Universal Effectiveness of Inducing Magnetic Moments in Graphene by Amino-Type sp3-Defects
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation
2.2. Instrumentation
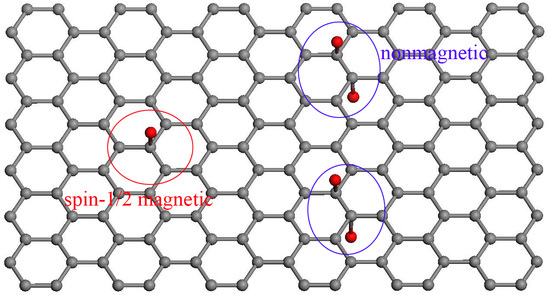
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hollen, S.M.; Gupta, J.A. Painting magnetism on a canvas of graphene. Science 2016, 352, 415–416. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Tang, N.J.; Zheng, Y.P.; Wan, X.G.; Liu, Y.; Liu, F.C.; Xu, Q.H.; Du, Y.W. Robust magnetic moments on the basal plane of the graphene sheet effectively induced by OH groups. Sci. Rep. 2015, 5, 8448. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Wang, X.; Lu, J.; Ni, Z.; Luo, Z.; Mao, H.; Wang, R.; Wang, Y.; Huang, H.; Qi, D.; et al. Room temperature ferromagnetism in partially hydrogenated epitaxial graphene. Appl. Phys. Lett. 2011, 98, 193113. [Google Scholar] [CrossRef]
- Nair, R.R.; Sepioni, M.; Tsai, I.L.; Lehtinen, O.; Keinonen, J.; Krasheninnikov, A.V.; Thomson, T.; Geim, A.K.; Grigorieva, I.V. Spin-half paramagnetism in graphene induced by point defects. Nat. Phys. 2012, 8, 199–202. [Google Scholar] [CrossRef]
- Tang, T.; Liu, F.C.; Liu, Y.; Li, X.Y.; Xu, Q.H.; Feng, Q.; Tang, N.J.; Du, Y.W. Identifying the magnetic properties of graphene oxide. Appl. Phys. Lett. 2014, 104, 123104. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, W.L.; Sun, Y.Y.; Zheng, Y.P.; Tang, N.J.; Du, Y.W. Creation of localized spins in graphene by ring-opening of epoxy derived hydroxyl. Sci. Rep. 2016, 6, 26862. [Google Scholar] [CrossRef] [PubMed]
- Ney, A.; Papakonstantinou, P.; Kumar, A.; Shang, N.-G.; Peng, N. Irradiation enhanced paramagnetism on graphene nanoflakes. Appl. Phys. Lett. 2011, 99, 102504. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.Y.; Zheng, Y.P.; Chen, J.; Zhang, W.L.; Tang, N.J.; Du, Y.W. Intrinsic magnetism of monolayer graphene oxide quantum dots. Appl. Phys. Lett. 2016, 108, 033105. [Google Scholar] [CrossRef]
- Rao, S.S.; Jammalamadaka, S.N.; Stesmans, A.; Moshchalkov, V.V.; van Tol, J.; Kosynkin, D.V.; Higginbotham-Duque, A.; Tour, J.M. Ferromagnetism in graphene nanoribbons: Split versus oxidative unzipped ribbons. Nano Lett. 2012, 12, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.X.; Chshiev, M.; Boukhvalov, D.W.; Waintal, X.; Roche, S. Inducing and optimizing magnetism in graphene nanomeshes. Phys. Rev. B 2011, 84, 214404. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, Q.; Tang, N.J.; Wan, X.G.; Liu, F.C.; Lv, L.Y.; Du, Y.W. Increased magnetization of reduced graphene oxide by nitrogen-doping. Carbon 2013, 60, 549–551. [Google Scholar] [CrossRef]
- Santos, E.J.G.; Ayuela, A.; Sánchez-Portal, D. Universal magnetic properties of sp3-type defects in covalently functionalized graphene. New J. Phys. 2012, 14, 043022. [Google Scholar] [CrossRef]
- Gonzalez-Herrero, H.; Gomez-Rodriguez, J.M.; Mallet, P.; Moaied, M.; Palacios, J.J.; Salgado, C.; Ugeda, M.M.; Veuillen, J.Y.; Yndurain, F.; Brihuega, I. Atomic-scale control of graphene magnetism by using hydrogen atoms. Science 2016, 352, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Khurana, G.; Kumar, N.; Kotnala, R.K.; Nautiyal, T.; Katiyar, R.S. Temperature tuned defect induced magnetism in reduced graphene oxide. Nanoscale 2013, 5, 3346–3351. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.Y.; Waller, G.; Liu, Y.; Liu, M.L.; Wong, C.P. Facile synthesis of nitrogen-doped graphene via pyrolysis of graphene oxide and urea, and its electrocatalytic activity toward the oxygen-reduction reaction. Adv. Energy Mater. 2012, 2, 884–888. [Google Scholar] [CrossRef]
- Samad, Y.A.; Li, Y.Q.; Schiffer, A.; Alhassan, S.M.; Liao, K. Graphene foam developed with a novel two-step technique for low and high strains and pressure-sensing applications. Small 2015, 11, 2380–2385. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.C.; Tang, N.J.; Tang, T.; Liu, Y.; Feng, Q.; Zhong, W.; Du, Y.W. Photochemical doping of graphene oxide with nitrogen for photoluminescence enhancement. Appl. Phys. Lett. 2013, 103, 123108. [Google Scholar] [CrossRef]
- Xu, X.F.; Gao, F.H.; Bai, X.H.; Liu, F.C.; Kong, W.J.; Li, M. Tuning the photoluminescence of graphene quantum dots by photochemical doping with nitrogen. Materials 2017, 10, 1328. [Google Scholar] [CrossRef] [PubMed]
- Parvez, K.; Wu, Z.S.; Li, R.J.; Liu, X.J.; Graf, R.; Feng, X.L.; Mullen, K. Exfoliation of graphite into graphene in aqueous solutions of inorganic salts. J. Am. Chem. Soc. 2014, 136, 6083–6091. [Google Scholar] [CrossRef] [PubMed]
- Matko, V.; Jezernik, K. Greatly improved small inductance measurement using quartz crystal parasitic capacitance compensation. Sensors 2010, 10, 3954–3960. [Google Scholar] [CrossRef] [PubMed]
- Matko, V. Next generation at-cut quartz crystal sensing devices. Sensors 2011, 11, 4474–4482. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Cheng, S.H.; Herding, C.; Zhu, J. Colossal negative magnetoresistance in dilute fluorinated graphene. Phys. Rev. B 2011, 83, 085410. [Google Scholar] [CrossRef]
- Yazyev, O.V. Emergence of magnetism in graphene materials and nanostructures. Rep. Prog. Phys. 2010, 73, 056501. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Nair, R.R.; Ren, W.C.; Jalil, R.; Riaz, I.; Kravets, V.G.; Britnell, L.; Blake, P.; Schedin, F.; Mayorov, A.S.; Yuan, S.J.; et al. Fluorographene: A two-dimensional counterpart of Teflon. Small 2010, 6, 2877–2884. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, P.O.; Foster, A.S.; Ayuela, A.; Krasheninnikov, A.; Nordlund, K.; Nieminen, R.M. Magnetic properties and diffusion of adatoms on a graphene sheet. Phys. Rev. Lett. 2003, 91, 017202. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.Y.; Yuan, J.M. Adsorption of molecular oxygen on doped graphene: Atomic, electronic, and magnetic properties. Phys. Rev. B 2010, 81, 165414. [Google Scholar] [CrossRef]
- Ma, Y.C.; Foster, A.S.; Krasheninnikov, A.V.; Nieminen, R.M. Nitrogen in graphite and carbon nanotubes: Magnetism and mobility. Phys. Rev. B 2005, 72, 205416. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, N.J.; Wan, X.G.; Feng, Q.; Li, M.; Xu, Q.H.; Liu, F.C.; Du, Y.W. Realization of ferromagnetic graphene oxide with high magnetization by doping graphene oxide with nitrogen. Sci. Rep. 2013, 3, 2566. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Tsai, I.L.; Sepioni, M.; Lehtinen, O.; Keinonen, J.; Krasheninnikov, A.V.; Neto, A.H.C.; Katsnelson, M.I.; Geim, A.K.; Grigorieva, I.V. Dual origin of defect magnetism in graphene and its reversible switching by molecular doping. Nat. Commun. 2013, 4, 3010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashtiban, R.J.; Dyson, M.A.; Nair, R.R.; Zan, R.; Wong, S.L.; Ramasse, Q.; Geim, A.K.; Bangert, U.; Sloan, J. Atomically resolved imaging of highly ordered alternating fluorinated raphene. Nat. Commun. 2014, 5, 5902. [Google Scholar]





| Samples (at %) | Pyridinic-N | Pyrrolic-N | Graphite-N | Amino-N | N | O | C |
|---|---|---|---|---|---|---|---|
| EG | - | - | - | - | 0.8 | 12.1 | 87.1 |
| TG | - | - | - | - | 0.7 | 5.5 | 93.8 |
| NG | 1.0 | 1.1 | 0.5 | 0 | 2.6 | 4.6 | 92.8 |
| sp3-NG | 0 | 0 | 0.3 | 2.6 | 2.9 | 8.5 | 88.6 |
| Impurities | Fe | Co | Ni | Cr | Mn | Al |
|---|---|---|---|---|---|---|
| EG | 0.61 | ND | 0.07 | 0.05 | 0.08 | 0.25 |
| TG | 0.32 | ND | 0.03 | 0.04 | 0.09 | 0.16 |
| NG | 0.31 | ND | 0.06 | 0.05 | 0.10 | 0.20 |
| sp3-NG | 0.50 | ND | 0.03 | 0.04 | 0.09 | 0.12 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, T.; Wu, L.; Gao, S.; He, F.; Li, M.; Wen, J.; Li, X.; Liu, F. Universal Effectiveness of Inducing Magnetic Moments in Graphene by Amino-Type sp3-Defects. Materials 2018, 11, 616. https://doi.org/10.3390/ma11040616
Tang T, Wu L, Gao S, He F, Li M, Wen J, Li X, Liu F. Universal Effectiveness of Inducing Magnetic Moments in Graphene by Amino-Type sp3-Defects. Materials. 2018; 11(4):616. https://doi.org/10.3390/ma11040616
Chicago/Turabian StyleTang, Tao, Liting Wu, Shengqing Gao, Fang He, Ming Li, Jianfeng Wen, Xinyu Li, and Fuchi Liu. 2018. "Universal Effectiveness of Inducing Magnetic Moments in Graphene by Amino-Type sp3-Defects" Materials 11, no. 4: 616. https://doi.org/10.3390/ma11040616