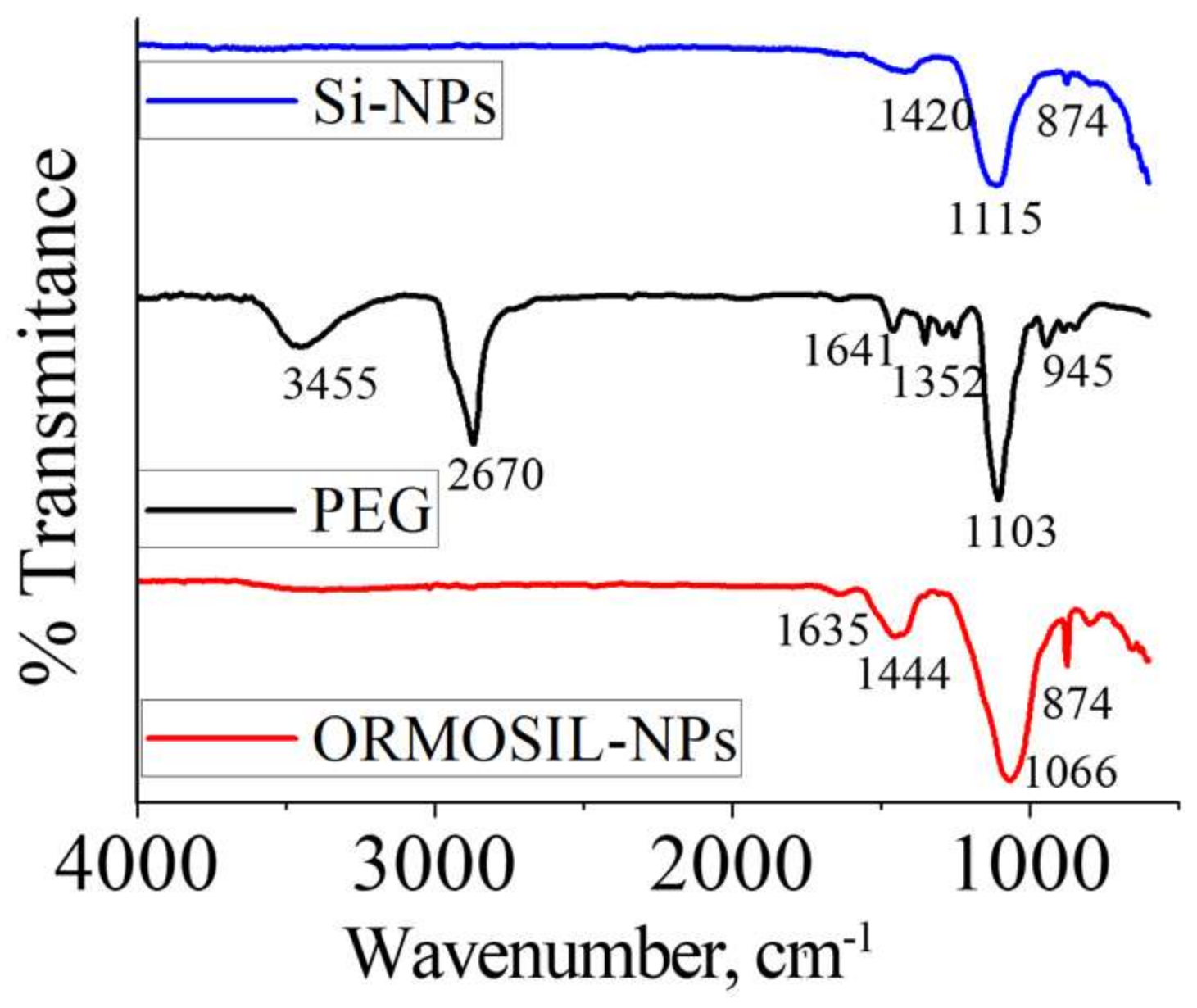
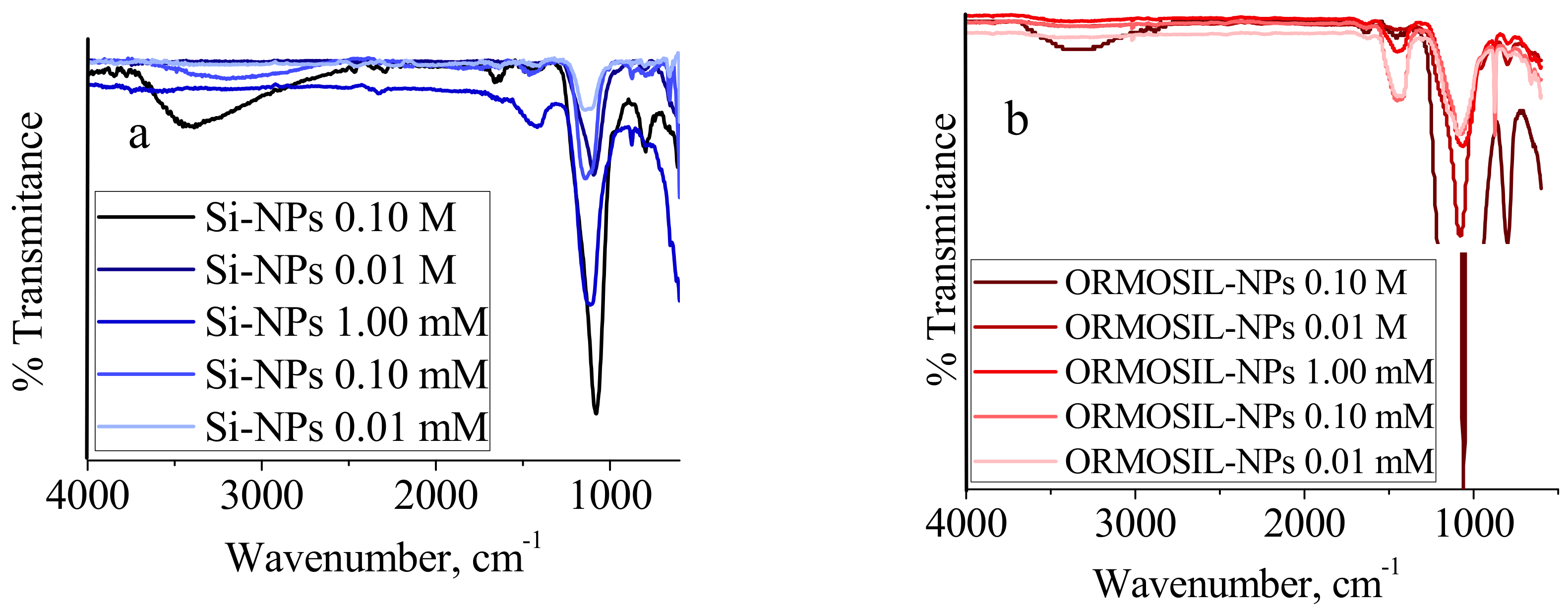
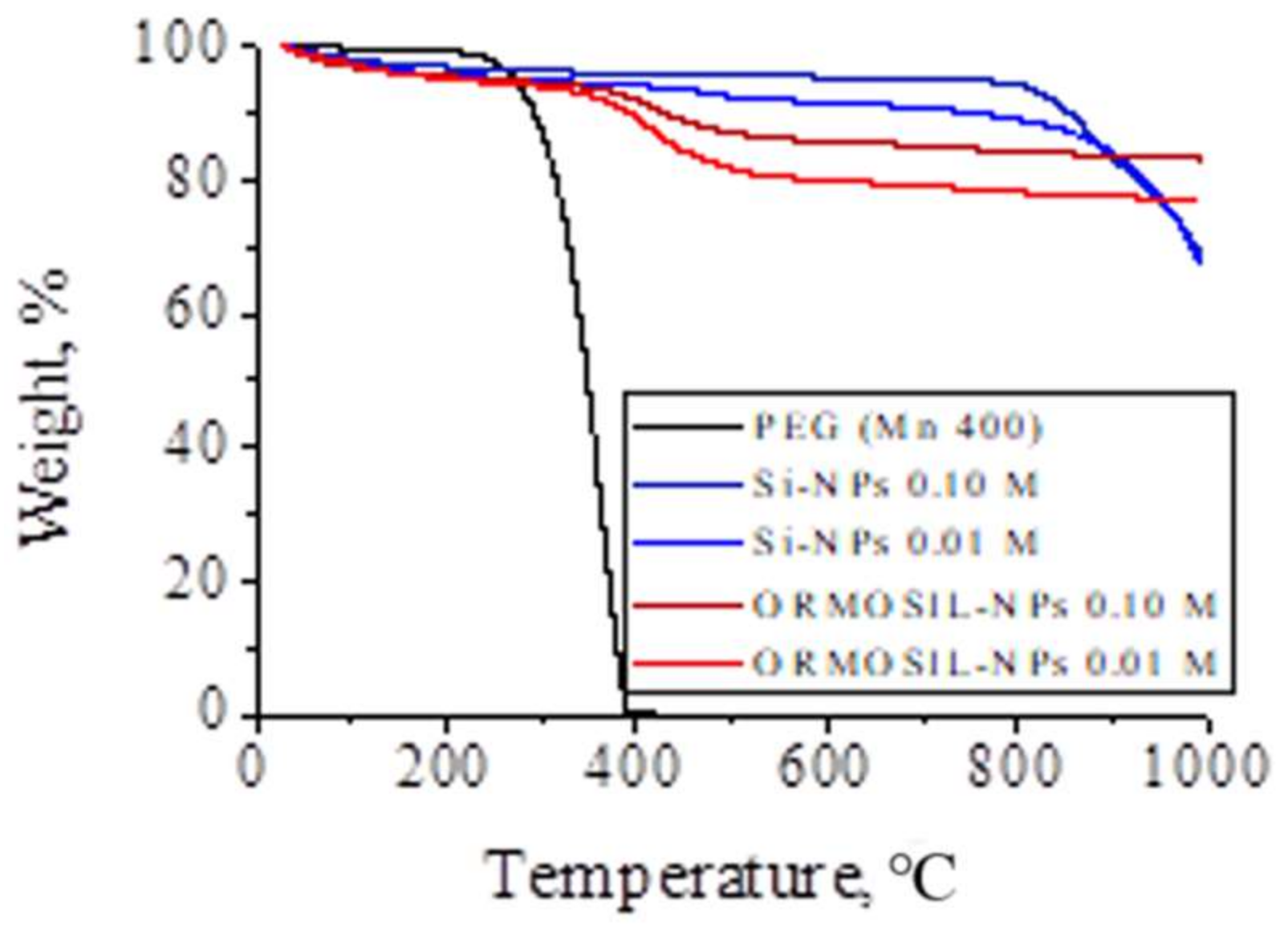
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Diab, R.; Canilho, N.; Pavel, I.A.; Haffner, F.B.; Girardon, M.; Pasc, A. Silica-based systems for oral delivery of drugs, macromolecules and cells. Adv. Colloid Interface Sci. 2017, 249, 346–362. [Google Scholar] [CrossRef] [PubMed]
- Spyrogianni, A.; Herrmann, I.K.; Keevend, K.; Pratsinis, S.E.; Wegner, K. The silanol content and in vitro cytolytic activity of flame-made silica. J. Colloid Interface Sci. 2017, 507, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Pagliaro, M.; Ciriminna, R.; Chi Man, M.W.; Campestrini, S. Better Chemistry through Ceramics: The Physical Bases of the Outstanding Chemistry of ORMOSIL. J. Phys. Chem. 2006, 110, 1976–1988. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.; Raja, S.; Rodrigues, A.S.; Fernandes, F.; Baleizão, C.; Farinha, J.P.S. NIR and visible perylenediimide-silica nanoparticles for laser scanning bioimaging. Dyes Pigments 2014, 110, 227–234. [Google Scholar] [CrossRef]
- Fedorenko, S.V.; Mustafina, A.R.; Mukhametshina, A.R.; Jilkin, M.E.; Mukhametzyanov, T.A.; Solovieva, A.O.; Pozmogova, T.N.; Shestopalova, L.V.; Shestopalov, M.A.; Kholin, K.V.; et al. Cellular imaging by green luminescence of Tb(III)-doped aminomodified silica nanoparticles. Mater. Sci. Eng. C 2017, 76, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Liu, X.; Liu, M.; Huang, L.; Xu, D.; Jiang, R.; Huang, Q.; Wen, Y.; Zhang, X.; Wei, Y. Surface grafting of zwitterionic polymers onto dye doped AIE-active luminescent silica nanoparticles through surface-initiated ATRP for biological imaging applications. Appl. Surf. Sci. 2017, 419, 188–196. [Google Scholar] [CrossRef]
- Zhao, W.; Fang, Y.; Zhu, Q.; Wang, K.; Liu, M.; Huang, X.; Shen, J. A novel glucose biosensor based on phosphonic acid-functionalized silica nanoparticles for sensitive detection of glucose in real samples. Electrochim. Acta 2013, 89, 278–283. [Google Scholar] [CrossRef]
- Wang, K.; Liu, P.; Ye, Y.; Li, J.; Zhao, W.; Huang, X. Fabrication of a novel laccase biosensor based on silica nanoparticles modified with phytic acid for sensitive detection of dopamine. Sens. Actuators B Chem. 2014, 197, 292–299. [Google Scholar] [CrossRef]
- Ohulchanskyy, T.Y.; Roy, I.; Goswami, L.N.; Chen, Y.; Bergey, E.J.; Pandey, R.K.; Oseroff, A.R.; Prasad, P.N. Organically Modified Silica Nanoparticles with Covalently Incorporated Photosensitizer for Photodynamic Therapy of Cancer. Nano Lett. 2007, 7, 2835–2842. [Google Scholar] [CrossRef] [PubMed]
- Ehi-Eromosele, C.O.; Ita, B.I.; Iweala, E.E.J. Silica coated LSMO magnetic nanoparticles for the pH-Responsive delivery of 5-Fluorouracil anticancer drug. Colloids Surf. A Physicochem. Eng. Asp. 2017, 530, 164–171. [Google Scholar] [CrossRef]
- He, Y.; Liang, S.; Long, M.; Xu, H. Mesoporous silica nanoparticles as potential carriers for enhanced drug solubility of paclitaxel. Mater. Sci. Eng. C 2017, 78, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Ingrid, B.; Kamil, Z.; Jarmila, K.; Alla, S.; Hana, A.; Pavel, U.; Pavla, P.; Martin, H.; Petr, Š.; Vladimír, K. Silica-based nanoparticles are efficient delivery system for temoporfin. Photodiagnosis Photodyn. Ther. 2017, 21, 275–284. [Google Scholar] [CrossRef]
- Zielińska, A.; Pereira, I.; Antunes, S.; Veiga, F.J.; Santos, A.C.; Nowak, I.; Silva, A.M.; Souto, E.B. Chapter 10—Mesoporous silica nanoparticles as drug delivery systems against melanoma. In Design of Nanostructures for Theranostics Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 437–466. ISBN 9780128136690. [Google Scholar]
- Hwang, J.; Son, J.; Seo, Y.; Jo, Y.; Lee, K.; Lee, D.; Khan, M.S.; Chavan, S.; Park, C.; Sharma, A.; et al. Functional silica nanoparticles conjugated with beta-glucan to deliver anti-tuberculosis drug molecules. J. Ind. Eng. Chem. 2018, 58, 376–385. [Google Scholar] [CrossRef]
- Ghasemi, S.; Farsangi, Z.J.; Beitollahi, A.; Mirkazemi, M.; Rezayat, S.M.; Sarkar, S. Synthesis of hollow mesoporous silica (HMS) nanoparticles as a candidate for sulfasalazine drug loading. Ceram. Int. 2017, 43, 11225–11232. [Google Scholar] [CrossRef]
- Jia, L.; Li, Z.; Shen, J.; Zheng, D.; Tian, X.; Guo, H.; Chang, P. Multifunctional mesoporous silica nanoparticles mediated co-delivery of paclitaxel and tetrandrine for overcoming multidrug resistance. Int. J. Pharm. 2015, 489, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Hargrove, D.; Lu, X. Improving paclitaxel pharmacokinetics by using tumor-specific mesoporous silica nanoparticles with intraperitoneal delivery. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, Y.; Zhao, Y.; Zhao, Q.; He, B.; Zhang, Q.; Wang, S. Effects of surface modification and size on oral drug delivery of mesoporous silica formulation. J. Colloid Interface Sci. 2018, 513, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liao, L.; Zhu, L.; Zhang, P.; Guo, K.; Kong, J.; Ji, C.; Liu, B. Size-dependent cellular uptake efficiency, mechanism, and cytotoxicity of silica nanoparticles toward HeLa cells. Talanta 2013, 107, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Manganelli, S.; Leone, C.; Toropov, A.A.; Toropova, A.P.; Benfenati, E. QSAR Model for Cytotoxicity of Silica Nanoparticles on Human Embryonic Kidney Cells. Mater. Today Proc. 2016, 3, 847–854. [Google Scholar] [CrossRef]
- Wang, W.; Zeng, C.; Feng, Y.; Zhou, F.; Liao, F.; Liu, Y.; Feng, S.; Wang, X. The size-dependent effects of silica nanoparticles on endothelial cell apoptosis through activating the p53-caspase pathway. Environ. Pollut. 2018, 233, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Liberman, A.; Mendez, N.; Trogler, W.C.; Kummel, A.C. Synthesis and surface functionalization of silica nanoparticles for nanomedicine. Surf. Sci. Rep. 2014, 69, 132–158. [Google Scholar] [CrossRef] [PubMed]
- Kolate, A.; Baradia, D.; Patil, S.; Vhora, I.; Kore, G.; Misra, A. PEG—A versatile conjugating ligand for drugs and drug delivery systems. J. Control. Release 2014, 192, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chapa-González, C.; Piñón-Urbina, A.L.; García-Casillas, P.E. Synthesis of Controlled-Size Silica Nanoparticles from Sodium Metasilicate and the Effect of the Addition of PEG in the Size Distribution. Materials 2018, 11, 510. https://doi.org/10.3390/ma11040510
Chapa-González C, Piñón-Urbina AL, García-Casillas PE. Synthesis of Controlled-Size Silica Nanoparticles from Sodium Metasilicate and the Effect of the Addition of PEG in the Size Distribution. Materials. 2018; 11(4):510. https://doi.org/10.3390/ma11040510
Chicago/Turabian StyleChapa-González, Christian, Ana Laura Piñón-Urbina, and Perla Elvia García-Casillas. 2018. "Synthesis of Controlled-Size Silica Nanoparticles from Sodium Metasilicate and the Effect of the Addition of PEG in the Size Distribution" Materials 11, no. 4: 510. https://doi.org/10.3390/ma11040510





