Mapping the Galvanic Corrosion of Three Coupled Metal Alloys Using Coupled Multielectrode Array: Influence of Chloride Ion Concentration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrochemical Setup
2.2. Coupled Multielectrode Arrays
2.3. Experimental Conditions
3. Results and Discussion
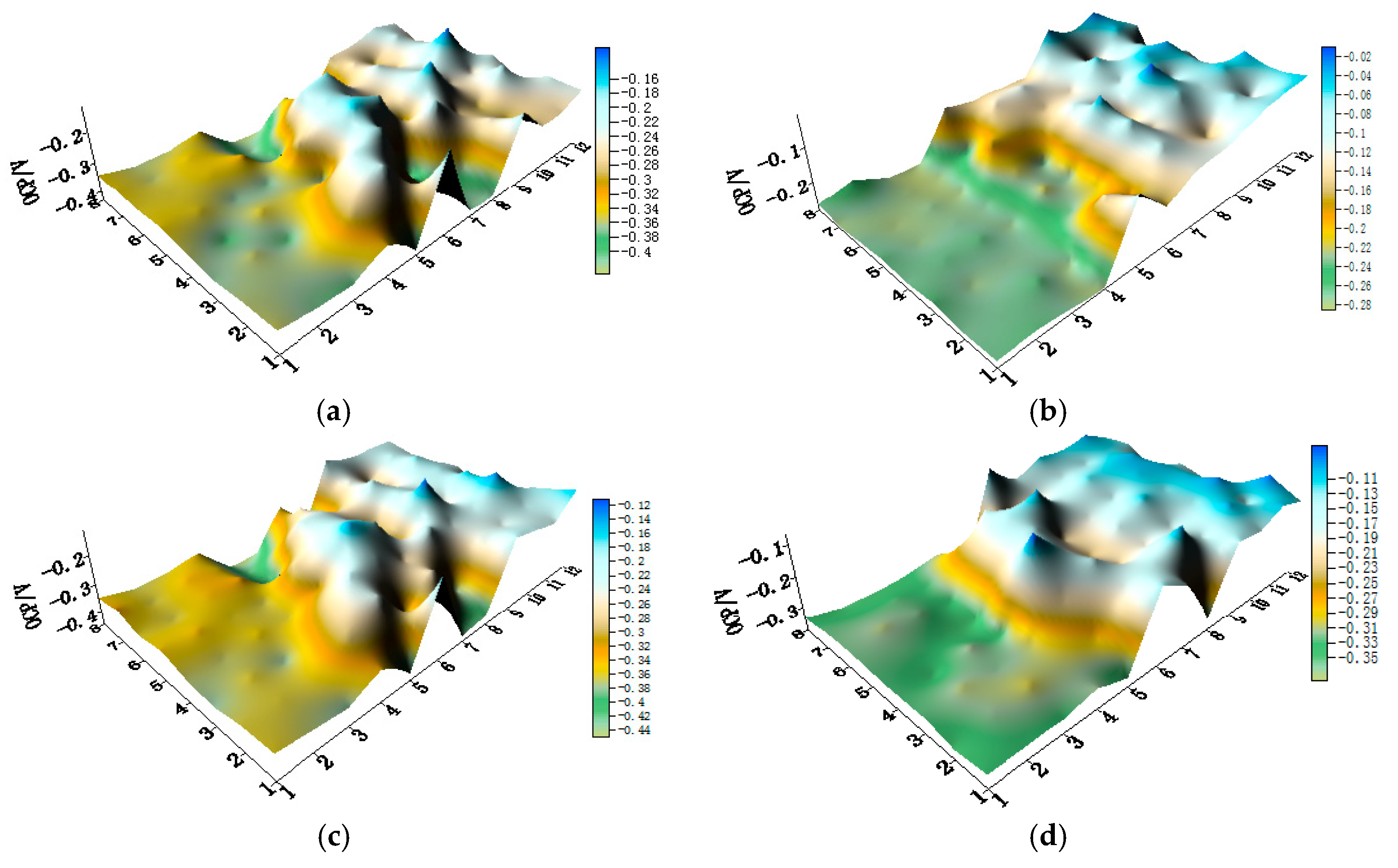
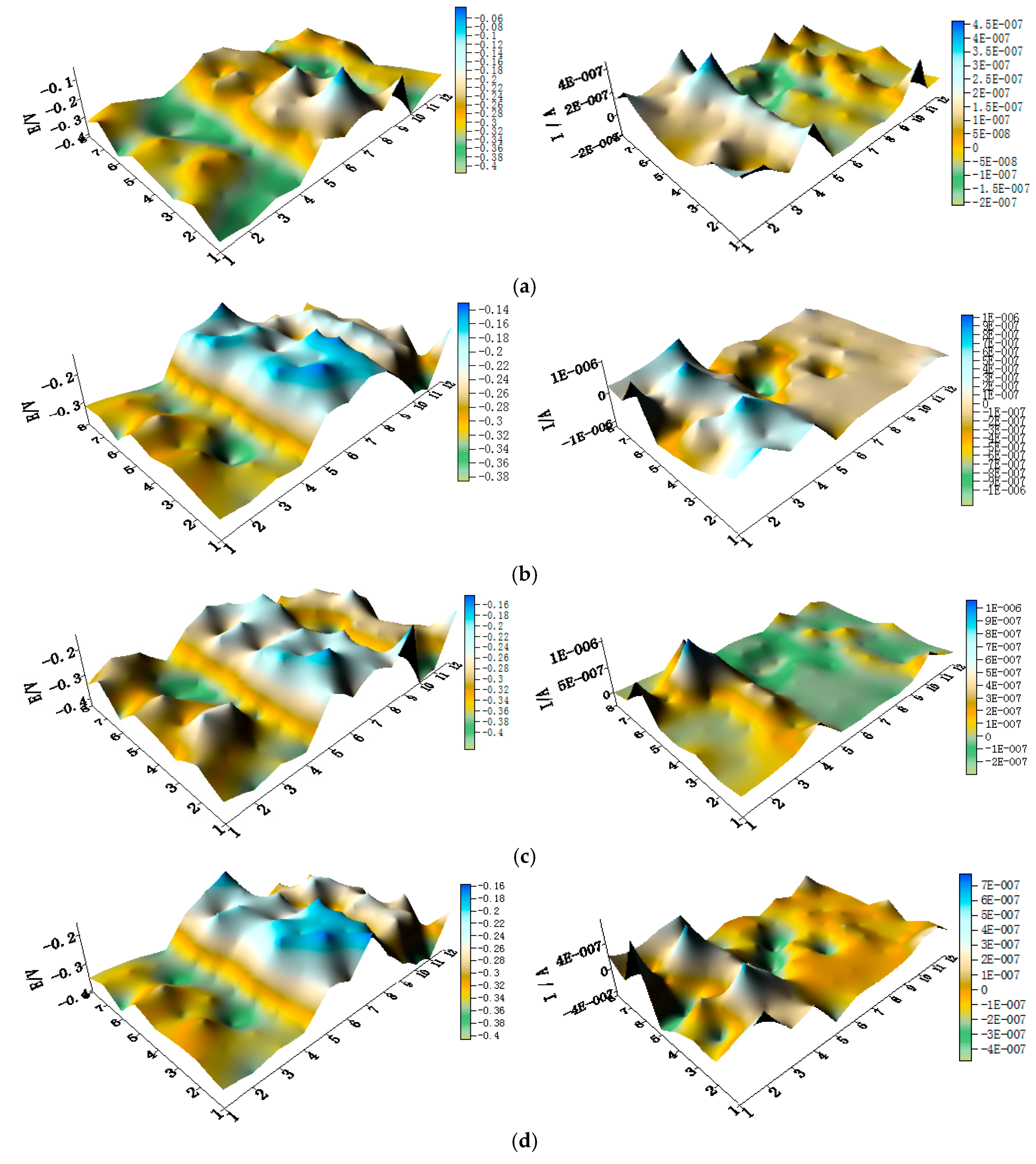
3.1. Corrosion Behavior of CMEA1 in Artificial Seawater with Different Concentrations of Chloride Ions
3.2. Corrosion Behavior of CMEA2 in Artificial Seawater with Different Concentrations of Chloride Ions
3.3. Corrosion Behavior of CMEA3 in Artificial Seawater with Different Concentrations of Chloride Ions
4. Conclusions
- In the three-alloy coupled system, the aluminum-brass (HAl77-2) wires are anodic compared to the titanium alloy (TA2) and 316L stainless steel (316L SS). TA2 acts as a primary cathode, and 316L SS acts as a secondary cathode accompanying the electrochemical inhomogeneity.
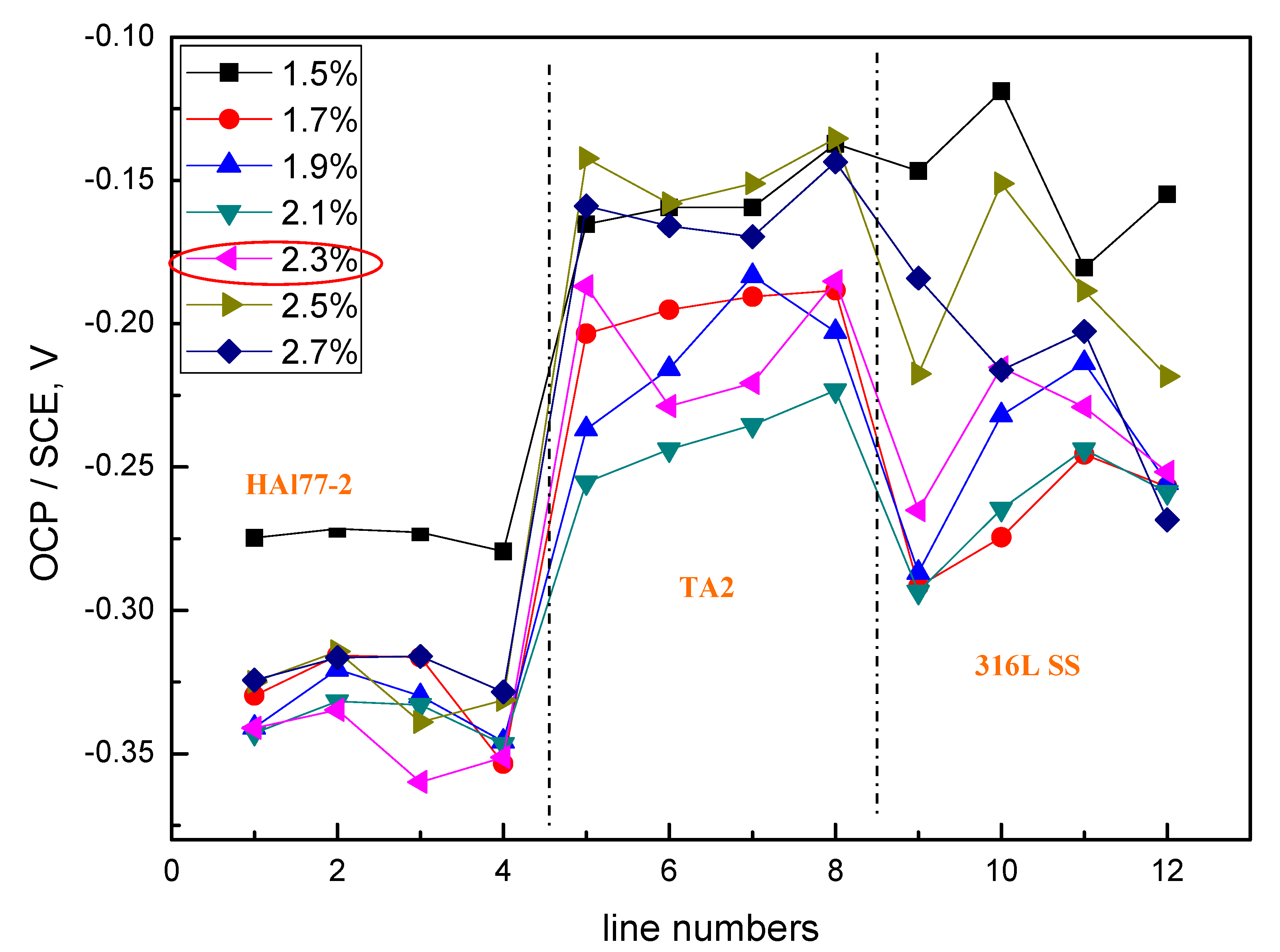
- Regardless of the relative positions of the three materials in the coupled systems, the corrosion of the three-material coupled systems always depends on the chloride concentrations in the artificial seawater—and the corrosion process is highest when the concentration of chloride is 2.3 wt %. When the mass concentration of chloride reaches 2.3%, the local currents of HAl77-2, 316L SS, and TA2 become extreme, and the corrosion of the anodic material is the most serious.
- The geometrical arrangement impacts the corrosion of the three-material coupled system. In the coupling CMEA1 system, when the anodic HAl77-2 wires are located at the edge and are connected to the 316L SS wires, the current value of the HAl77-2 wires decreases from the first-line wires to the fourth-line wires, and the fifth-line wires (316L SS) display anodic potential and current. As a result, the main anodic area enlarges and is even adjacent to the 316L SS wires near the HAl77-2. In the CMEA2 system, fourth-line (HAl77-2) higher current values are obtained when the anodic HAl77-2 wires are also located on the edge and are directly connected with cathodic TA2—this indicates local anodic corrosion. Furthermore, the CMEA3 system has the most severe corrosion when the anodic material HAl77-2 wires are located between the other two materials (relative to the other two geometrical arrangements).
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sharon, H.; Reddy, K.S. A review of solar energy driven desalination technologies. Renew. Sustain. Energy Rev. 2015, 41, 1080–1118. [Google Scholar] [CrossRef]
- Miller, S.; Shemer, H.; Semiat, R. Energy and environmental issues in desalination. Desalination 2015, 366, 2–8. [Google Scholar] [CrossRef]
- Ali, M.T.; Fath, H.E.S.; Armstrong, P.R. A comprehensive techno-economical review of indirect solar desalination. Renew. Sustain. Energy Rev. 2011, 15, 4187–4199. [Google Scholar] [CrossRef]
- Pearce, M.; Brennan, F. Novel findings in desalination. Desalination 2015, 360, 13–18. [Google Scholar] [CrossRef]
- Yan, M.; Sun, C.; Dong, J.; Xu, J.; Ke, W. Electrochemical investigation on steel corrosion in iron-rich clay. Corros. Sci. 2015, 97, 62–73. [Google Scholar] [CrossRef]
- Kuznetsov, V.; Maljusch, A.; Souto, R.M.; Bandarenka, A.S.; Schuhmann, W. Characterisation of localised corrosion processes using scanning electrochemical impedance microscopy. Electrochem. Commun. 2014, 44, 38–41. [Google Scholar] [CrossRef]
- Frankenthal, R.P.; Pickering, H.W. On the Mechanism of Localized Corrosion of Iron and Stainless Steel. J. Electrochem. Soc. 1972, 119, 1304–1310. [Google Scholar] [CrossRef]
- Wexler, S.B.D.; Galvele, J.R. Anodic Behavior of Aluminum Straining and a Mechanism for Pitting. J. Electrochem. Soc. 1974, 121, 1271–1276. [Google Scholar] [CrossRef]
- Astarita, A.; Curioni, M.; Squillace, A.; Bellucci, F.; Thompson, G.E.; Beamish, K.A. Corrosion behaviour of stainless steel–titanium alloy linear friction welded joints: Galvanic coupling. Mater. Corros. 2015, 66, 111–117. [Google Scholar] [CrossRef]
- Sridhar, N.; Yang, L. Coupled Multielectrode Array Systems and Sensors for Real-Time Corrosion Monitoring—A Review. In Proceedings of the NACE International Corrosion Conference Series Corrosion, San Diego, CA, USA, 12–16 March 2006; Paper n. 06681. pp. 1–46. [Google Scholar]
- Tan, Y.J. Wire beam electrode: A new tool for studying localised corrosion and other heterogeneous electrochemical processes. Corros. Sci. 1998, 41, 229–247. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Wang, J. A novel device for the wire beam electrode method and its application in the ennoblement study. Corros. Sci. 2009, 51, 1475–1479. [Google Scholar] [CrossRef]
- Wang, Y.H.; Liu, Y.Y.; Wang, W.; Zhong, L.; Wang, J. Influences of the three-phase boundary on the electrochemical corrosion characteristics of carbon steel under droplets. Mater. Corros. 2013, 64, 309–313. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Liu, Y.; Zhong, L.; Wang, J. Study of localized corrosion of 304 stainless steel under chloride solution droplets using the wire beam electrode. Corros. Sci. 2011, 53, 2963–2968. [Google Scholar] [CrossRef]
- Muster, T.H.; Bradbury, A.; Trinchi, A.; Cole, I.S.; Markley, T.; Lau, D.; Dligatch, S.; Bendavid, A.; Martin, P. The atmospheric corrosion of zinc: The effects of salt concentration, droplet size and droplet shape. Electrochim. Acta 2011, 56, 1866–1873. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Wang, J. The influence of local glucose oxidase activity on the potential/current distribution on stainless steel: A study by the wire beam electrode method. Electrochim. Acta 2009, 54, 5598–5604. [Google Scholar] [CrossRef]
- Tan, Y. Experimental methods designed for measuring corrosion in highly resistive and inhomogeneous media. Corros. Sci. 2011, 53, 1145–1155. [Google Scholar] [CrossRef]
- Zhang, D.L.; Wang, W.; Li, Y. An electrode array study of electrochemical inhomogeneity of zinc in zinc/steel couple during galvanic corrosion. Corros. Sci. 2010, 52, 1277–1284. [Google Scholar] [CrossRef]
- Tan, Y.J.; Aung, N.N.; Liu, T. Novel corrosion experiments using the wire beam electrode. (I) Studying electrochemical noise signatures from localised corrosion processes. Corros. Sci. 2006, 48, 23–38. [Google Scholar] [CrossRef]
- Souto, R.M.; González-García, Y.; Bastos, A.C.; Simões, A.M. Investigating corrosion processes in the micrometric range: A SVET study of the galvanic corrosion of zinc coupled with iron. Corros. Sci. 2007, 49, 4568–4580. [Google Scholar] [CrossRef]
- Thébault, F.; Vuillemin, B.; Oltra, R.; Ogle, K.; Allely, C. Investigation of self-healing mechanism on galvanized steels cut edges by coupling SVET and numerical modeling. Electrochim. Acta 2008, 53, 5226–5234. [Google Scholar] [CrossRef]
- Ogle, K.; Morel, S.; Jacquet, D. Observation of Self-Healing Functions on the Cut Edge of Galvanized Steel Using SVET and pH Microscopy. J. Electrochem. Soc. 2006, 153, B1–B5. [Google Scholar] [CrossRef]
- Simões, A.M.; Bastos, A.C.; Ferreira, M.G.; González-García, Y.; González, S.; Souto, R.M. Use of SVET and SECM to study the galvanic corrosion of an iron–zinc cell. Corros. Sci. 2007, 49, 726–739. [Google Scholar] [CrossRef]
- Izquierdo, J.; Nagy, L.; González, S.; Santana, J.J.; Nagy, G.; Souto, R.M. Resolution of the apparent experimental discrepancies observed between SVET and SECM for the characterization of galvanic corrosion reactions. Souto. Electrochem. Commun. 2013, 27, 50–53. [Google Scholar] [CrossRef]
- Izquierdo, J.; Nagy, L.; Varga, Á.; Santana, J.J.; Nagy, G.; Souto, R.M. Spatially resolved measurement of electrochemical activity and pH distributions in corrosion processes by scanning electrochemical microscopy using antimony microelectrode tips. Electrochim Acta 2011, 56, 8846–8850. [Google Scholar] [CrossRef]
- Souto, R.M.; González-García, Y.; Battistel, D.; Daniele, S. On the use of mercury-coated tips in scanning electrochemical microscopy to investigate galvanic corrosion processes involving zinc and iron. Corros. Sci. 2012, 55, 401–406. [Google Scholar] [CrossRef] [Green Version]
- Ogle, K.; Baudu, V.; Garrigues, L.; Philippe, X. Localized Electrochemical Methods Applied to Cut Edge Corrosion. J. Electrochem. Soc. 2000, 147, 3654–3660. [Google Scholar] [CrossRef]
- Blanc, C.; Orazem, M.E.; Pébère, N.; Tribollet, B.; Vivier, V.; Wu, S. The origin of the complex character of the Ohmic impedance. Electrochim. Acta 2010, 55, 6313–6321. [Google Scholar] [CrossRef] [Green Version]
- Lacroix, L.; Blanc, C.; Pébère, N.; Tribollet, B.; Vivier, V. Localized Approach to Galvanic Coupling in an Aluminum–Magnesium System. J. Electrochem. Soc. 2009, 156, C259–C265. [Google Scholar] [CrossRef] [Green Version]
- Jorcin, J.B.; Blanc, C.; Pébère, N.; Tribollet, B.; Vivier, V. Galvanic Coupling between Pure Copper and Pure Aluminum Experimental Approach and Mathematical Model. J. Electrochem. Soc. 2008, 155, C46–C51. [Google Scholar] [CrossRef] [Green Version]
- Mouanga, M.; Puiggali, M.; Tribollet, B.; Vivier, V.; Pébère, N.; Devos, O. Galvanic corrosion between zinc and carbon steel investigated by local electrochemical impedance spectroscopy. Electrochim. Acta 2013, 88, 6–14. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Wu, G.; Cheng, X.; Zhang, Z.K.; Li, M.Y.; Ji, S.D.; Huang, Z. Galvanic corrosion behaviour of carbon fibre reinforced polymer/magnesium alloys coupling. Corros. Sci. 2015, 98, 672–677. [Google Scholar] [CrossRef]
- Du, X.; Yang, Q.; Chen, Y.; Yang, Y.; Zhang, Z.; Nonferr, T. Galvanic corrosion behavior of copper/titanium galvanic couple in artificial seawater. Met. Soc. 2014, 24, 570–581. [Google Scholar] [CrossRef]
- Hasan, B.O.; Petrol, J. Galvanic corrosion of carbon steel-brass couple in chloride containing water and the effect of different parameters. Sci. Eng. 2014, 124, 137–145. [Google Scholar] [CrossRef]
- Idrac, J.; Mankowski, G.; Thompson, G.; Skeldon, P.; Kihn, Y.; Blanc, C. Galvanic corrosion of aluminium–copper model alloys. Electrochim. Acta 2007, 52, 7626–7633. [Google Scholar] [CrossRef] [Green Version]
- Yadav, A.P.; Katayama, H.; Noda, K.; Masuda, H.; Nishikata, A.; Tsuru, T. Surface potential distribution over a zinc/steel galvanic couple corroding under thin layer of electrolyte. Electrochim. Acta 2007, 52, 3121–3129. [Google Scholar] [CrossRef]
- Ju, H.; Yang, Y.; Liu, Y.; Liu, S.; Duan, J.; Li, Y. Mapping the Galvanic Corrosion of Three Metals Coupled with a Wire Beam Electrode: The Influence of Temperature and Relative Geometrical Position. Materials 2018, 11, 357. [Google Scholar] [CrossRef] [PubMed]


















| HAl77-2 | Composition | Cu | C | Zn | |||||||
| wt % | 59.33 | 0.93 | 39.74 | ||||||||
| 316LSS | Composition | C | Si | Mn | S | P | Ni | Mo | Cr | Cu | Fe |
| wt % | 0.02 | 0.33 | 1.5 | 0.02 | 0.029 | 10.1 | 2.1 | 16.7 | 0.4 | 68.80 | |
| TA2 | Composition | C | Si | Ti | |||||||
| wt % | 0.26 | 1.06 | 98.68 |
| Composition (g/L) | NaCl | MgCl2·6H2O | CaCl2 | |
|---|---|---|---|---|
| Cl− (wt %) | ||||
| 1.5 | 19.46 | 8.80 | 0.92 | |
| 1.7 | 21.95 | 9.94 | 1.04 | |
| 1.9 | 24.53 | 11.11 | 1.16 | |
| 2.1 | 27.11 | 12.28 | 1.28 | |
| 2.3 | 29.98 | 13.52 | 1.42 | |
| 2.5 | 32.59 | 14.75 | 1.54 | |
| 2.7 | 35.26 | 16.03 | 1.67 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, H.; Duan, J.; Yang, Y.; Cao, N.; Li, Y. Mapping the Galvanic Corrosion of Three Coupled Metal Alloys Using Coupled Multielectrode Array: Influence of Chloride Ion Concentration. Materials 2018, 11, 634. https://doi.org/10.3390/ma11040634
Ju H, Duan J, Yang Y, Cao N, Li Y. Mapping the Galvanic Corrosion of Three Coupled Metal Alloys Using Coupled Multielectrode Array: Influence of Chloride Ion Concentration. Materials. 2018; 11(4):634. https://doi.org/10.3390/ma11040634
Chicago/Turabian StyleJu, Hong, JinZhuo Duan, Yuanfeng Yang, Ning Cao, and Yan Li. 2018. "Mapping the Galvanic Corrosion of Three Coupled Metal Alloys Using Coupled Multielectrode Array: Influence of Chloride Ion Concentration" Materials 11, no. 4: 634. https://doi.org/10.3390/ma11040634





