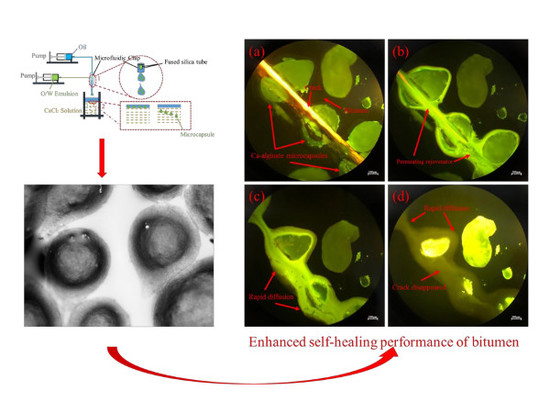
Microfluidic Synthesis of Ca-Alginate Microcapsules for Self-Healing of Bituminous Binder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of O/W Emulsions
2.3. Fabrication of Ca-Alginate Microcapsules by Microfluidic
2.4. Characterization of Ca-Alginate Microcapsules
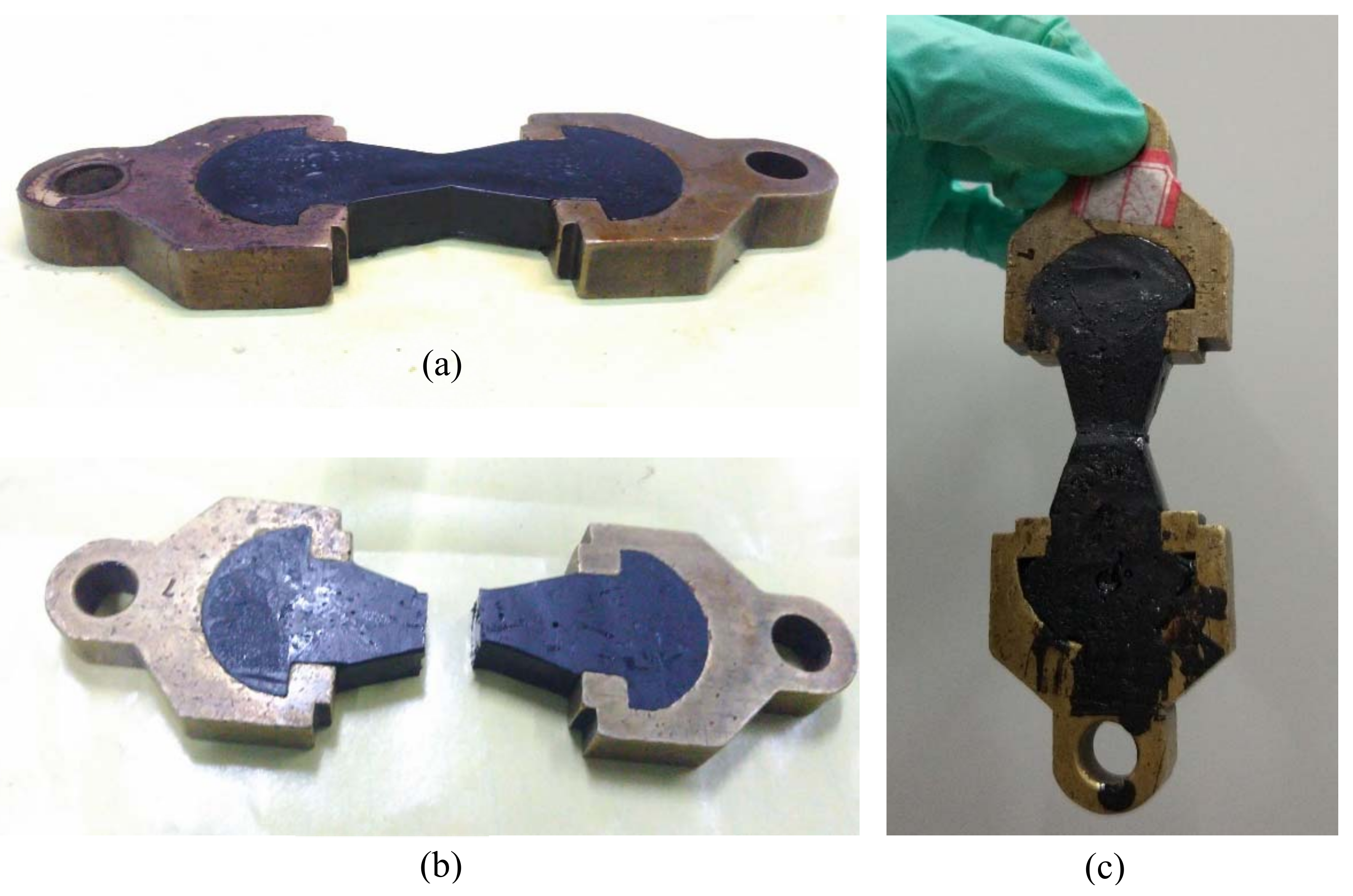
2.5. Self-Healing Evaluation of Bitumen Containing Ca-Alginate Microcapsules
3. Results and Discussion
3.1. Synthesis Mechanism of the Microcapsules
3.2. Effect of Surfactant on the Size of the Microcapsules
3.3. Effect of the Flow Rates of Continuous and Dispersed Phases on Microcapsules’ Size
3.4. Size Predictive Mathematical Model of the Microcapsules
3.5. Self-Healing Property of Bitumen with Multinuclear Ca-Alginate Microcapsules
3.5.1. Micro Self-Healing Process of Bituminous Binder with Microcapsules
3.5.2. Tensile Stress-Recovery Test of Bituminous Binder with Microcapsules
4. Conclusions
- Multinuclear Ca-alginate microcapsules containing rejuvenator were successfully synthesized by a microfluidic droplet device.
- The microcapsules with size ranging from 139 μm to 482 μm has be synthesized. The addition of surfactant Tween80 could effectively improve stability of the emulsion and reduce the size of the microcapsules. The size could be easily controlled by changing the flow rates of continuous phase and dispersed phase.
- The formation mechanism of the emulsion droplets in the microfluidic device was explained by force analysis of the droplets, and the prediction model of the microcapsules size was thus obtained.
- Micro self-healing process of bituminous binder with those microcapsules was monitored and showed the enhanced self-healing performance. Tensile stress-recovery test revealed that the recovery rate increased by 32.08% (in the case of 5% microcapsules), which meant that the multinuclear Ca-alginate microcapsules containing rejuvenator we fabricated could effectively enhance the self-healing property of bituminous binder.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2002, 409, 794–797. [Google Scholar]
- Blaiszik, B.J.; Caruso, M.M.; Mcilroy, D.A.; Moore, J.S.; White, S.R.; Sottos, N.R. Microcapsules filled with reactive solutions for self-healing materials. Polymer 2009, 50, 990–997. [Google Scholar]
- Suryanarayana, C.; Rao, K.C.; Kumar, D. Preparation and characterization of microcapsules containing linseed oil and its use in self-healing coatings. Prog. Org. Coat. 2008, 63, 72–78. [Google Scholar]
- Wei, H.; Wang, Y.; Guo, J.; Shen, N.; Jiang, D.; Zhang, X.; Yan, X.; Zhu, J.; Wang, Q.; Shao, L. Advanced micro/nanocapsules for self-healing smart anticorrosion coatings. J. Mater. Chem. A 2014, 3, 469–480. [Google Scholar]
- Amendola, V.; Meneghetti, M. Self-healing at the nanoscale. Nanoscale 2009, 1, 74–88. [Google Scholar] [PubMed]
- Sun, D.; An, J.; Wu, G.; Yang, J. Double-layered reactive microcapsules with excellent thermal and non-polar solvent resistance for self-healing coatings. J. Mater. Chem. A 2015, 3, 4435–4444. [Google Scholar]
- Xu, G.; Wang, H. Molecular dynamics study of oxidative aging effect on asphalt binder properties. Fuel 2017, 188, 1–10. [Google Scholar]
- Petersen, J.C.; Harnsberger, P.M. Asphalt aging: Dual oxidation mechanism and its interrelationships with asphalt composition and oxidative age hardening. Transp. Res. Rec. J. Transp. Res. Board 1998, 1638, 47–55. [Google Scholar]
- Das, P.K.; Balieu, R.; Kringos, N.; Birgisson, B. On the oxidative ageing mechanism and its effect on asphalt mixtures morphology. Mater. Struct. 2014, 48, 1–15. [Google Scholar]
- Li, R.; Wang, C.; Wang, P.; Pei, J. Preparation of a novel flow improver and its viscosity-reducing effect on bitumen. Fuel 2016, 181, 935–941. [Google Scholar]
- Wang, Y.; Su, J.; Schlangen, E.; Han, N.; Han, S.; Li, W. Fabrication and characterization of self-healing microcapsules containing bituminous rejuvenator by a nano-inorganic/organic hybrid method. Constr. Build. Mater. 2016, 121, 471–482. [Google Scholar]
- Sun, D.; Pang, Q.; Zhu, X.; Tian, Y.; Lu, T.; Yang, Y. Enhanced self-healing process of sustainable asphalt materials containing microcapsules. ACS Sustain. Chem. Eng. 2017, 5, 9881–9893. [Google Scholar]
- Su, J.F.; Schlangen, E. Synthesis and physicochemical properties of high compact microcapsules containing rejuvenator applied in asphalt. Chem. Eng. J. 2012, 198, 289–300. [Google Scholar]
- Aguirre, M.A.; Hassan, M.M.; Shirzad, S.; Daly, W.H.; Mohammad, L.N. Micro-encapsulation of asphalt rejuvenators using melamine-formaldehyde. Constr. Build. Mater. 2016, 114, 29–39. [Google Scholar]
- Shirzad, S.; Hassan, M.M.; Aguirre, M.A.; Mohammad, L.N.; Daly, W.H. Evaluation of sunflower oil as a rejuvenator and its microencapsulation as a healing agent. J. Mater. Civ. Eng. 2016, 28, 4016116. [Google Scholar]
- Micaelo, R.; Al-Mansoori, T.; Garcia, A. Study of the mechanical properties and self-healing ability of asphalt mixture containing calcium-alginate capsules. Constr. Build. Mater. 2016, 123, 734–744. [Google Scholar]
- Al-Mansoori, T.; Micaelo, R.; Artamendi, I.; Norambuena-Contreras, J.; Garcia, A.; Al-Mansoori, T.; Micaelo, R.; Artamendi, I.; Norambuena-Contreras, J.; Garcia, A. Microcapsules for self-healing of asphalt mixture without compromising mechanical performance. Constr. Build. Mater. 2017, 155, 1091–1100. [Google Scholar]
- Zhang, M.; Xing, F.; Shi, K.Y.; Du, X.X. Study on organic microcapsule based self-healing cementitious composite. Adv. Mater. Res. 2011, 239, 764–767. [Google Scholar]
- Li, R.; Zhou, T.; Pei, J. Design, preparation and properties of microcapsules containing rejuvenator for asphalt. Constr. Build. Mater. 2015, 99, 143–149. [Google Scholar]
- Ren, P.W.; Ju, X.J.; Xie, R.; Chu, L.Y. Monodisperse alginate microcapsules with oil core generated from a microfluidic device. J. Colloid Interface Sci. 2010, 343, 392–395. [Google Scholar] [PubMed]
- Yeh, K.W.; Chang, C.P.; Yamamoto, T.; Dobashi, T. Release model of alginate microcapsules containing volatile tea-tree oil. Colloids Surf. A Physicochem. Eng. Asp. 2011, 380, 152–155. [Google Scholar]
- Romanowsky, M.B.; Abate, A.R.; Rotem, A.; Holtze, C.; Weitz, D.A. High throughput production of single core double emulsions in a parallelized microfluidic device. Lab Chip 2012, 12, 802–807. [Google Scholar] [PubMed]
- Noppakundilograt, S.; Piboon, P.; Graisuwan, W.; Nuisin, R.; Kiatkamjornwong, S. Encapsulated eucalyptus oil in ionically cross-linked alginate microcapsules and its controlled release. Carbohydr. Polym. 2015, 131, 23–33. [Google Scholar] [PubMed]
- Chew, S.C.; Tan, C.P.; Long, K.; Nyam, K.L. In-vitro evaluation of kenaf seed oil in chitosan coated-high methoxyl pectin-alginate microcapsules. Ind. Crop. Prod. 2015, 76, 230–236. [Google Scholar]
- Schmit, A.; Courbin, L.; Marquis, M.; Renard, D.; Panizza, P. A pendant drop method for the production of calibrated double emulsions and emulsion gels. RSC Adv. 2014, 4, 28504–28510. [Google Scholar]
- Marquis, M.; Alix, V.; Capron, I.; Cuenot, S.; Zykwinska, A. Microfluidic encapsulation of pickering oil microdroplets into alginate microgels for lipophilic compound delivery. ACS Biomater. Sci. Eng. 2016, 2, 535–543. [Google Scholar]
- Martins, E.; Renard, D.; Davy, J.; Marquis, M.; Poncelet, D. Oil core microcapsules by inverse gelation technique. J. Microencapsul. 2015, 32, 86–95. [Google Scholar] [PubMed]
- Martins, E.; Poncelet, D.; Rodrigues, R.C.; Renard, D. Oil encapsulation in core-shell alginate capsules by inverse gelation II: Comparison between dripping techniques using W/O or O/W emulsions. J. Microencapsul. 2017, 34, 522–534. [Google Scholar] [PubMed]
- Abang, S.; Chan, E.S.; Poncelet, D. Effects of process variables on the encapsulation of oil in ca-alginate capsules using an inverse gelation technique. J. Microencapsul. 2012, 29, 417–428. [Google Scholar] [PubMed]
- Celli, G.B.; Teixeira, A.G.; Duke, T.G.; Brooks, M.S.L. Encapsulationof lycopene from watermelon in calcium-alginate microparticles using an optimised inverse-gelation method by response surface methodology. Int. J. Food Sci. Technol. 2016, 51, 1523–1529. [Google Scholar]
- Vandenberg, G.W.; De, L.N.J. Evaluation of protein release from chitosan-alginate microcapsules produced using external or internal gelation. J. Microencapsul. 2008, 18, 433–441. [Google Scholar]
- Li, X.; Wu, Z.; He, Y.; Ye, B.C.; Wang, J. Preparation and characterization of monodisperse microcapsules with alginate and bentonite via external gelation technique encapsulating Pseudomonas putida Rs-198. J. Biomater. Sci. Polym. Ed. 2017, 28, 1556–1571. [Google Scholar] [PubMed]
- Standard Test Methods of Bitumen and Bituminous Mixtures for Highway Engineering: JTG E20-2011; China Highway & Transportation Standards: Beijing, China, 2011.
- Mirchi, V.; Saraji, S.; Goual, L.; Piri, M. Dynamic interfacial tension and wettability of shale in the presence of surfactants at reservoir conditions. Fuel 2015, 148, 127–138. [Google Scholar]
- Nowak, E.; Xie, Z.; Kovalchuk, N.M.; Matar, O.K.; Mjh, S. Bulk advection and interfacial flows in the binary coalescence of surfactant-laden and surfactant-free drops. Soft Matter 2017, 13, 4616–4628. [Google Scholar] [PubMed]
- Herdes, C.; Santiso, E.E.; James, C.; Eastoe, J.; Müller, E.A. Modelling the interfacial behaviour of dilute light-switching surfactant solutions. J. Colloid Interface Sci. 2015, 445, 16–23. [Google Scholar] [PubMed]
- Neeson, M.J.; Chan, D.Y.; Tabor, R.F. Compound pendant drop tensiometry for surface tension measurement at zero Bond number. Langmuir ACS J. Surf. Colloids 2014, 30, 15388–15391. [Google Scholar]
- Lee, H.J.; Kim, Y.R. Viscoelastic continuum damage model of asphalt concrete with healing. J. Eng. Mech. 1998, 124, 1224–1232. [Google Scholar]
- Bonnaure, F.P. Laboratory investigation of the influence of rest periods on the fatigue characteristics of bituminous mixes. In Proceedings of the Asphalt Paving Technology, Kansas City, MO, USA; 1982. Available online: https://trid.trb.org/view/725881 (accessed on 19 April 2018).
- García, Á.; Schlangen, E.; van de Ven, M.; Sierra-Beltrán, G. Preparation of capsules containing rejuvenators for their use in asphalt concrete. J. Hazard. Mater. 2010, 184, 603–611. [Google Scholar] [PubMed]










| Bitumen | Penetration/0.1 mm (20 °C) | Softening Point/°C | Ductility (15 °C) |
|---|---|---|---|
| 70A | 68.7 | 48.5 | >100 cm |
| Apparent Viscosity/Pa s (60 °C) | Flash Point/°C | Saturates/% | Aromatics/% | Density/g·cm−3 (15 °C) |
|---|---|---|---|---|
| 0.285 | 240 | 21.07 | 67.4 | 0.935 |
| /mL·h−1 | /mL·h−1 | |||
|---|---|---|---|---|
| 3 | 5 | 7 | 9 | |
| 3 | 385 ± 4 μm | 221 ± 11 μm | 172 ± 5 μm | 139 ± 8 μm |
| 5 | 421 ± 12 μm | 272 ± 8 μm | 203 ± 3 μm | 159 ± 8 μm |
| 7 | 460 ± 8 μm | 290 ± 5 μm | 218 ± 9 μm | 161 ± 13 μm |
| 9 | 482 ± 16 μm | 311 ± 3 μm | 232 ± 11 μm | 169 ± 5 μm |
| Specimen | Tensile Stress (N) | Tensile Stress after Recovery (N) | Strength Recovery Rate (%) |
|---|---|---|---|
| 0 | 70.23 | 33.58 | 47.81 |
| 1% | 68.85 | 37.96 | 55.13 |
| 3% | 65.93 | 44.68 | 68.81 |
| 5% | 61.48 | 49.12 | 79.89 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, B.; Wu, S.; Dong, L.; Wang, Q.; Liu, Q. Microfluidic Synthesis of Ca-Alginate Microcapsules for Self-Healing of Bituminous Binder. Materials 2018, 11, 630. https://doi.org/10.3390/ma11040630
Shu B, Wu S, Dong L, Wang Q, Liu Q. Microfluidic Synthesis of Ca-Alginate Microcapsules for Self-Healing of Bituminous Binder. Materials. 2018; 11(4):630. https://doi.org/10.3390/ma11040630
Chicago/Turabian StyleShu, Benan, Shaopeng Wu, Lijie Dong, Qing Wang, and Quantao Liu. 2018. "Microfluidic Synthesis of Ca-Alginate Microcapsules for Self-Healing of Bituminous Binder" Materials 11, no. 4: 630. https://doi.org/10.3390/ma11040630