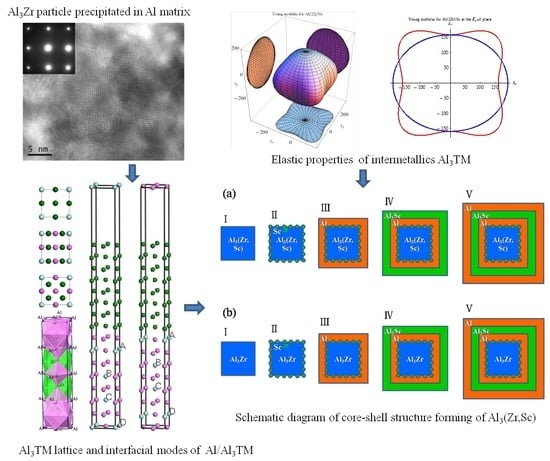
Intermetallic Growth and Interfacial Properties of the Grain Refiners in Al Alloys
Abstract
:1. Introduction
2. Method and Models of Calculation
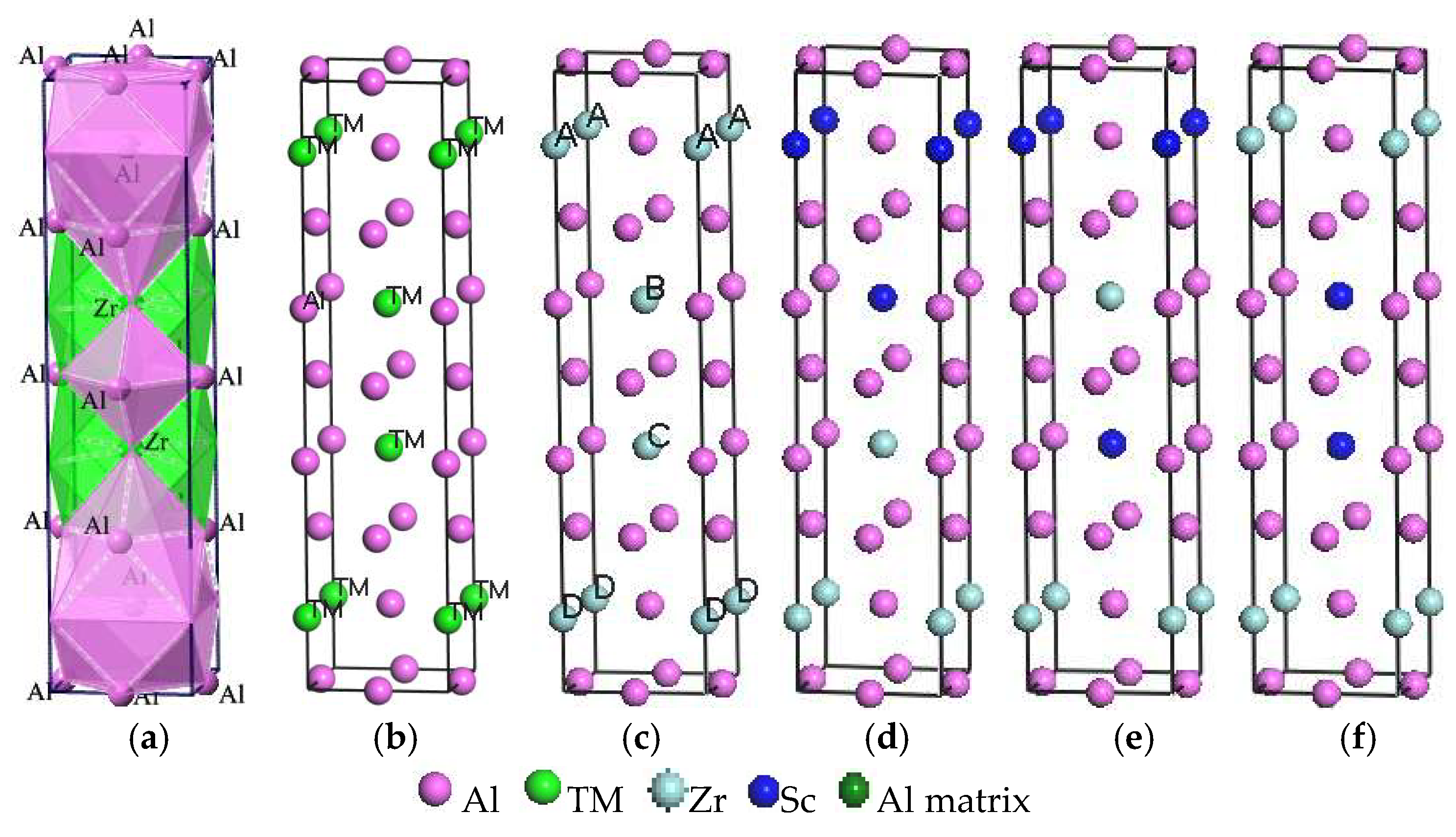
2.1. Models Construction
2.2. Energy Calculation Method
2.3. Elastic Properties Calculation Method
2.4. Griffith Rupture Work and Interfacial Energy Calculation Method
3. Results and Discussion
3.1. Energy
3.2. Elastic Properties
3.3. Griffith Rupture Work and Interfacial Energy of Al(001)/Al3TM(001)
3.4. Sc-Doped Al3(Zr,Sc) Phase and Al/Al3(Zr,Sc) Interface
3.4.1. Energy of Al3(Zr, Sc)
3.4.2. Griffith Rupture Work and Interfacial Energy of Al(001)/Al3(Zr, Sc)(001)
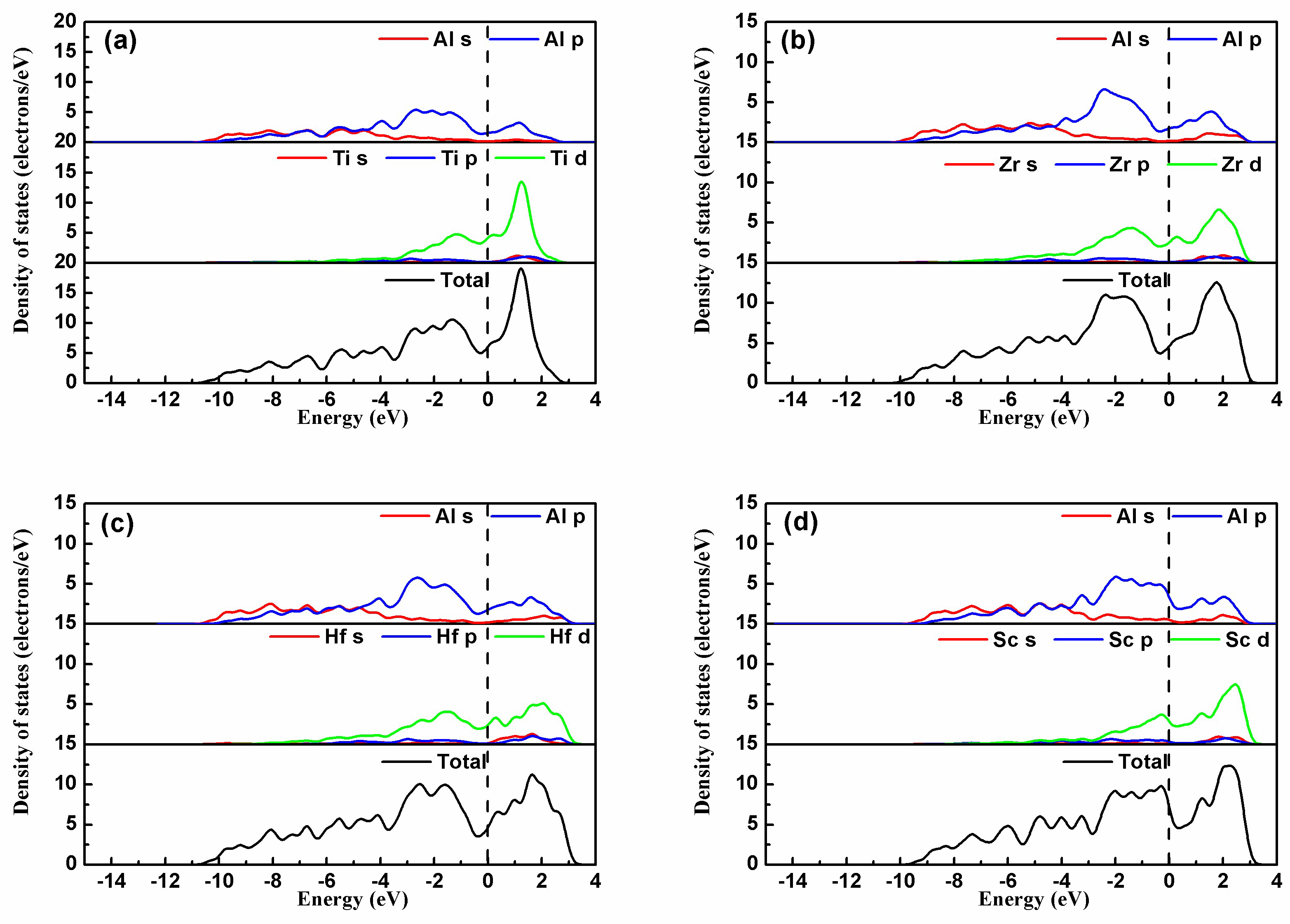
3.5. Electronic Structure
3.5.1. Density of States
3.5.2. Planar-Averaged Difference Charge
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dinaharan, I.; Kumar, G.A.; Vijay, S.J.; Murugan, N. Development of Al3Ti and Al3Zr intermetallic particulate reinforced aluminum alloy AA6061 in situ composites using friction stir processing. Mater. Design 2014, 63, 213–222. [Google Scholar] [CrossRef]
- Zheng, Q.; Yang, C.; Wang, S.; Yu, A.; Chen, H.; He, Y. Effect of compound inoculants Ti and Zr on as cast microstructure and mechanical properties of Al-Cu alloy. Mater. Res. Innov. 2014, 18, 59–63. [Google Scholar] [CrossRef]
- Duan, Y.L.; Xu, G.F.; Tang, L.; Liu, Y.; Xu, J.W.; Deng, Y.; Yin, Z.M. Excellent high strain rate superplasticity of Al-Mg-Sc-Zr alloy sheet produced by an improved asymmetrical rolling process. J. Alloys Compd. 2017, 715, 311–321. [Google Scholar] [CrossRef]
- Deng, Y.; Yin, Z.M.; Pan, Q.L.; Xu, G.F.; Duan, Y.L.; Wang, Y.J. Nano-structure evolution of secondary Al3(Sc1-xZrx) particles during superplastic deformation and their effects on deformation mechanism in Al-Zn-Mg alloys. J. Alloys Compd. 2017, 695, 142–153. [Google Scholar] [CrossRef]
- Sun, F.F.; Nash, G.L.; Li, Q.Y.; Liu, E.Z.; He, C.N.; Shi, C.S.; Zhao, N.Q. Effect of Sc and Zr additions on microstructures and corrosion behavior of Al-Cu-Mg-Sc-Zr alloys. J. Mater. Sci. Technol. 2017, 33, 1015–1022. [Google Scholar] [CrossRef]
- Li, C.M.; Zeng, S.M.; Chen, Z.Q.; Cheng, N.P.; Chen, T.X. First-principles calculations of elastic and thermodynamic properties of the four main intermetallic phases in Al-Zn-Mg-Cu alloys. Comput. Mater. Sci. 2014, 93, 210–220. [Google Scholar] [CrossRef]
- Boulechfar, R.; Meradji, H.; Ghemid, S.; Drablia, S.; Bouhafs, B. First principle calculations of structural, electronic and thermodynamic properties of Al3(TixV1-x) alloy in D0 and L1 structures. Solid. State. Sci. 2013, 16, 1–5. [Google Scholar] [CrossRef]
- Yuan, X.L.; Wei, D.Q.; Cheng, Y.; Ji, G.F.; Zhang, Q.M.; Gong, Z.Z. Pressure effects on elastic and thermodynamic properties of Zr3Al intermetallic compound. Comput. Mater. Sci. 2012, 58, 125–130. [Google Scholar] [CrossRef]
- Ghosh, G.; van de Walle, A.; Asta, M. First-principles calculations of the structural and thermodynamic properties of bcc, fcc and hcp solid solutions in the Al-TM (TM = Ti, Zr and Hf) systems: A comparison of cluster expansion and supercell methods. Acta Mater. 2008, 56, 3202–3221. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.Q.; Luo, X. First-principles study of the Al(001)/Al3Ti(001) interfacial properties. Comput. Mater. Sci. 2012, 62, 136–141. [Google Scholar] [CrossRef]
- Asta, M.; Foiles, S.M.; Quong, A.A. First-principles calculations of bulk and interfacial thermodynamic properties for fcc-based Al-Sc alloys. Phys. Rev. B 1998, 57, 11265–11275. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Zhou, Y.; Chen, G.X. First-principles study of Al/A13Ti heterogeneous nucleation interface. Appl. Surf. Sci. 2014, 307, 593–600. [Google Scholar] [CrossRef]
- Colinet, C.; Pasturel, A. Ab initio calculation of the formation energies of L1, D0, D0 and one dimensional long period structures in TiAl3 compound. Intermetallics 2002, 10, 751–764. [Google Scholar] [CrossRef]
- Amador, C.; Hoyt, J.J.; Chakoumakos, B.C.; Defontaine, D. Theoretical and Experimental-Study of Relaxations in Al3ti and Al3Zr Ordered Phases. Phys. Rev. Lett. 1995, 74, 4955–4958. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Desch, P.B.; Schwarz, R.B. Metastable Phases in the Al3X(X = Ti, Zr, and Hf) Intermetallic System. Scripta. Metall. Mater. 1991, 25, 2513–2516. [Google Scholar] [CrossRef]
- Liu, Z.L.; Qiu, D.; Wang, F.; Taylor, J.A.; Zhang, M.X. The grain refining mechanism of cast zinc through silver inoculation. Acta. Mater. 2014, 79, 315–326. [Google Scholar] [CrossRef]
- Ghosh, G.; Asta, M. First-principles calculation of structural energetics of Al-TM (TM = Ti, Zr, Hf) intermetallics. Acta Mater. 2005, 53, 3225–3252. [Google Scholar] [CrossRef]
- Colinet, C.; Pasturel, A. Phase stability and electronic structure in ZrAl3 compound. J. Alloys. Compd. 2001, 319, 154–161. [Google Scholar] [CrossRef]
- Kematick, R.J.; Franzen, H.F. Thermodynamic Study of the Zirconium Aluminum System. J. Solid State Chem. 1984, 54, 226–234. [Google Scholar] [CrossRef]
- Ma, Y.; Rømming, C.; Lebech, B.; Gjønnes, J.; Taftø, J. Structure refinement of Al3Zr using single-crystal X-ray diffraction, powder neutron diffraction and CBED. Acta Crystallogr. 2010, 48, 11–16. [Google Scholar] [CrossRef]
- Colinet, C.; Pasturel, A. Phase stability and electronic structure of the compound. Phys. Rev. B 2001, 64, 197–201. [Google Scholar] [CrossRef]
- Vanderbilt, D.; Louie, S.G. Total energies of diamond (111) surface reconstructions by a linear combination of atomic orbitals method. Phys. Rev. B 1984, 30, 6118–6130. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6887. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. ERRATA:Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillonin-Zone Integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Fischer, T.H.; Almlof, J. General methods for geometry and wave function optimization. J. Phys. Chem. 1992, 96, 9768–9774. [Google Scholar] [CrossRef]
- Medvedeva, N.I.; Gornostyrev, Y.N.; Novikov, D.L.; Mryasov, O.N.; Freeman, A.J. Ternary site preference energies, size misfits and solid solution hardening in NiAl and FeAl. Acta Mater. 1998, 46, 3433–3442. [Google Scholar] [CrossRef]
- Chunmei, L.I.; Chen, Z.Q.; Zeng, S.M.; Cheng, N.P.; Chen, T.X. Intermetallic phase formation and evolution during homogenization and solution in Al-Zn-Mg-Cu alloys. Sci. China Tech. Sci. 2013, 56, 2827–2838. [Google Scholar]
- Deyirmenjian, V.B.; Heine, V.V.; Payne, M.C.; Milman, V.V.; Lyndenbell, R.M.; Finnis, M.W. Ab initio atomistic simulation of the strength of defective aluminum and tests of empirical force models. Phys. Rev. B 1995, 52, 15191–15207. [Google Scholar] [CrossRef]
- Milman, V.; Warren, M.C. Elastic properties of TiB2 and MgB2. J. Phys. Condens. Matter 2001, 13, 5585–5595. [Google Scholar] [CrossRef]
- Aydin, S.; Simsek, M. First-principles calculations of elemental crystalline boron phases under high pressure: Orthorhombic B28 and tetragonal B48. J. Alloy Compd. 2011, 509, 5219–5229. [Google Scholar] [CrossRef]
- Duan, Y.H.; Sun, Y.; Guo, Z.Z.; Peng, M.J.; Zhu, P.X.; He, J.H. Elastic constants of AlB2-type compounds from first-principles calculations. Comput. Mater. Sci. 2012, 51, 112–116. [Google Scholar] [CrossRef]
- Hashibon, A.; Elsässer, C.; Mishin, Y.; Gumbsch, P. First-principles study of thermodynamical and mechanical stabilities of thin copper film on tantalum. Phys. Rev. B 2007, 76, 4692. [Google Scholar] [CrossRef]
- Cacciamani, G.; Riani, P.; Borzone, G.; Parodi, N.; Saccone, A.; Ferro, R.; Pisch, A.; Schmid-Fetzer, R. Thermodynamic measurements and assessment of the Al–Sc system. Intermetallics 1999, 7, 101–108. [Google Scholar] [CrossRef]
- Fu, C.L. Electronic, elastic, and fracture properties of trialuminide alloys: Al3Sc and Al3Ti. J. Mater. Res. 1990, 5, 971–979. [Google Scholar] [CrossRef]
- Asta, M.; De, F.D.; Van, S.M.; Sluiter, M.; Methfessel, M. First-principles phase-stability study of fcc alloys in the Ti-Al system. Phys. Rev. B 1992, 46, 5055–5072. [Google Scholar] [CrossRef]
- Watson, R.E.; Weinert, M. Transition-metal aluminide formation: Ti, V, Fe, and Ni aluminides. Phys. Rev. B 1998, 58, 5981–5988. [Google Scholar] [CrossRef]
- Nassik, M.; Chrifi-Alaoui, F.Z.; Mahdouk, K.; Gachon, J.C. Calorimetric study of the aluminium–titanium system. J. Alloy Compd. 2003, 350, 151–154. [Google Scholar] [CrossRef]
- Clouet, E.; Sanchez, J.; Sigli, C. First principles study of the solubility of Zr in Al. Phys. Rev. B 2012, 65, 094105. [Google Scholar] [CrossRef]
- Murray, J.; Peruzzi, A.; Abriata, J.P. The Al-Zr (aluminum-zirconium) system. J. Phase Equilib. 1992, 13, 277–291. [Google Scholar] [CrossRef]
- Meschel, S.V.; Kleppa, O.J. Standard enthalpies of formation of 4d aluminides by direct synthesis calorimetry. J. Alloy Compd. 1993, 191, 111–116. [Google Scholar] [CrossRef]
- Meschel, S.V.; Kleppa, O.J. Standard enthalpies of formation of 5d aluminides by high-temperature direct synthesis calorimetry. J. Alloy Compd. 1993, 197, 75–81. [Google Scholar] [CrossRef]
- Balducci, G.; Ciccioli, A.; Gigli, G.; Gozzi, D.; Anselmi-Tamburini, U. Thermodynamic study of intermetallic phases in the Hf-Al system. J. Alloy Compd. 1995, 220, 117–121. [Google Scholar] [CrossRef]
- Pugh, S.F.; XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Philos. Mag. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Boulechfar, R.; Ghemid, S.; Meradji, H.; Bouhafs, B. FP-LAPW investigation of structural, electronic, and thermodynamic properties of Al3V and Al3Ti compounds. Physica B 2010, 405, 4045–4050. [Google Scholar] [CrossRef]
- Wang, J.; Shang, S.L.; Wang, Y. First-principles calculations of binary Al compounds: Enthalpies of formation and elastic properties. Calphad 2011, 35, 562–573. [Google Scholar] [CrossRef]
- Nakamura, M.; Kimura, K. Elastic constants of TiAl3, and ZrAl3, single crystals. J. Mater. Sci. 1991, 26, 2208–2214. [Google Scholar] [CrossRef]
- Mao, Z.; Chen, W.; Seidman, D.N. First-principles study of the nucleation and stability of ordered precipitates in ternary Al–Sc–Li alloys. Acta Mater. 2011, 59, 3012–3023. [Google Scholar] [CrossRef]
- Hasegawa, M.; Yagi, T. Systematic study of formation and crystal structure of 3d-transition metal nitrides synthesized in a supercritical nitrogen fluid under 10 GPa and 1800 K using diamond anvil cell and YAG laser heating. J. Alloys Compd. 2005, 403, 131–142. [Google Scholar] [CrossRef]
- Nye, F. Physical Properties of Crystals; Clarendon Press: Oxford, UK, 1964; pp. 145–146. [Google Scholar]
- Yin, D.Q.; Yang, Y.; Peng, X.H.; Qin, Y.; Wang, Z.C. Tensile and fracture process of the TiN/VN interface from first principles. Ceram. Int. 2014, 40, 14453–14462. [Google Scholar] [CrossRef]
- Ouyang, G.; Tan, X.; Wang, C.X. Charge-induced transition between miscible and immiscible in nanometer-sized alloying particles. Chem. Phys. Lett. 2006, 420, 65–70. [Google Scholar] [CrossRef]
- Li, J.H.; Oberdorfer, B.; Wurster, S.; Schumacher, P. Samenvatting Impurity effects on the nucleation and growth of primary Al3(Sc, Zr) phase in Al alloys. J. Mater. Sci. 2014, 49, 5961–5977. [Google Scholar] [CrossRef]
- Radmilovic, V.; Tolley, A.; Dahmen, U. HREM and HAADF Imaging of Al3(Sc, Zr) Core/Shell Structure. Microsc. Microanal. 2005, 11, 1712–1713. [Google Scholar] [CrossRef]






| Phases | Al | Al3Ti | Al3Zr | Al3Hf | Al3Sc | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cal. [This Work] | Cal. | Exp. | Cal. [This Work] | Cal. | Exp. | Cal. [This Work] | Cal. | Exp. | Cal. [This Work] | Cal. | Exp. | Cal. [This Work] | Cal. | Exp. | |
| Crystal System | Cubic | Cubic | Cubic | Tetragonal | Tetragonal | Tetragonal | Tetragonal | Tetragonal | Tetragonal | Tetragonal | Tetragonal | Tetragonal | Tetragonal | Tetragonal | Tetragonal |
| Space Group | FM-3M | FM-3M | FM-3M | I4/MMM | I4/MMM | I4/MMM | I4/MMM | I4/MMM | I4/MMM | I4/MMM | I4/MMM | I4/MMM | I4/MMM | I4/MMM | I4/MMM |
| a = b(Å) (deviation) | 4.053 | 4.046 [12] | 4.049 [12] | 3.907 (−3.60%) | 3.885 [13] 3.81 [14] | 3.89 [15] | 4.028 (−0.62%) | 3.999 [16] 4.008 [17] 4.02 [18] | 4.007 [19] 3.999 [20] | 4.004 (–1.22%) | 3.990 [17] 3.987 [21] | 4.01 [21] | 4.055 (0.04%) | ||
| c(Å) | 4.053 | 4.046 [12] | 4.049 [12] | 16.714 | 16.823 [13] 16.459 [14] | 16.922 [15] | 17.384 | 17.283 [16] 17.297 [17] 17.36 [18] | 17.286 [19] 17.283 [20] | 17.224 | 17.172 [17] 17.179 [21] | 17.653 [21] | 17.283 | ||
| V(Å3) | 66.59 | 255.18 | 282.10 | 276.43 [16] | 276.09 | 284.16 | |||||||||
| Phases | Al3Ti | Al3Zr | Al3Hf | Al3Sc | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cal. [This Work] | Cal. | Exp. | Cal. [This Work] | Cal. | Exp. | Cal. [This Work] | Cal. | Exp. | Cal. [This Work] | Cal. | Exp. | |
| ΔH (eV/atom) | −0.44 | −0.53 | −0.44 | −0.45 | −0.45 [9] | −0.45 [34] | ||||||
| ΔH (kJ/mol) | −42.39 | −38.90 [16] −41.45 [35] −41.90 [36] −39.51 [37] | −39.2 [38] | −50.65 | −49.11 [16] −51.06 [13] −53.45 [39] | −49 ± 4 [40] −48.4 ± 1.3 [41] | −42.85 | −39.63 [16] −40.00 [20] | −40. 6 ± 0.8 [42] −44.7 ± 2.4 [43] | −43.41 | ||
| Eb (eV/atom) | 4.524 | 5.157 | 4.107 | 4.048 | ||||||||
| Eb (kJ/mol) | 436.27 | 495.85 | 396.07 | 423.80 |
| Phases | Al3Ti | Al3Zr | Al3Hf | Al3Sc | Al12Zr3Sc | ||||
|---|---|---|---|---|---|---|---|---|---|
| This Work | Other Work | This Work | Other Work | This Work | Other Work | This Work | Other Work | This Work | |
| BV | 102.4 | 100.8 | 106.5 | 92.1 | 96.7 | ||||
| BR | 102.2 | 100.6 | 106.0 | 91.8 | 96.5 | ||||
| B | 102.3 | 102 [45] 103 [5] 107 [46] | 100.7 | 105.3 [46] 102.2 [47] | 106.3 | 108.2 [46] | 91.9 | 91.8 [48] | 96.6 |
| GV | 81.8 | 84.6 | 77.8 | 74.1 | 79.2 | ||||
| GR | 81.7 | 83.3 | 77.4 | 71.7 | 77.0 | ||||
| G | 81.8 | 88.5 [46] | 84.0 | 83.2 [46] 85.1 [47] | 77.6 | 80.3 [46] | 72.9 | 71.7 [48] | 78.1 |
| E | 193.8 | 208.5 [46] | 197.2 | 197.6 [46] 201.8 [47] | 187.2 | 193.3 [46] | 173.1 | 184.6 | |
| B/G | 1.25 | 1.22 [48] [46] | 1.20 | 1.26 [46,47] | 1.37 | 1.35 [46] | 1.26 | 1.24 | |
| ν | 0.18 | 0.17 | 0.21 | 0.19 | 0.18 | ||||
| Interfaces | Stacking Site | Al3Ti(001)/Al(001) | Al3Zr(001)/Al(001) | Al3Hf(001)/Al(001) | Al3Sc(001)/Al(001) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Type | D-ter | I-ter | D-ter | I-ter | D-ter | I-ter | D-ter | I-ter | |
| Wad(J/m2) | Top | 2.610 | 2.877 | 2.783 | 2.894 | 2.752 | 2.886 | 2.491 | 2.985 |
| Bridge | - | - | - | - | - | - | - | - | |
| Central | - | 2.450 | - | 2.772 | - | 2.758 | - | 2.740 | |
| σsuf(J/m2) | Al3TM | 2.030 | 1.923 | 1.824 | 1.751 | 1.835 | 1.780 | 1.445 | 1.444 |
| γint(J/m2) | Top | 0.458 | 0.084 | 0.079 | −0.105 | 0.121 | −0.068 | −0.008 | −0.503 |
| Bridge | - | - | - | - | - | - | - | - | |
| Central | - | 0.511 | - | 0.017 | - | 0.06 | - | −0.258 | |
| Phases | ΔH(eV/atom) | ΔH (kJ/mol) | Eb(eV/atom) | Eb (kJ/mol) |
|---|---|---|---|---|
| Al3Zr (Sc1-1) | −0.532 | −51.14 | −4.996 | −480.35 |
| Al3Zr (Sc2-1) | −0.504 | −48.44 | −4.799 | −461.42 |
| Al3Zr (Sc2-2) | −0.509 | −48.98 | −4.804 | −461.96 |
| Al3Zr (Sc2-3) | −0.509 | −48.98 | −4.804 | −461.96 |
| Al3Zr (Sc3-1) | −0.481 | −46.27 | −4.608 | −443.03 |
| Al3Sc | −0.450 | −43.27 | −4.408 | −423.80 |
| Al3Zr | −0.524 | −50.42 | −5.157 | −495.85 |
| Model | Wad (J/m2) | Model | Wad (J/m2) | Model | Wad (J/m2) |
|---|---|---|---|---|---|
| Al/Al3Zr (Sc1-1) (I-ter) | 3.108 | Al/Al3Zr (Sc2-1) (I-ter) | 2.998 | Al/Al3Zr (Sc3-1) (I-ter) | 3.008 |
| Al/Al3Zr (Sc1-2) (I-ter) | 2.996 | Al/Al3Zr (Sc2-2) (I-ter) | 3.000 | Al/Al3Zr (Sc3-2) (I-ter) | 3.014 |
| Al/Al3Zr (Sc1-3) (I-ter) | 3.022 | Al/Al3Zr (Sc2-3) (I-ter) | 3.021 | Al/Al3Zr (Sc3-3) (I-ter) | 3.021 |
| Al/Al3Zr (Sc2-4) (I-ter) | 3.008 | Al/Al3Zr (Sc3-4) (I-ter) | 3.014 | ||
| Al/Al3Zr(Sc1-1) (D-ter) | 2.501 | Al/Al3Zr(Sc2-1) (D-ter) | 2.502 | Al/Al3Zr(Sc3-1) (D-ter) | 2.498 |
| Al/Al3Zr(Sc1-2) (D-ter) | 2.825 | Al/Al3Zr(Sc2-2) (D-ter) | 2.532 | Al/Al3Zr(Sc3-2) (D-ter) | 2.485 |
| Al/Al3Zr(Sc1-3) (D-ter) | 2.829 | Al/Al3Zr(Sc2-3) (D-ter) | 2.886 | Al/Al3Zr(Sc3-3) (D-ter) | 2.503 |
| Al/Al3Zr(Sc2-4) (D-ter) | 2.503 | Al/Al3Zr(Sc3-4) (D-ter) | 2.838 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Cheng, N.; Chen, Z.; Xie, Z.; Hui, L. Intermetallic Growth and Interfacial Properties of the Grain Refiners in Al Alloys. Materials 2018, 11, 636. https://doi.org/10.3390/ma11040636
Li C, Cheng N, Chen Z, Xie Z, Hui L. Intermetallic Growth and Interfacial Properties of the Grain Refiners in Al Alloys. Materials. 2018; 11(4):636. https://doi.org/10.3390/ma11040636
Chicago/Turabian StyleLi, Chunmei, Nanpu Cheng, Zhiqian Chen, Zhongjing Xie, and Liangliang Hui. 2018. "Intermetallic Growth and Interfacial Properties of the Grain Refiners in Al Alloys" Materials 11, no. 4: 636. https://doi.org/10.3390/ma11040636