Synergistic Effect of Nitrogen Doping and MWCNT Intercalation for the Graphene Hybrid Support for Pt Nanoparticles with Exemplary Oxygen Reduction Reaction Performance
Abstract
:1. Introduction
2. Experimental
2.1. Material Synthesis
2.2. Structural Characterization
2.3. Electrochemical Measurements
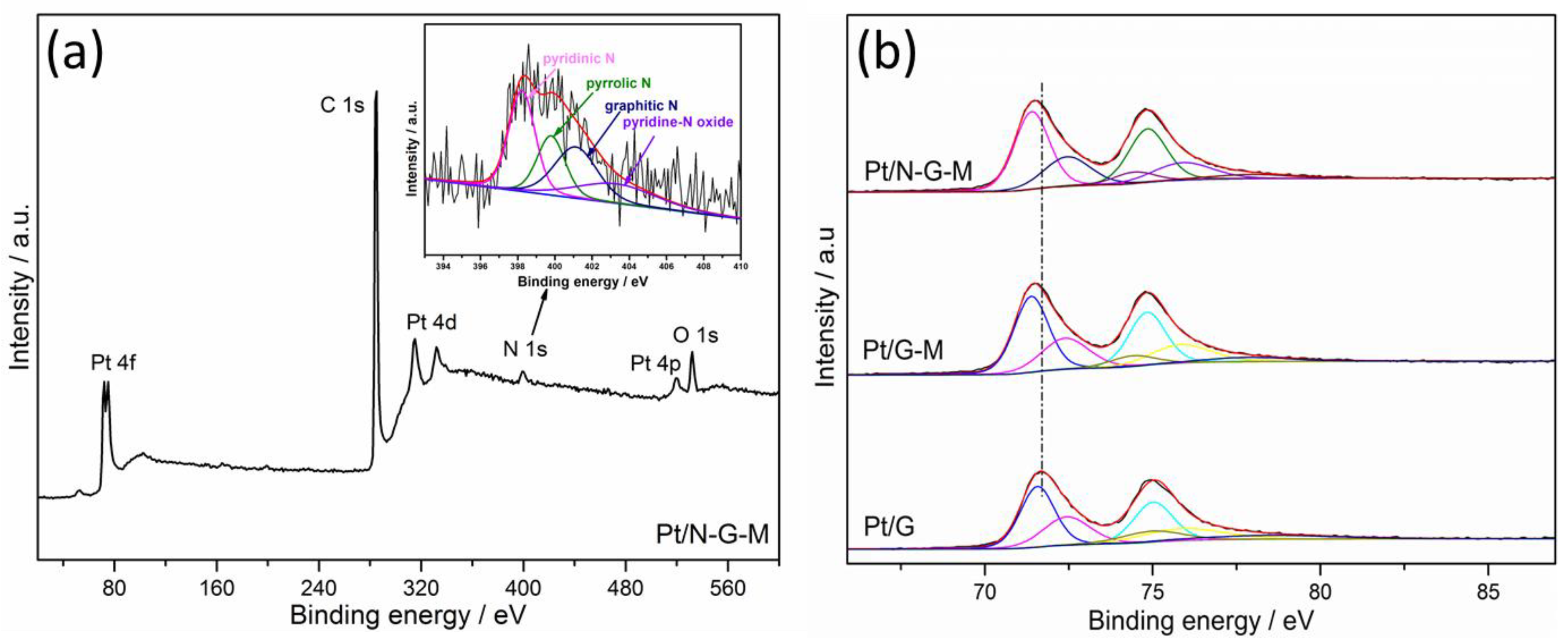
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smalley, R.E. Future global energy prosperity: The terawatt challenge. MRS Bull. 2005, 30, 412–417. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Hamelin, J. Improved carbon nanostructures as a novel catalyst support in the cathode side of PEMFC: A critical review. Carbon 2015, 94, 705–728. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.A.; Hassan, F.M.; Higgins, D.; Choi, J.Y.; Pritzker, M.; Knights, S.; Ye, S.; Chen, Z. Multigrain platinum nanowires consisting of oriented nanoparticles anchored on sulfur-doped graphene as a highly active and durable oxygen reduction electrocatalyst. Adv. Mater. 2015, 27, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Bing, Y.; Liu, H.; Zhang, L.; Ghosh, D.; Zhang, J. Nanostructured Pt-alloy electrocatalysts for PEM fuel cell oxygen reduction reaction. Chem. Soc. Rev. 2010, 39, 2184–2202. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Pollet, B.G. Support materials for PEMFC and DMFC electrocatalysts—A review. J. Power Sources 2012, 208, 96–119. [Google Scholar] [CrossRef]
- Hartl, K.; Hanzlik, M.; Arenz, M. IL-TEM investigations on the degradation mechanism of Pt/C electrocatalysts with different carbon supports. Energy Environ. Sci. 2011, 4, 234–238. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, G.; Geng, D.; Chen, Y.; Li, R.; Cai, M.; Sun, X. A highly durable platinum nanocatalyst for proton exchange membrane fuel cells: Multiarmed starlike nanowire single crystal. Angew. Chem. 2011, 123, 442–446. [Google Scholar] [CrossRef]
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D. Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef] [PubMed]
- Hasche, F.; Oezaslan, M.; Strasser, P. Activity, stability and degradation of multi walled carbon nanotube (MWCNT) supported Pt fuel cell electrocatalysts. Phys. Chem. Chem. Phys. 2010, 12, 15251–15258. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qu, J.; Fu, X.; Wang, Q.; Zhong, G.; Peng, F. Low Pt content catalyst supported on nitrogen and phosphorus-codoped carbon nanotubes for electrocatalytic O2 reaction in acidic medium. Mater. Lett. 2015, 142, 115–118. [Google Scholar] [CrossRef]
- Park, J.-H.; Ju, Y.-W.; Park, S.-H.; Jung, H.-R.; Yang, K.-S.; Lee, W.-J. Effects of electrospun polyacrylonitrile-based carbon nanofibers as catalyst support in PEMFC. J. Appl. Electrochem. 2009, 39, 1229. [Google Scholar] [CrossRef]
- Sebastián, D.; Ruíz, A.G.; Suelves, I.; Moliner, R.; Lázaro, M.J.; Baglio, V.; Stassi, A.; Aricò, A.S. Enhanced oxygen reduction activity and durability of Pt catalysts supported on carbon nanofibers. Appl. Catal. B Environ. 2012, 115, 269–275. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, J.; Yang, S.; Li, G.; Qiao, J. Highly active and stable platinum catalyst supported on porous carbon nanofibers for improved performance of PEMFC. Electrochim. Acta 2015, 177, 181–189. [Google Scholar] [CrossRef]
- Shanahan, P.V.; Xu, L.; Liang, C.; Waje, M.; Dai, S.; Yan, Y. Graphitic mesoporous carbon as a durable fuel cell catalyst support. J. Power Sources 2008, 185, 423–427. [Google Scholar] [CrossRef]
- Song, S.; Liang, Y.; Li, Z.; Wang, Y.; Fu, R.; Wu, D.; Tsiakaras, P. Effect of pore morphology of mesoporous carbons on the electrocatalytic activity of Pt nanoparticles for fuel cell reactions. Appl. Catal. B Environ. 2010, 98, 132–137. [Google Scholar] [CrossRef]
- Hsueh, Y.-J.; Yu, C.-C.; Lee, K.-R.; Tseng, C.-J.; Su, B.-J.; Wu, S.-K.; Weng, L.-C. Ordered porous carbon as the catalyst support for proton-exchange membrane fuel cells. Int. J. Hydrogen Energy 2013, 38, 10998–11003. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, R.; Chen, W. Graphene-supported nanoelectrocatalysts for fuel cells: Synthesis, properties, and applications. Chem. Rev. 2014, 114, 5117–5160. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xiao, M.; Zhao, X.; Liu, C.; Ge, J.; Xing, W. Strongly coupled Pt nanotubes/N-doped graphene as highly active and durable electrocatalysts for oxygen reduction reaction. Nano Energy 2015, 13, 318–326. [Google Scholar] [CrossRef]
- Li, Z.-F.; Xin, L.; Yang, F.; Liu, Y.; Liu, Y.; Zhang, H.; Stanciu, L.; Xie, J. Hierarchical polybenzimidazole-grafted graphene hybrids as supports for Pt nanoparticle catalysts with excellent PEMFC performance. Nano Energy 2015, 16, 281–292. [Google Scholar] [CrossRef]
- Jiang, L.; Fan, Z. Design of advanced porous graphene materials: From graphene nanomesh to 3D architectures. Nanoscale 2014, 6, 1922–1945. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Dong, S. Recent progress in graphene-based nanomaterials as advanced electrocatalysts towards oxygen reduction reaction. Nanoscale 2013, 5, 1753–1767. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Shao, Y.; Wan, H.; Rieke, P.C.; Viswanathan, V.V.; Towne, S.A.; Saraf, L.V.; Liu, J.; Lin, Y.; Wang, Y. Design of graphene sheets-supported Pt catalyst layer in PEM fuel cells. Electrochem. Commun. 2011, 13, 258–261. [Google Scholar] [CrossRef]
- Jafri, R.I.; Arockiados, T.; Rajalakshmi, N.; Ramaprabhu, S. Nanostructured Pt dispersed on graphene-multiwalled carbon nanotube hybrid nanomaterials as electrocatalyst for PEMFC. J. Electrochem. Soc. 2010, 157, B874–B879. [Google Scholar] [CrossRef]
- Zhou, Y.; Neyerlin, K.; Olson, T.S.; Pylypenko, S.; Bult, J.; Dinh, H.N.; Gennett, T.; Shao, Z.; O’Hayre, R. Enhancement of Pt and Pt-alloy fuel cell catalyst activity and durability via nitrogen-modified carbon supports. Energy Environ. Sci. 2010, 3, 1437–1446. [Google Scholar] [CrossRef]
- Jafri, R.I.; Rajalakshmi, N.; Ramaprabhu, S. Nitrogen doped graphene nanoplatelets as catalyst support for oxygen reduction reaction in proton exchange membrane fuel cell. J. Mater. Chem. 2010, 20, 7114–7117. [Google Scholar] [CrossRef]
- He, D.; Jiang, Y.; Lv, H.; Pan, M.; Mu, S. Nitrogen-doped reduced graphene oxide supports for noble metal catalysts with greatly enhanced activity and stability. Appl. Catal. B Environ. 2013, 132–133, 379–388. [Google Scholar] [CrossRef]
- Vinayan, B.P.; Nagar, R.; Rajalakshmi, N.; Ramaprabhu, S. Novel Platinum–Cobalt alloy nanoparticles dispersed on nitrogen-doped graphene as a cathode electrocatalyst for PEMFC applications. Adv. Funct. Mater. 2012, 22, 3519–3526. [Google Scholar] [CrossRef]
- Bai, J.; Zhu, Q.; Lv, Z.; Dong, H.; Yu, J.; Dong, L. Nitrogen-doped graphene as catalysts and catalyst supports for oxygen reduction in both acidic and alkaline solutions. Int. J. Hydrogen Energy 2013, 38, 1413–1418. [Google Scholar] [CrossRef]
- Lee, W.H.; Yang, H.N.; Park, K.W.; Choi, B.S.; Yi, S.C.; Kim, W.J. Synergistic effect of boron/nitrogen co-doping into graphene and intercalation of carbon black for Pt-BCN-Gr/CB hybrid catalyst on cell performance of polymer electrolyte membrane fuel cell. Energy 2016, 96, 314–324. [Google Scholar] [CrossRef]
- Fu, K.; Wang, Y.; Mao, L.; Jin, J.; Yang, S.; Li, G. Facile one-pot synthesis of graphene-porous carbon nanofibers hybrid support for Pt nanoparticles with high activity towards oxygen reduction. Electrochim. Acta 2016, 215, 427–434. [Google Scholar] [CrossRef]
- Xu, Y.; Bai, H.; Lu, G.; Li, C.; Shi, G. Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J. Am. Chem. Soc. 2008, 130, 5856–5857. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.-S.; Oh, J.-G.; Hong, Y.-G.; Kim, H. Investigation of carbon-supported Pt nanocatalyst preparation by the polyol process for fuel cell applications. Electrochim. Acta 2007, 52, 7278–7285. [Google Scholar] [CrossRef]
- Wu, M.; Wang, J.; Wu, Z.; Xin, H.L.; Wang, D. Synergistic enhancement of nitrogen and sulfur co-doped graphene with carbon nanosphere insertion for the electrocatalytic oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 7727–7731. [Google Scholar] [CrossRef]
- Niu, W.; Li, L.; Liu, X.; Zhou, W.; Li, W.; Lu, J.; Chen, S. One-pot synthesis of graphene/carbon nanospheres/graphene sandwich supported Pt3Ni nanoparticles with enhanced electrocatalytic activity in methanol oxidation. Int. J. Hydrogen Energy 2015, 40, 5106–5114. [Google Scholar] [CrossRef]
- Ratso, S.; Kruusenberg, I.; Joost, U.; Saar, R.; Tammeveski, K. Enhanced oxygen reduction reaction activity of nitrogen-doped graphene/multi-walled carbon nanotube catalysts in alkaline media. Int. J. Hydrogen Energy 2016, 41, 22510–22519. [Google Scholar] [CrossRef]
- Wang, H.; Kakade, B.A.; Tamaki, T.; Yamaguchi, T. Synthesis of 3D graphite oxide-exfoliated carbon nanotube carbon composite and its application as catalyst support for fuel cells. J. Power Sources 2014, 260, 338–348. [Google Scholar] [CrossRef]
- Vinayan, B.P.; Ramaprabhu, S. Platinum-TM (TM = Fe, Co) alloy nanoparticles dispersed nitrogen doped (reduced graphene oxide-multiwalled carbon nanotube) hybrid structure cathode electrocatalysts for high performance PEMFC applications. Nanoscale 2013, 5, 5109–5118. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Li, Y.; Huang, X.; Wang, Q.; Mei, A.; Shen, P.K. Highly stable electrocatalysts supported on nitrogen-self-doped three-dimensional graphene-like networks with hierarchical porous structures. J. Mater. Chem. A 2015, 3, 1492–1497. [Google Scholar] [CrossRef]
- Bayram, E.; Yilmaz, G.; Mukerjee, S. A solution-based procedure for synthesis of nitrogen doped graphene as an efficient electrocatalyst for oxygen reduction reactions in acidic and alkaline electrolytes. Appl. Catal. B Environ. 2016, 192, 26–34. [Google Scholar] [CrossRef]
- Xing, T.; Zheng, Y.; Li, L.H.; Cowie, B.C.; Gunzelmann, D.; Qiao, S.Z.; Huang, S.; Chen, Y. Observation of active sites for oxygen reduction reaction on nitrogen-doped multilayer graphene. ACS Nano 2014, 8, 6856–6862. [Google Scholar] [CrossRef] [PubMed]
- Bo, X.; Han, C.; Zhang, Y.; Guo, L. Confined nanospace synthesis of less aggregated and porous nitrogen-doped graphene as metal-free electrocatalysts for oxygen reduction reaction in alkaline solution. ACS Appl. Mater. Interfaces 2014, 6, 3023–3030. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.Y.; Wu, H.B.; Yan, Y.; Lou, X.W.; Wang, X. Ultrathin and ultralong single-crystal platinum nanowire assemblies with highly stable electrocatalytic activity. J. Am. Chem. Soc. 2013, 135, 9480–9485. [Google Scholar] [CrossRef] [PubMed]
- Peera, S.G.; Arunchander, A.; Sahu, A.K. Platinum nanoparticles supported on nitrogen and fluorine co-doped graphite nanofibers as an excellent and durable oxygen reduction catalyst for polymer electrolyte fuel cells. Carbon 2016, 107, 667–679. [Google Scholar] [CrossRef]
- Xu, J.; Fu, G.; Tang, Y.; Zhou, Y.; Chen, Y.; Lu, T. One-pot synthesis of three-dimensional platinum nanochain networks as stable and active electrocatalysts for oxygen reduction reactions. J. Mater. Chem. 2012, 22, 13585–13590. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zhu, E.; McLouth, T.; Chiu, C.Y.; Huang, X.; Huang, Y. Stabilization of high-performance oxygen reduction reaction Pt electrocatalyst supported on reduced graphene oxide/carbon black composite. J. Am. Chem. Soc. 2012, 134, 12326–12329. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Jiang, M.; Camargo, P.H.; Cho, E.C.; Tao, J.; Lu, X.; Zhu, Y.; Xia, Y. Pd-Pt bimetallic nanodendrites with high activity for oxygen reduction. Science 2009, 324, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xin, H.L.; Yu, Y.; Wang, H.; Rus, E.; Muller, D.A.; Abruna, H.D. Pt-decorated PdCo@ Pd/C core−shell nanoparticles with enhanced stability and electrocatalytic activity for the oxygen reduction reaction. J. Am. Chem. Soc. 2010, 132, 17664–17666. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, K.; Wang, Y.; Qian, Y.; Mao, L.; Jin, J.; Yang, S.; Li, G. Synergistic Effect of Nitrogen Doping and MWCNT Intercalation for the Graphene Hybrid Support for Pt Nanoparticles with Exemplary Oxygen Reduction Reaction Performance. Materials 2018, 11, 642. https://doi.org/10.3390/ma11040642
Fu K, Wang Y, Qian Y, Mao L, Jin J, Yang S, Li G. Synergistic Effect of Nitrogen Doping and MWCNT Intercalation for the Graphene Hybrid Support for Pt Nanoparticles with Exemplary Oxygen Reduction Reaction Performance. Materials. 2018; 11(4):642. https://doi.org/10.3390/ma11040642
Chicago/Turabian StyleFu, Kang, Yang Wang, Ying Qian, Linchang Mao, Junhong Jin, Shenglin Yang, and Guang Li. 2018. "Synergistic Effect of Nitrogen Doping and MWCNT Intercalation for the Graphene Hybrid Support for Pt Nanoparticles with Exemplary Oxygen Reduction Reaction Performance" Materials 11, no. 4: 642. https://doi.org/10.3390/ma11040642



