Ultralight Graphene/Carbon Nanotubes Aerogels with Compressibility and Oil Absorption Properties
Abstract
:1. Introduction
2. Materials and Methods
3. Results
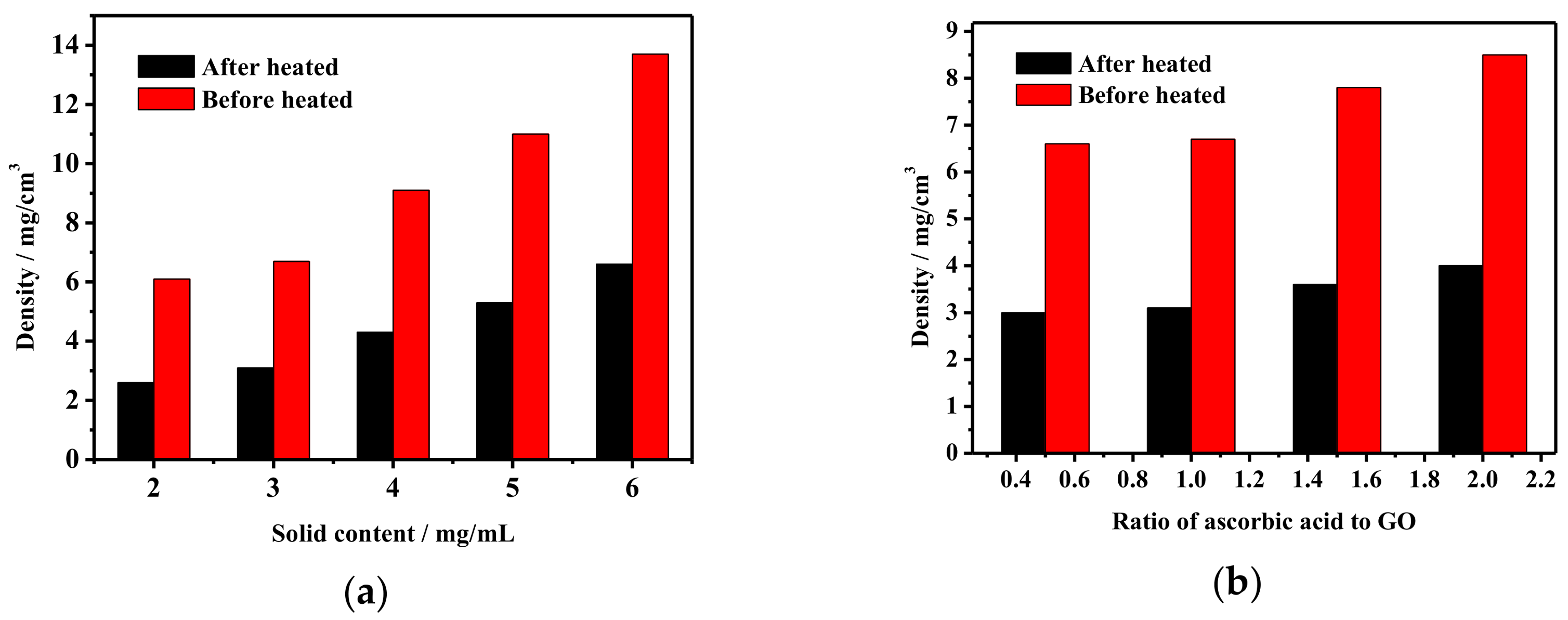
3.1. The Density of Grapheme/CNTs Aerogels
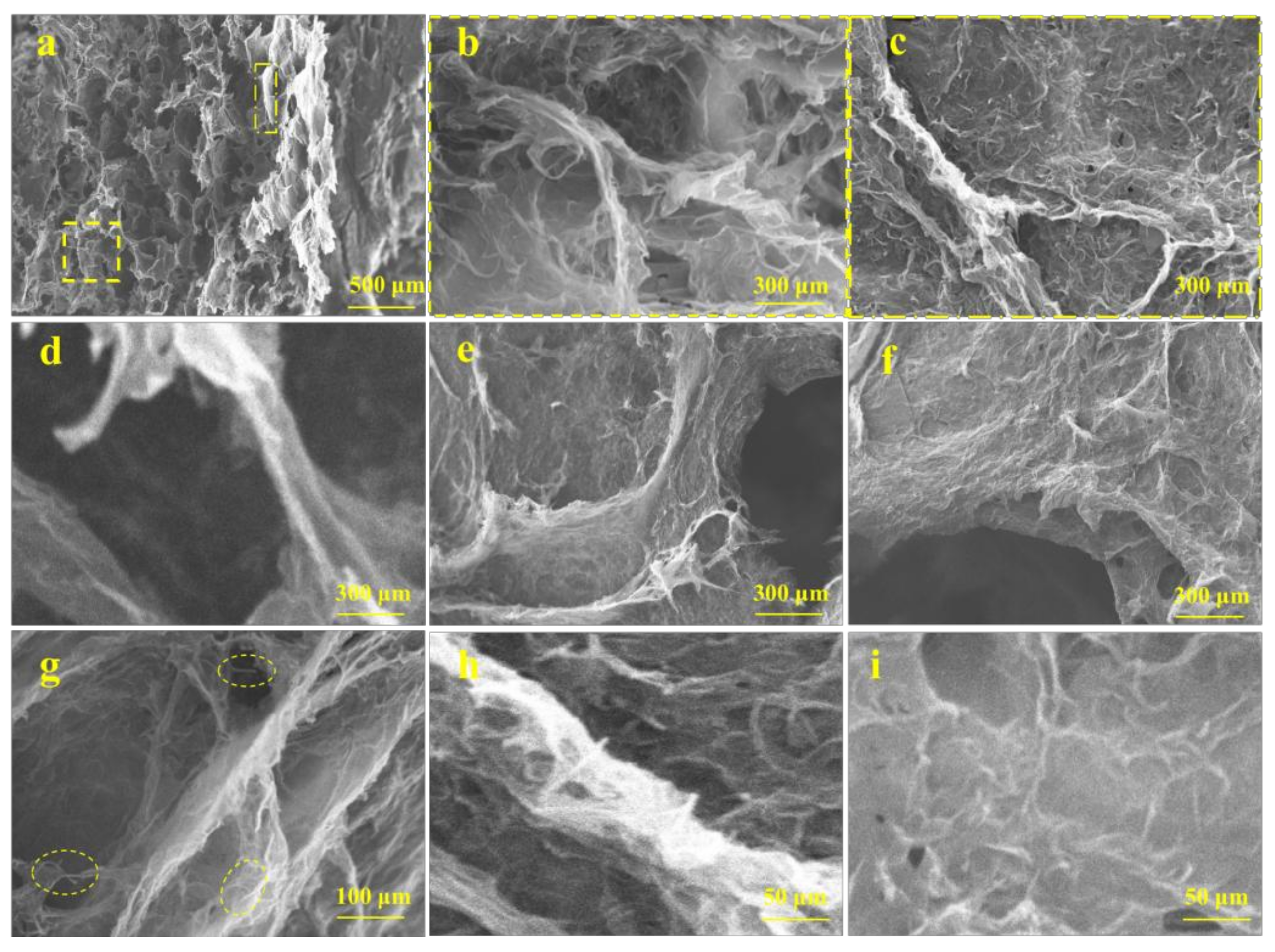
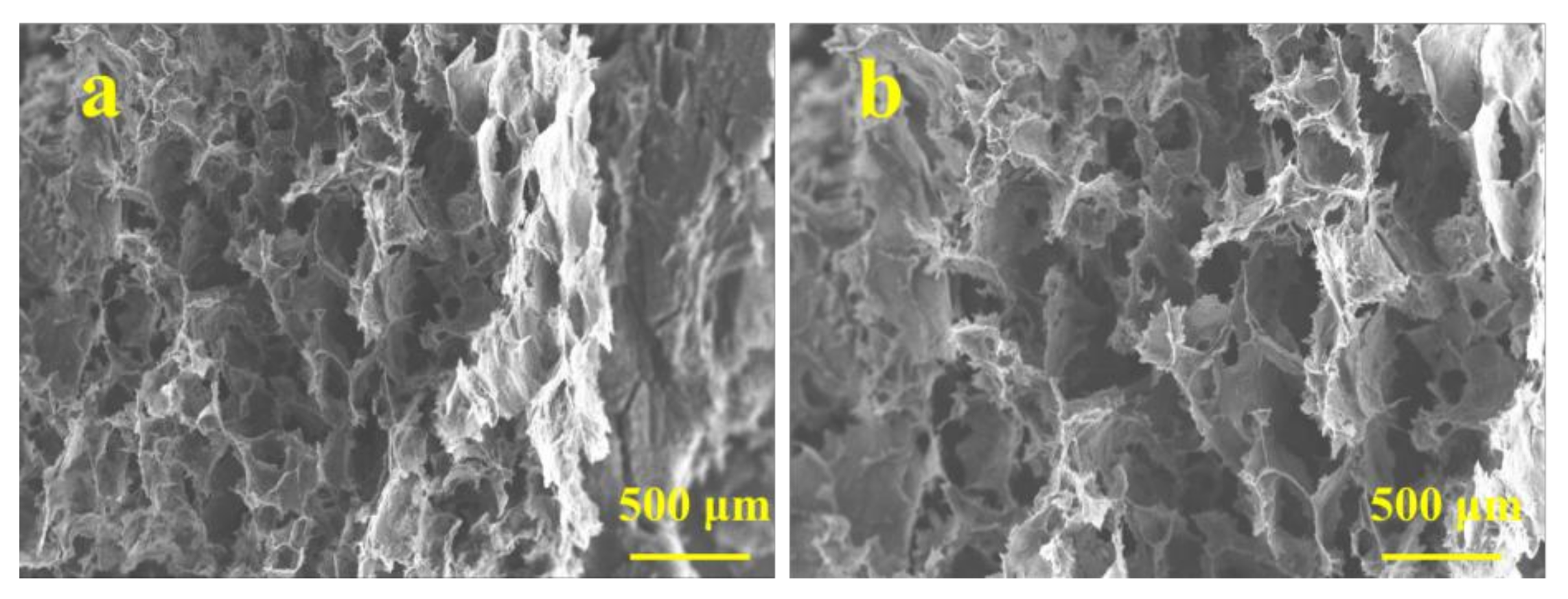
3.2. Macrostructure and Microstructure of Graphene/CNTs Aerogels
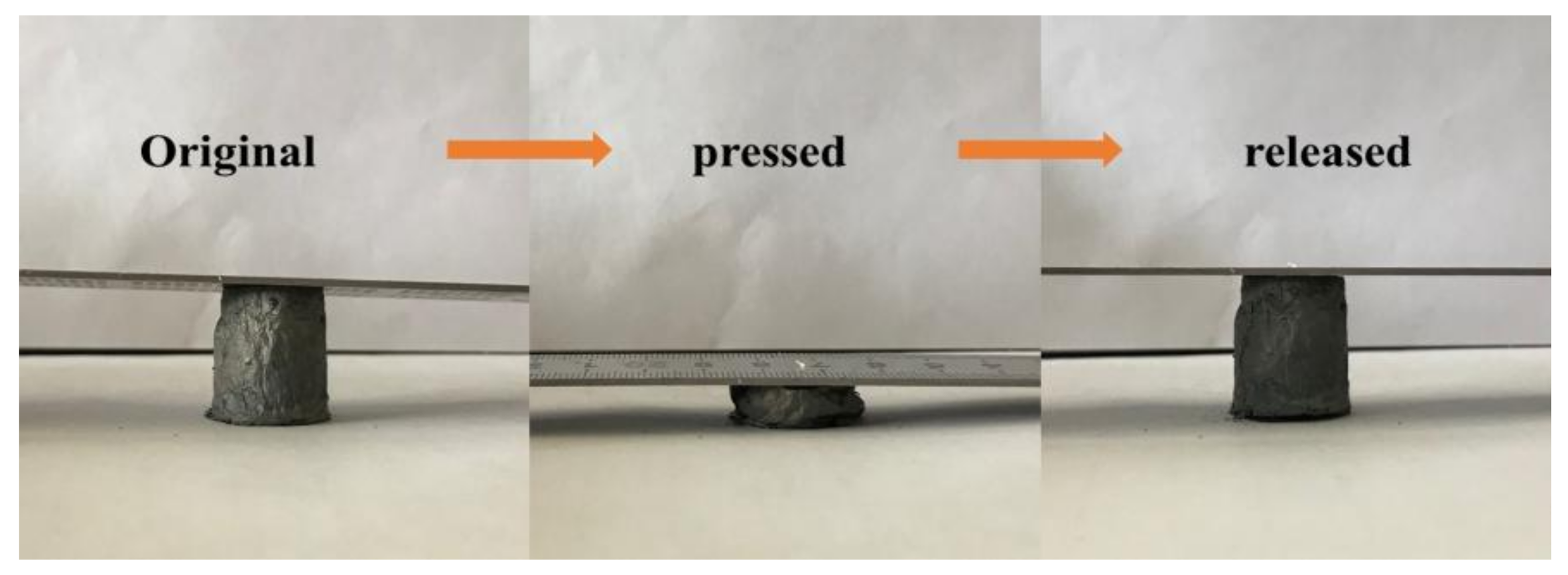
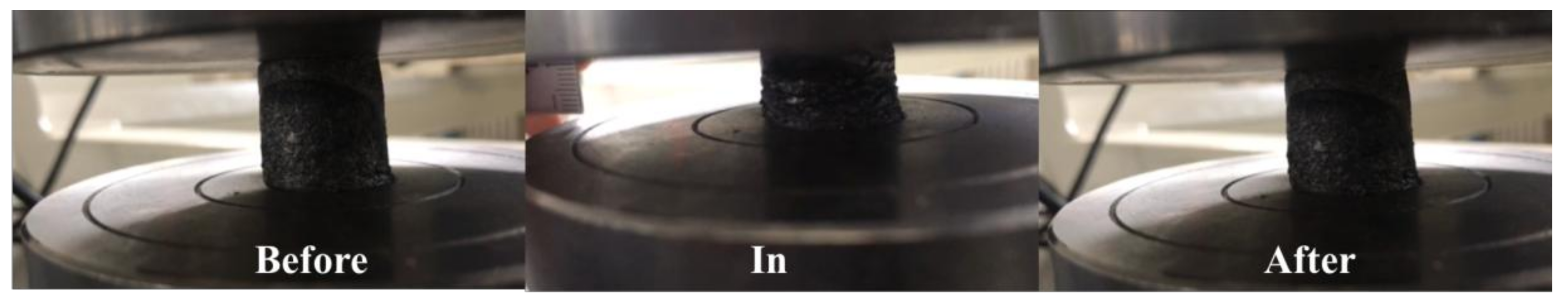
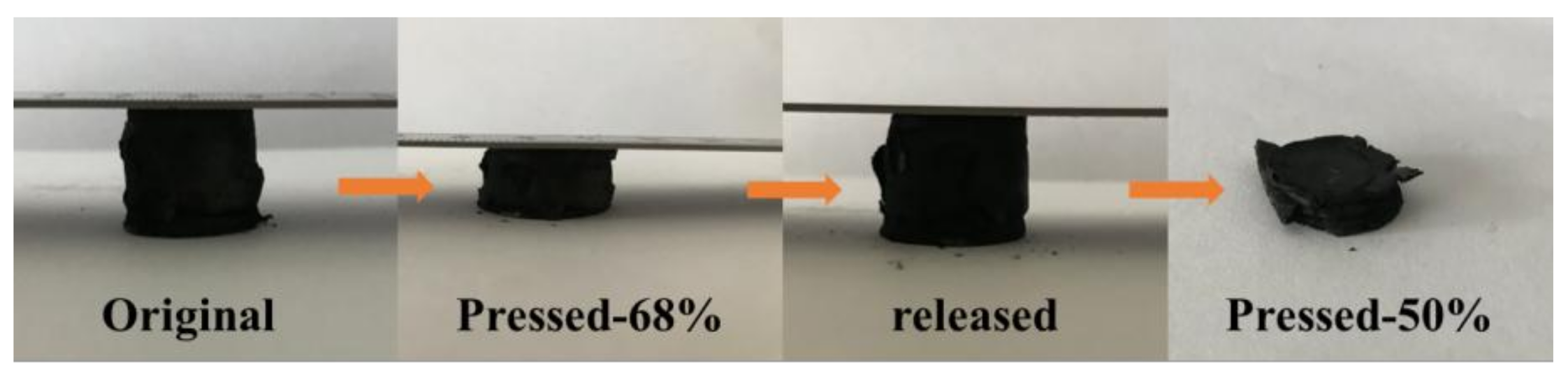
3.3. Compressibility and Influencing Factors of Graphene/CNTs Aerogels
3.4. Hydrophobicity and Hydrophobicity of Grapheme/CNTs Aerogels
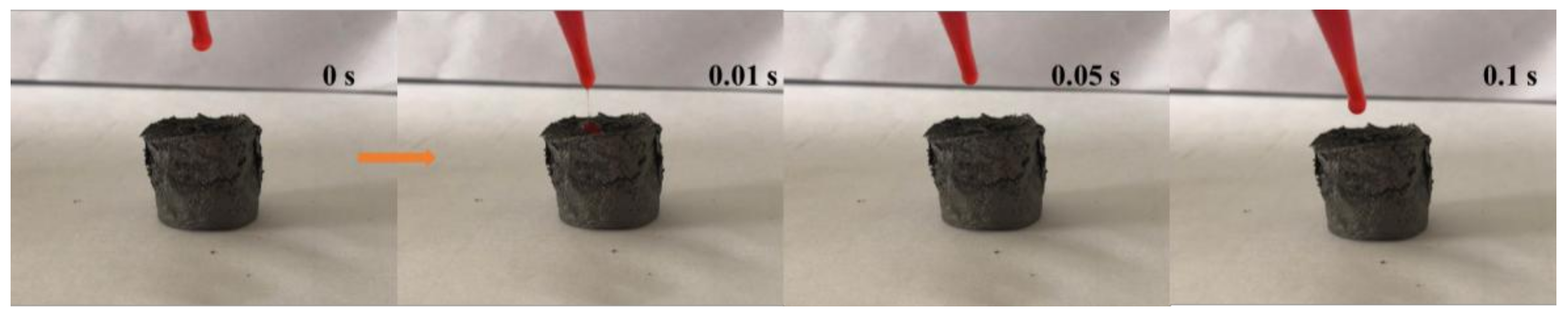
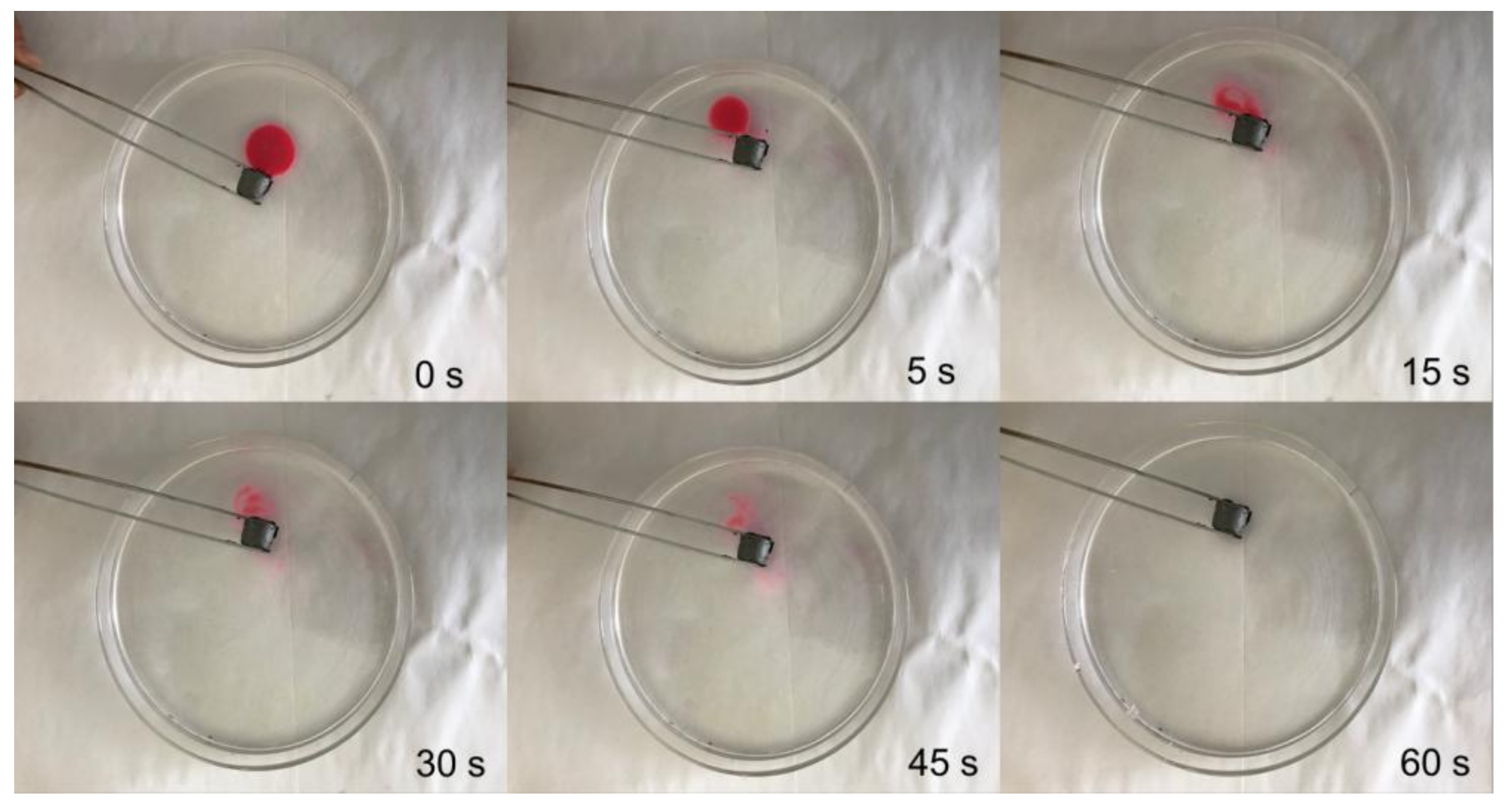
3.5. Oil Absorption Capacity and Influencing Factors of Grapheme/CNTs Aerogels
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hu, K.; Szkopek, T.; Cerruti, M. Tuning the aggregation of graphene oxide dispersions to synthesize elastic, low density graphene aerogels. J. Mater. Chem. A 2017, 5, 23123–23130. [Google Scholar] [CrossRef]
- Wang, B.; Al Abdulla, W.; Wang, D.; Zhao, X.S. A three-dimensional porous LiFePO cathode material modified with a nitrogen-doped graphene aerogel for high-power lithium ion batteries. Energy Environ. Sci. 2015, 8, 869–875. [Google Scholar] [CrossRef]
- Xin, Z.; Li, W.; Fang, W.; He, X.; Zhao, L.; Chen, H.; Zhang, W.; Sun, Z. Enhanced specific surface area by hierarchical porous graphene aerogel/carbon foam for supercapacitor. J. Nanopart. Res. 2017, 19, 386. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, L.; Zhang, L.; Yan, J.; Lu, H.; Fan, W.; Liu, T. Immobilization of NiS nanoparticles on N-doped carbon fiber aerogels as advanced electrode materials for supercapacitors. Nano Res. 2016, 9, 2747–2759. [Google Scholar] [CrossRef]
- Repp, S.; Harputlu, E.; Gurgen, S.; Castellano, M.; Kremer, N.; Pompe, N.; Wörner, J.; Hoffmann, A.; Thomann, R.; Emen, F.; et al. Synergetic effects of Fe3+ doped spinel Li4Ti5O12 nanoparticles on reduced graphene oxide for high surface electrode hybrid supercapacitors. Nanoscale 2018, 10, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Genc, R.; Alas, M.O.; Harputlu, E.; Repp, S.; Kremer, N.; Castellano, M.; Colak, S.; Ocakoglu, K.; Erdem, E. High-capacitance hybrid supercapacitor based on multi-colored fluorescent carbon-dots. Sci. Rep. 2017, 7, 11222. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Li, L.; Zhou, Y.; Yu, M.; Wang, J.; Liu, J.; Zhou, J.; Fan, Z.; Shao, Z. A cellulose-based hybrid 2D material aerogel for a flexible all-solid-state supercapacitor with high specific capacitance. RSC Adv. 2017, 7, 43512–43520. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, T.; Qian, F.; Han, T.Y.J.; Duoss, E.B.; Kuntz, J.D.; Spadaccini, C.M.; Worsley, M.A.; Li, Y. Supercapacitors based on three-dimensional hierarchical graphene aerogels with periodic macropores. Nano Lett. 2016, 16, 3448–3456. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhao, Z.; Wan, W.; Gogotsi, Y.; Qiu, J. Ultralight and highly compressible graphene aerogels. Adv. Mater. 2013, 25, 2219–2223. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, N.; Zeng, W.; Long, F.; Song, Z.; Su, J.; Li, L.; Zou, Z.; Fang, G.; Xiong, L.; et al. Superelastic and ultralight electron source from modifying 3D reduced graphene aerogel microstructure. Nano Energy. 2017, 33, 280–287. [Google Scholar] [CrossRef]
- Chen, Z.; Li, H.; Tian, R.; Duan, H.; Guo, Y.; Chen, Y.; Zhou, J.; Zhang, C.; DUGNANI, R.; Liu, H. Three dimensional graphene aerogels as binder-less, freestanding, elastic and high-performance electrodes for lithium-ion batteries. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Bak, B.M.; Wie, J.J.; Kong, J.; Park, H.S. Energy storage: Reversibly compressible, highly elastic, and durable graphene aerogels for energy storage devices under limiting conditions. Adv. Funct. Mater. 2015, 25, 1159. [Google Scholar] [CrossRef]
- Wan, Y.J.; Zhu, P.L.; Yu, S.H.; Sun, R.; Wong, C.P.; Liao, W.H. Ultralight, super-elastic and volume-preserving cellulose fiber/graphene aerogel for high-performance electromagnetic interference shielding. Carbon 2017, 115, 629–639. [Google Scholar] [CrossRef]
- Yu, M.F.; Files, B.S.; Arepalli, S.; Ruoff, R.S. Tensile loading of ropes of single wall carbon nanotubes and their mechanical properties. Phys. Rev. Lett. 2014, 84, 5552–5555. [Google Scholar] [CrossRef] [PubMed]
- Kulik, A.J.; Kis, A.; Lukic, B.; Lee, K.; Forró, L. Mechanical properties of carbon nanotubes. In Fundamentals of Friction and Wear; Gnecco, E., Meyer, E., Eds.; Springer: Heidelberg, Germany, 2007; pp. 406–410. [Google Scholar]
- Pham, C.V.; Repp, S.; Thomann, R.; Krueger, M.; Weber, S.; Erdem, E. Charge transfer and surface defect healing within ZnO nanoparticle decorated graphene hybrid materials. Nanoscale 2016, 8, 9682–9687. [Google Scholar] [CrossRef] [PubMed]
- Dennany, L.; Sherrell, P.; Chen, J.; Innis, P.C.; Wallace, G.G.; Minett, A.I. EPR characterisation of platinum nanoparticle functionalised carbon nanotube hybrid materials. PCCP 2010, 12, 4135–4141. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.N.; Khan, U.; Gun’ko, Y.K. Mechanical reinforcement of polymers using carbon nanotubes. Adv. Mater. 2006, 18, 689–706. [Google Scholar] [CrossRef]
- Worsley, M.A.; Satcher, J.H., Jr.; Baumann, T.F. Synthesis and characterization of monolithic carbon aerogel nanocomposites containing double-walled carbon nanotubes. Langmuir 2008, 24, 9763–9766. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, F.; Tang, J. Sodium alginate decorated carbon nanotubes-graphene composite aerogel for heavy metal ions detection. Electrochemistry 2015, 83, 84–90. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, X.; Gong, C.; Zhang, J.; Zhang, J.; Gu, X.; Tong, L.; Zhou, J.; Zhang, Z. Preparation of graphene-based composite aerogel and the effects of carbon nanotubes on preserving porous structure of aerogel and improving its capacitor performance. J. Mater. Chem. A 2015, 3, 13445–13452. [Google Scholar] [CrossRef]




| CNTs Content (%) | Maximum Recoverable Elastic Deformation of Aaerogels (%) |
|---|---|
| 0 | 10 |
| 5 | 40 |
| 10 | 50 |
| 30 | 70 |
| 50 | 90 |
| 70 | 65 |
| 90 | 45 |
| 100 | 0 |
| CNTs Content (%) | Water Contact Angles of Aerogels (°) |
|---|---|
| 0 | 92 |
| 5 | 101 |
| 10 | 115 |
| 30 | 120 |
| 50 | 123 |
| 70 | 122 |
| 90 | 124 |
| 100 | 122 |
| Oil Species | Oil Density (g/mL) | Dynamic Viscosity (cP) |
|---|---|---|
| n-Hexane | 0.66 | 307 |
| Ethanol | 0.79 | 1.2 |
| Methylbenzene | 0.87 | 0.69 |
| Vacuum pump oil | 0.88 | 350 |
| Diesel oil | 0.83 | 4.3 |
| Gasoline | 0.7 | 0.8 |
| Vegetable oil | 0.9 | 100 |
| Trichloromethane | 1.48 | 0.56 |
| Carbon tetrachloride | 1.59 | 0.97 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Yu, L.; Liu, D. Ultralight Graphene/Carbon Nanotubes Aerogels with Compressibility and Oil Absorption Properties. Materials 2018, 11, 641. https://doi.org/10.3390/ma11040641
Zhao D, Yu L, Liu D. Ultralight Graphene/Carbon Nanotubes Aerogels with Compressibility and Oil Absorption Properties. Materials. 2018; 11(4):641. https://doi.org/10.3390/ma11040641
Chicago/Turabian StyleZhao, Da, Li Yu, and Dongxu Liu. 2018. "Ultralight Graphene/Carbon Nanotubes Aerogels with Compressibility and Oil Absorption Properties" Materials 11, no. 4: 641. https://doi.org/10.3390/ma11040641




