Effect of Reaction Temperature on Structure, Appearance and Bonding Type of Functionalized Graphene Oxide Modified P-Phenylene Diamine
Abstract
:1. Introduction
2. Experimental
2.1. Reagents
2.2. Preparation of Graphene Oxide (GO)
2.3. Preparation of FGO
2.4. Characterization
3. Results and Discussion
3.1. Structural Changes of FGO at Different Reaction Temperatures
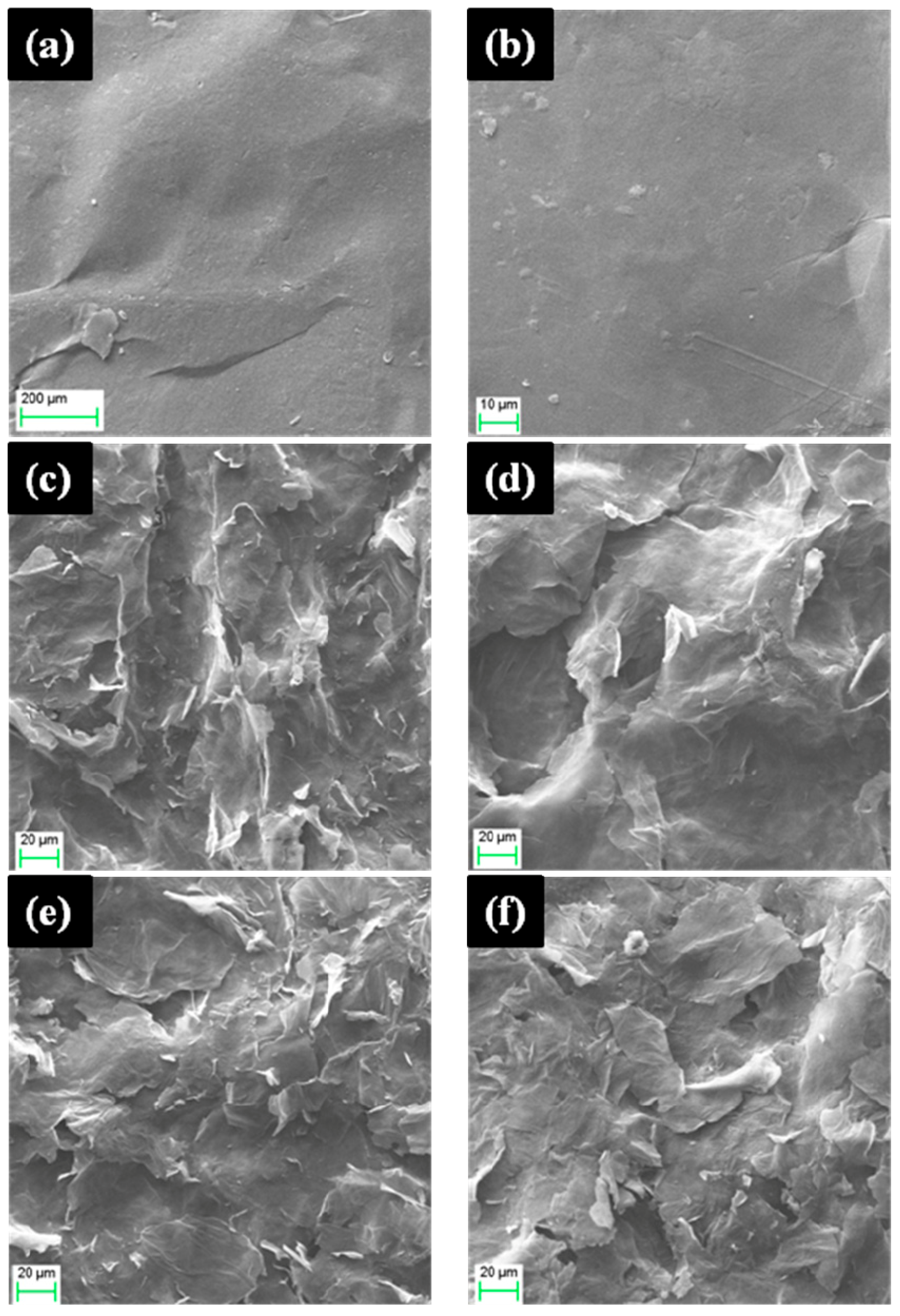
3.2. Effect of Reaction Temperature on FGO Appearance
3.3. Influence of Temperature on Oxygen-Containing Functional Groups and Types of Interactions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hu, T.; Gerber, I.C. Theoretical study of the interaction of electron donor and acceptor molecules with graphene. J. Phys. Chem. C 2017, 117, 2411–2420. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Layer area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2009, 9, 3087. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- El-Kady, M.F.; Shao, Y.; Kaner, R.B. Graphene for batteries, supercapacitors and beyond. Nat. Rev. Mater. 2016, 1, 16033. [Google Scholar] [CrossRef]
- Gonçalves, J.A.; Nascimento, R.; Matos, M.J.S.; de Oliveira, A.B.; Chacham, H.; Batista, R.J.C. Edge-reconstructed, few-layered graphene nanoribbons: Stability and electronic properties. J. Phys. Chem. C 2017, 121, 5836–5840. [Google Scholar] [CrossRef]
- Bottari, G.; Herranz, M.A.; Wibmer, L.; Volland, M.; Rodriguez-Perez, L.; Guldi, D.M.; Hirsch, A.; Martin, N.; D’Souza, F.; Torres, T. Chemical functionalization and characterization of graphene-based materials. Chem. Soc. Rev. 2017, 46, 4464–4500. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Wu, Z.P.; Bartolucci, S.F.; Basu, S.; Mukherjee, R.; Gupta, T.; Hundekar, P.; Shi, Y.; Lu, T.-M.; Koratkar, N. Protecting silicon film anodes in lithium-ion batteries using an atomically thin graphene drape. ACS Nano 2017, 11, 5051–5061. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ji, H.; Cheng, H.-M.; Ruoff, R.S. Mass production and industrial applications of graphene materials. Natl. Sci. Rev. 2017, 0, 1–12. [Google Scholar] [CrossRef]
- Emiru, T.F.; Ayele, D.W. Controlled synthesis, characterization and reduction of graphene oxide: A convenient method for large scale production. Egypt. J. Basic Appl. Sci. 2017, 4, 74–79. [Google Scholar] [CrossRef]
- Phiri, J.; Gane, P.; Maloney, T.C. General overview of graphene: Production, properties and application in polymer composites. Mater. Sci. Eng. B 2017, 215, 9–28. [Google Scholar] [CrossRef]
- Guo, H.; Li, X.; Li, B.; Wang, J.; Wang, S. Thermal conductivity of graphene/poly(vinylidene fluoride) nanocomposite membrane. Mater. Des. 2017, 114, 355–363. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, X.; Wu, X.; Lu, C. Self-stabilized polyaniline@graphene aqueous colloids for the construction of assembled conductive network in rubber matrix and its chemical sensing application. Compos. Sci. Technol. 2016, 125, 1–8. [Google Scholar] [CrossRef]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef] [PubMed]
- Parhizkar, N.; Shahrabi, T.; Ramezanzadeh, B. A new approach for enhancement of the corrosion protection properties and interfacial adhesion bonds between the epoxy coating and steel substrate through surface treatment by covalently modified amino functionalized graphene oxide film. Corros. Sci. 2017, 123, 55–75. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, S.; Zhang, Q.; Ming, P.; Wan, S.; Peng, J.; Jiang, L.; Cheng, Q. Graphene-based artificial nacre nanocomposites. Chem. Soc. Rev. 2016, 45, 2378–2395. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Huang, Y.; Shi, G.; Jiang, L.; Qu, L. Graphene-based functional architectures: Sheets regulation and macrostructure construction toward actuators and power generators. Acc. Chem. Res. 2017, 50, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Lee, S.; Lee, Y.S. Mechanical properties of epoxy composites reinforced with ammonia-treated graphene oxides. Carbon Lett. 2017, 21, 1–7. [Google Scholar] [CrossRef]
- Manna, R.; Srivastava, S.K. Fabrication of functionalized graphene filled carboxylated nitrile rubber nanocomposites as flexible dielectric materials. Mater. Chem. Front. 2017, 1, 780–788. [Google Scholar] [CrossRef]
- Zahirifar, J.; Karimi-Sabet, J.; Moosavian, S.M.A.; Hadi, A.; Khadiv-Parsi, P. Fabrication of a novel octadecylamine functionalized graphene oxide/pvdf dual-layer flat sheet membrane for desalination via air gap membrane distillation. Desalination 2018, 428, 227–239. [Google Scholar] [CrossRef]
- Liu, S.; Li, D.; Wang, L.; Yang, H.; Han, X.; Liu, B. Ethylenediamine-functionalized graphene oxide incorporated acid-base ion exchange membranes for vanadium redox flow battery. Electrochim. Acta 2017, 230, 204–211. [Google Scholar] [CrossRef]
- Deng, W.; Zhang, Y.; Tan, Y.; Ma, M. Three-dimensional nitrogen-doped graphene derived from poly-o-phenylenediamine for high-performance supercapacitors. J. Electroanal. Chem. 2017, 787, 103–109. [Google Scholar] [CrossRef]
- Pandit, S.; De, M. Interaction of amino acids and graphene oxide: Trends in thermodynamic properties. J. Phys. Chem. C 2017, 121, 600–608. [Google Scholar] [CrossRef]
- Shanmugharaj, A.M.; Yoon, J.H.; Yang, W.J.; Ryu, S.H. Synthesis, characterization, and surface wettability properties of amine functionalized graphene oxide films with varying amine chain lengths. J. Colloid Interface Sci. 2013, 401, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Miyabe, T.; Fukutsuka, T.; Sugie, Y. Preparation and characterization of alkylamine-intercalated graphite oxides. Carbon 2007, 45, 1005–1012. [Google Scholar] [CrossRef]
- Hung, W.-S.; Tsou, C.-H.; De Guzman, M.; An, Q.-F.; Liu, Y.-L.; Zhang, Y.-M.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Cross-linking with diamine monomers to prepare composite graphene oxide-framework membranes with varying d-spacing. Chem. Mater. 2014, 26, 2983–2990. [Google Scholar] [CrossRef]
- Eng, A.Y.S.; Chua, C.K.; Pumera, M. Facile labelling of graphene oxide for superior capacitive energy storage and fluorescence applications. Phys. Chem. Chem. Phys. 2016, 18, 9673–9681. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, L.; Wu, D.; Xia, W.; Zhao, H.; Jia, D. Hydrothermal synthesis of nitrogen-doped graphene hydrogels using amino acids with different acidities as doping agents. J. Mater. Chem. A 2014, 2, 8352–8361. [Google Scholar] [CrossRef]
- Peng, T.; Sun, H.; Peng, T.; Liu, B.; Zhao, X. Structural regulation and electroconductivity change of nitrogen-doping reduced graphene oxide prepared using p-phenylene diamine as modifier. Nanomaterials 2017, 7, 292. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, L.; Song, B.; Moon, K.-S.; Hu, N.; Liao, G.; Shi, T.; Wong, C. Mechanistic investigation of the graphene functionalization using p-phenylenediamine and its application for supercapacitors. Nano Energy 2015, 17, 160–170. [Google Scholar] [CrossRef]
- Hu, N.; Wang, Y.; Chai, J.; Gao, R.; Yang, Z.; Kong, E.S.-W.; Zhang, Y. Gas sensor based on p-phenylenediamine reduced graphene oxide. Sens. Actuators B Chem. 2012, 163, 107–114. [Google Scholar] [CrossRef]
- Liu, B.; Sun, H.J.; Peng, T.J. Factor group analysis of molecular vibrational modes of graphene and density functional calculations. Acta Phys. Chim. Sin. 2012, 28, 799–804. [Google Scholar]
- Eigler, S.; Dotzer, C.; Hirsch, A. Visualization of defect densities in reduced graphene oxide. Carbon 2012, 50, 3666–3673. [Google Scholar] [CrossRef]
- Tomita, S.; Sakurai, T.; Ohta, H.; Fujii, M.; Hayashi, S. Structure and electronic properties of carbon onions. J. Chem. Phys. 2001, 114, 7477–7482. [Google Scholar] [CrossRef]
- Roghani-Mamaqani, H.; Haddadi-Asl, V. In-plane functionalizing graphene nanolayers with polystyrene by atom transfer radical polymerization: Grafting from hydroxyl groups. Polym. Compos. 2014, 35, 386–395. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, H.; Huang, Z.; Wang, W.; Zeng, F.; Kuang, Y. Graphene covalently functionalized with poly(p-phenylenediamine) as high performance electrode material for supercapacitors. J. Mater. Chem. A 2013, 1, 3454–3462. [Google Scholar] [CrossRef]
- Han, Z.; Tang, Z.; Li, P.; Yang, G.; Zheng, Q.; Yang, J. Ammonia solution strengthened three-dimensional macro-porous graphene aerogel. Nanoscale 2013, 5, 5462–5467. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Dikin, D.A.; Nguyen, S.B.T.; Ruoff, R.S. Graphene oxide sheets chemically cross-linked by polyallylamine. J. Phys. Chem. C 2016, 113, 15801–15804. [Google Scholar] [CrossRef]
- Zhang, D.D.; Zu, S.Z.; Han, B.H. Inorganic–organic hybrid porous materials based on graphite oxide sheets. Carbon 2009, 47, 2993–3000. [Google Scholar] [CrossRef]
- Ma, H.-L.; Zhang, H.-B.; Hu, Q.-H.; Li, W.-J.; Jiang, Z.-G.; Yu, Z.-Z.; Dasari, A. Functionalization and reduction of graphene oxide with p-phenylene diamine for electrically conductive and thermally stable polystyrene composites. ACS Appl. Mater. Interfaces 2012, 4, 1948–1953. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Zhu, J.; Liu, N.; Li, Y. Facile functionalization of graphene oxide with ethylenediamine as a solid base catalyst for knoevenagel condensation reaction. Catal. Commun. 2015, 64, 105–109. [Google Scholar] [CrossRef]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.-J.; Liu, B.; Peng, T.-J.; Zhao, X.-L. Effect of Reaction Temperature on Structure, Appearance and Bonding Type of Functionalized Graphene Oxide Modified P-Phenylene Diamine. Materials 2018, 11, 647. https://doi.org/10.3390/ma11040647
Sun H-J, Liu B, Peng T-J, Zhao X-L. Effect of Reaction Temperature on Structure, Appearance and Bonding Type of Functionalized Graphene Oxide Modified P-Phenylene Diamine. Materials. 2018; 11(4):647. https://doi.org/10.3390/ma11040647
Chicago/Turabian StyleSun, Hong-Juan, Bo Liu, Tong-Jiang Peng, and Xiao-Long Zhao. 2018. "Effect of Reaction Temperature on Structure, Appearance and Bonding Type of Functionalized Graphene Oxide Modified P-Phenylene Diamine" Materials 11, no. 4: 647. https://doi.org/10.3390/ma11040647




