An Electrochemical Study on the Copolymer Formed from Piperazine and Aniline Monomers
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
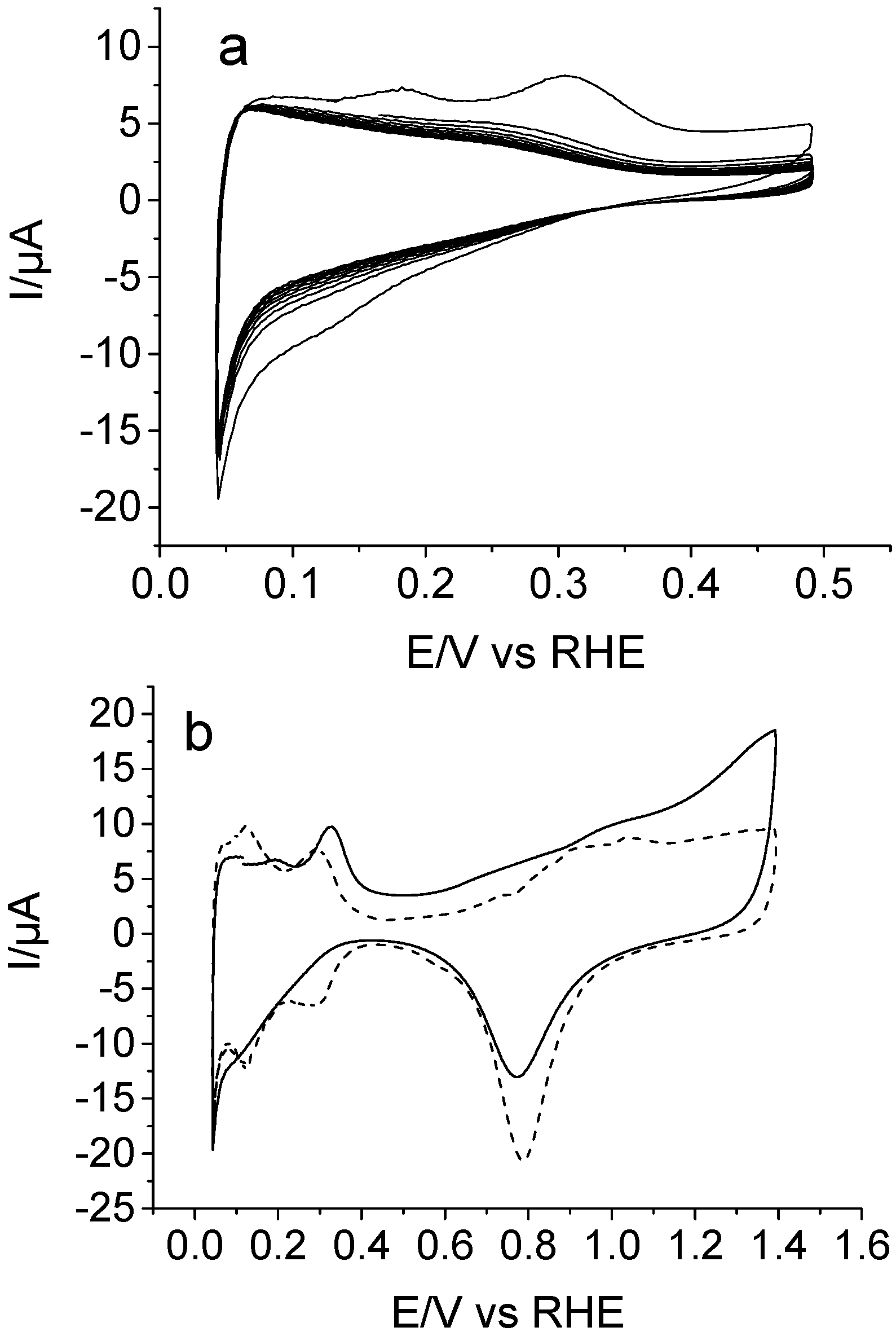
3.1. Electrochemical Behavior of Piperazine on Pt
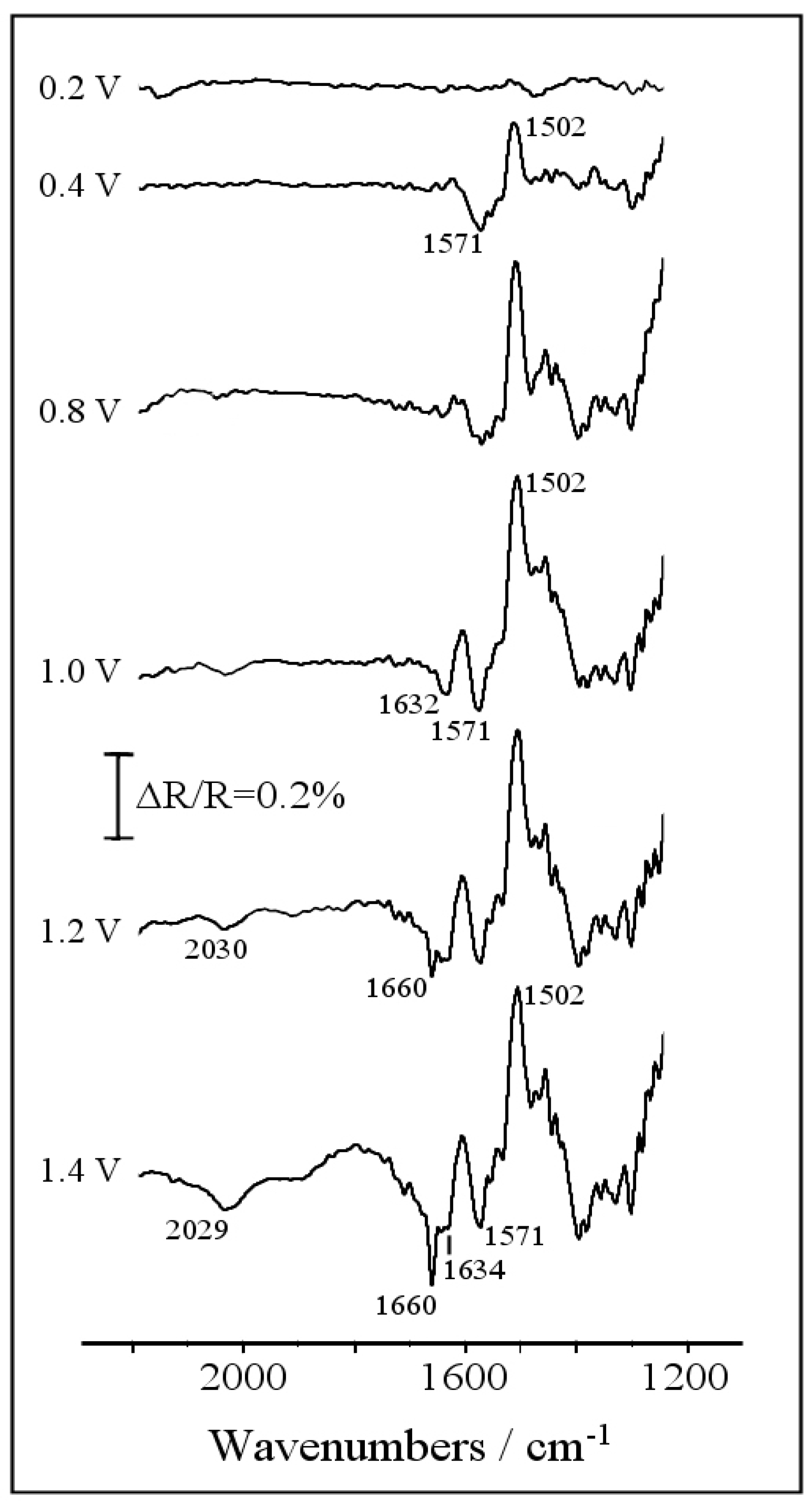
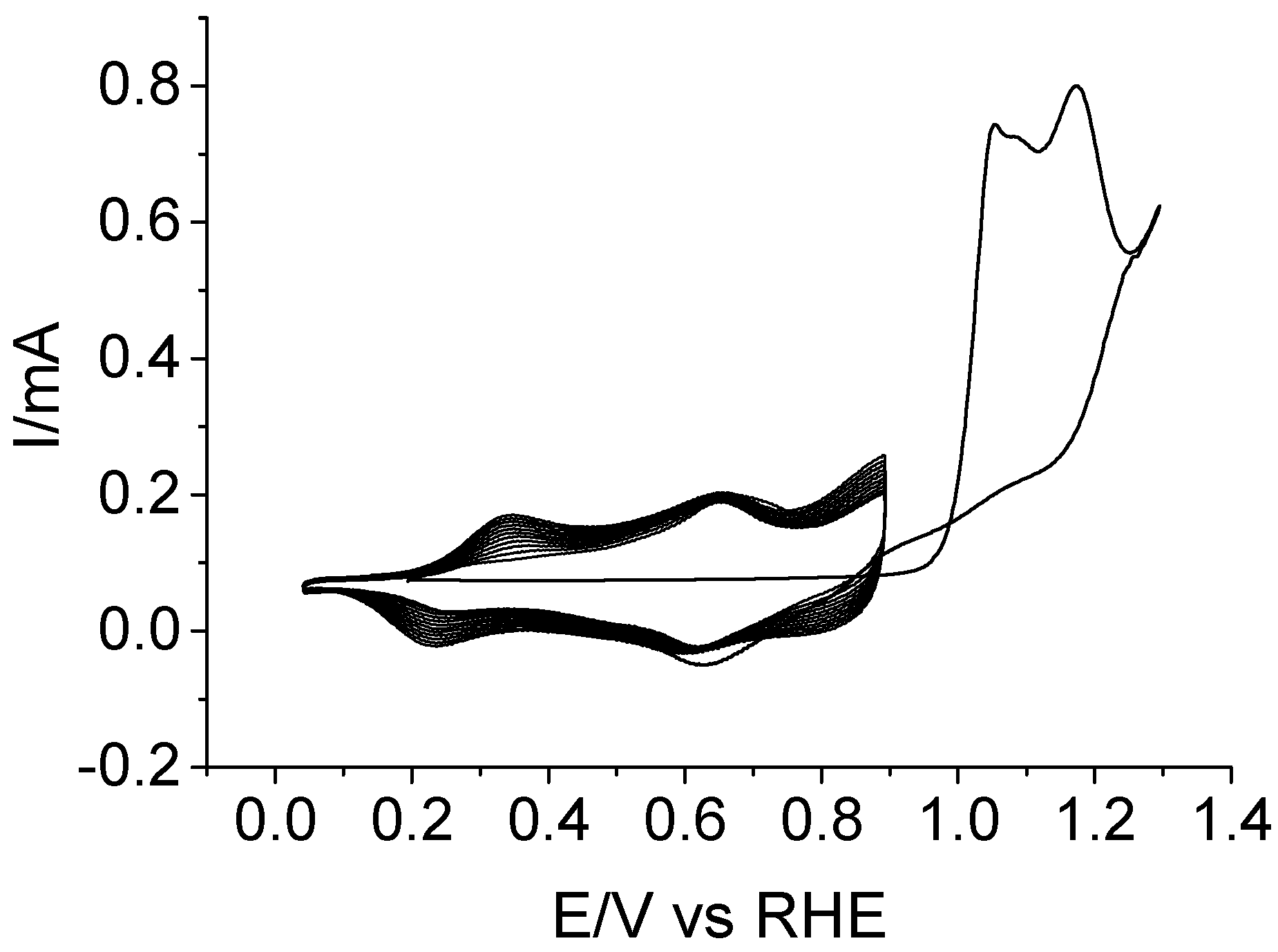
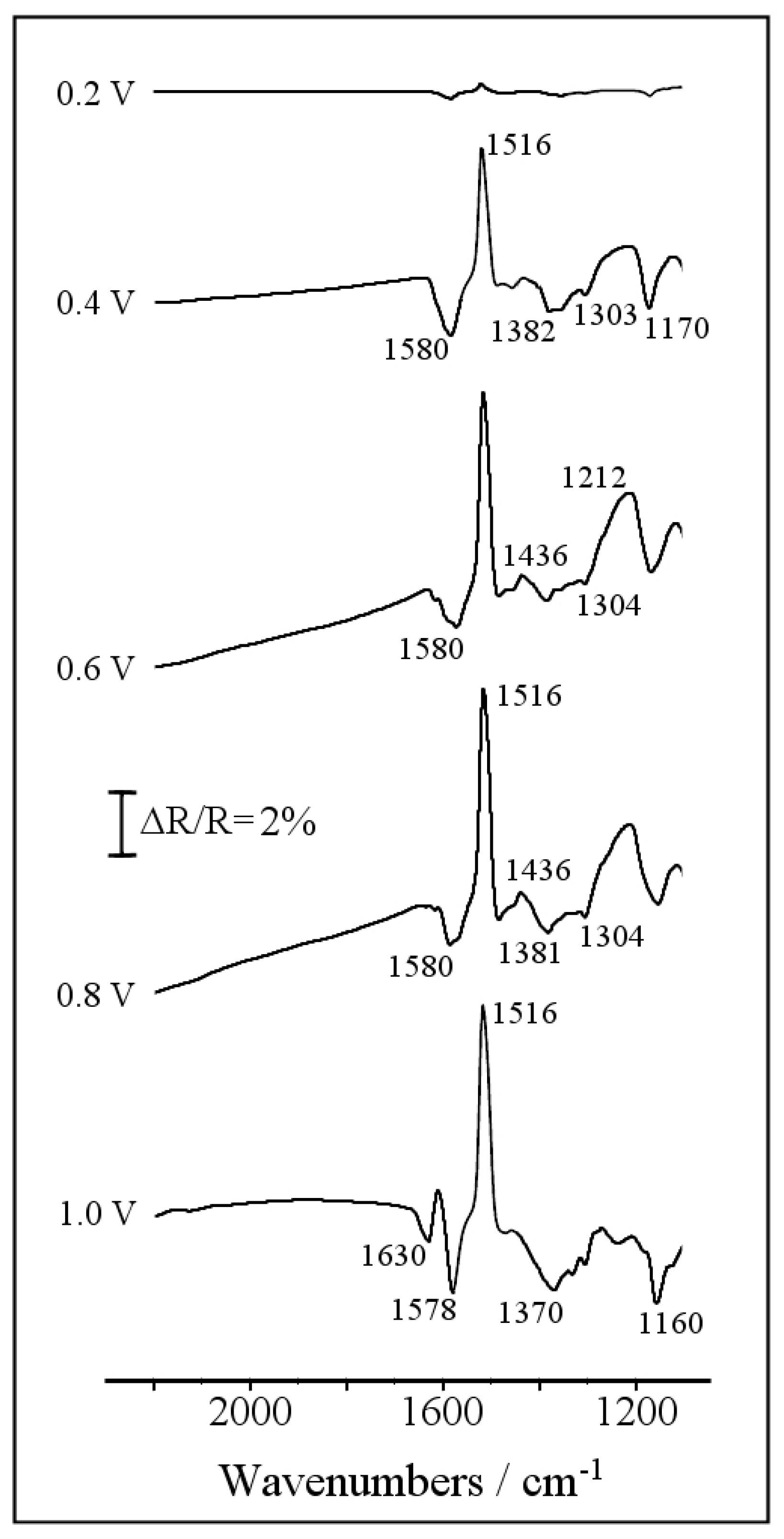
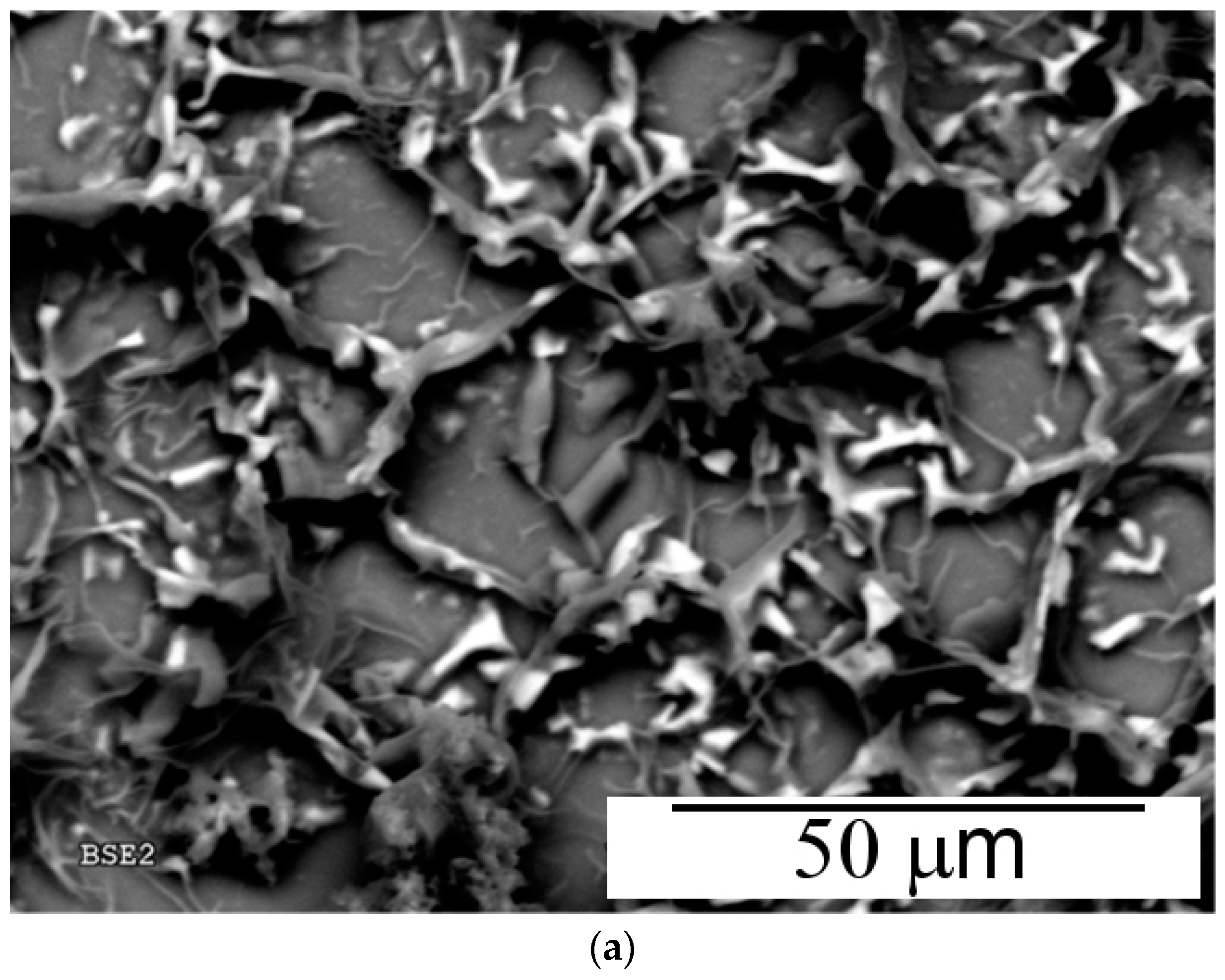
3.2. Electrochemical Copolymerization of Piperazine and Aniline
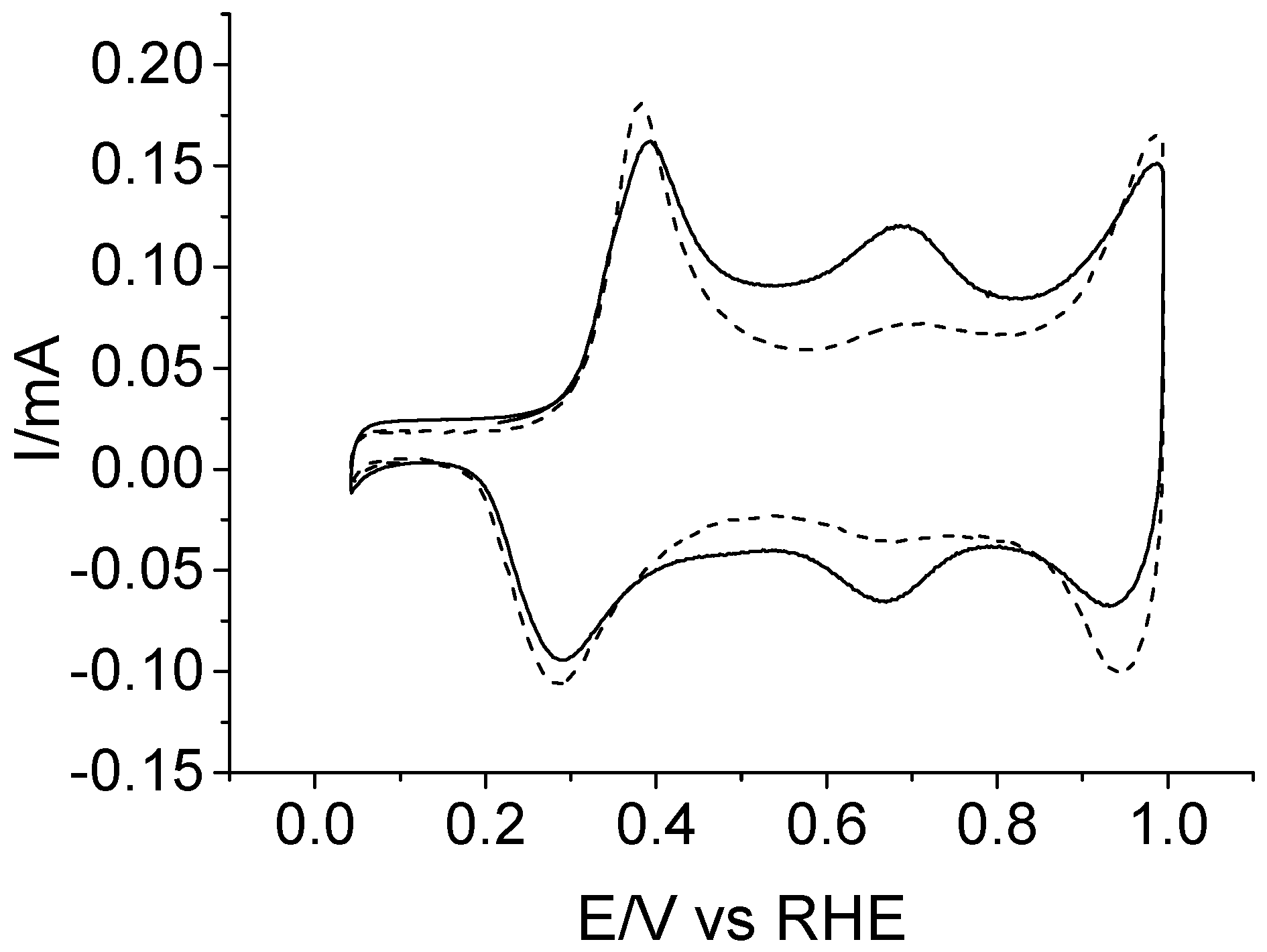
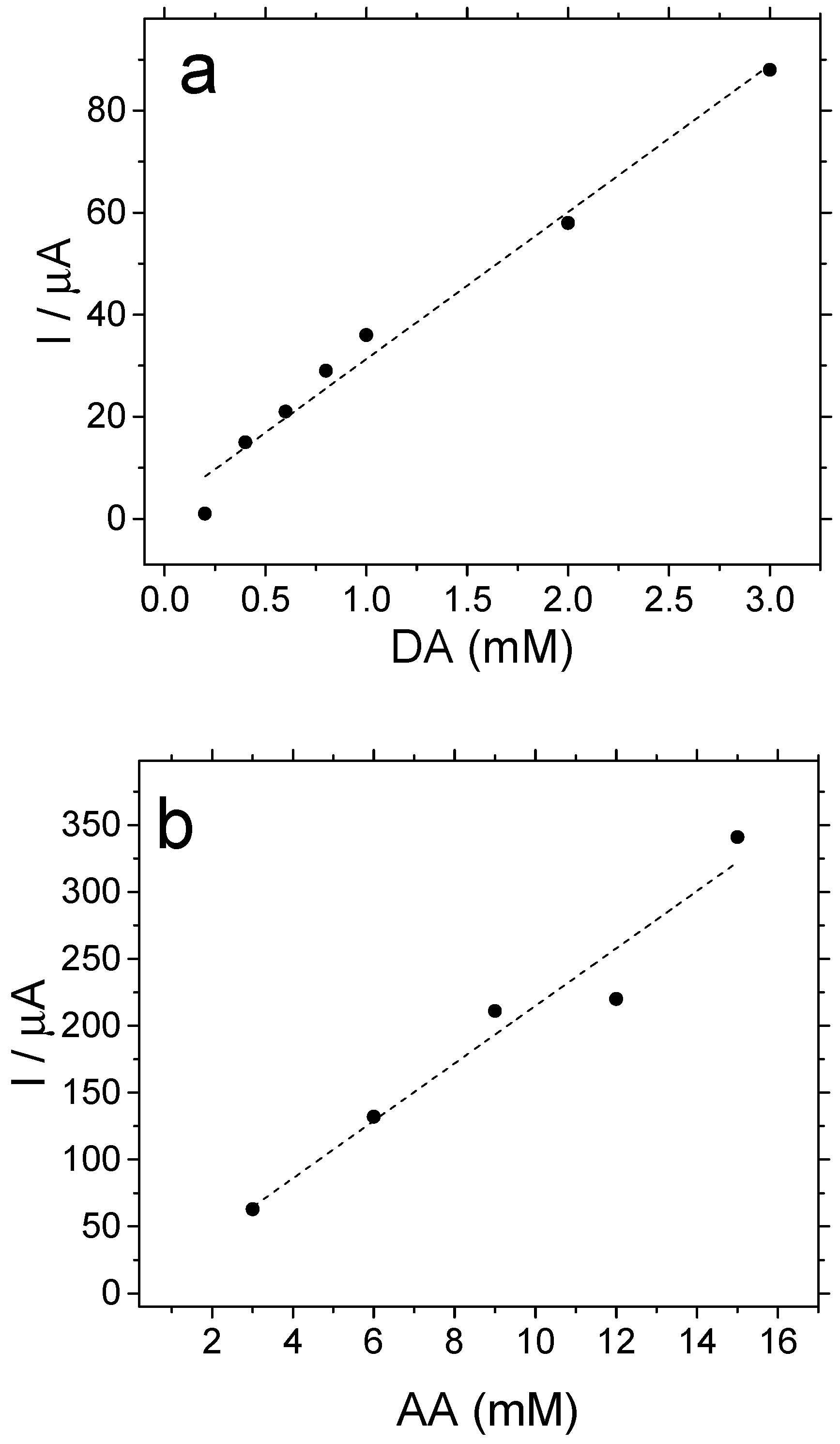
3.3. Testing the Electrocatalytic Properties of the Copolymer towards Dopamine and Ascorbic Acid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed]
- Janata, J.; Josowicz, M. Conducting polymers in electronic chemical sensors. Nat. Mater. 2003, 2, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Otero, T.F. Biomimetic Conducting Polymers: Synthesis, Materials, Properties, Functions, and Devices. Polym. Rev. 2013, 53, 311–351. [Google Scholar] [CrossRef] [Green Version]
- Joulazadeh, M.; Navarchian, A.H. Ammonia detection of one-dimensional nano-structured polypyrrole/metal oxide nanocomposites sensors. Synth. Met. 2015, 210, 404–411. [Google Scholar] [CrossRef]
- Le, T.-H.; Kim, Y.; Yoon, H. Electrical and Electrochemical Properties of Conducting Polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef]
- Yoon, H. Current Trends in Sensors Based on Conducting Polymer Nanomaterials. Nanomaterials 2013, 3, 524–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerard, M.; Chaubey, A.; Malhotra, B.D. Application of conducting polymers to biosensors. Biosens. Bioelectron. 2002, 17, 345–359. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, S. Biosensors based on direct electron transfer in redox proteins. Microchim. Acta 2007, 159, 1–17. [Google Scholar] [CrossRef]
- Nöll, T.; Nöll, G. Strategies for “wiring” redox-active proteins to electrodes and applications in biosensors, biofuel cells, and nanotechnology. Chem. Soc. Rev. 2011, 40, 3564–3576. [Google Scholar] [CrossRef] [PubMed]
- López-Bernabeu, S.; Gamero-Quijano, A.; Huerta, F.; Morallón, E.; Montilla, F. Enhancement of the direct electron transfer to encapsulated cytochrome c by electrochemical functionalization with a conducting polymer. J. Electroanal. Chem. 2017, 793, 34–40. [Google Scholar] [CrossRef]
- Gu, D.; Yang, G.; He, Y.; Qi, B.; Wang, G.; Su, Z. Triphenylamine-based pH chemosensor: Synthesis, crystal structure, photophysical properties and computational studies. Synth. Met. 2009, 159, 2497–2501. [Google Scholar] [CrossRef]
- Li, S.; Ge, Y.; Piletsky, S.A.; Lunec, J. (Eds.) Molecularly Imprinted Sensors: Overview and Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Ghosh, K.; Tarafdar, D.; Samadder, A.; Khuda-Bukhsh, A.R. Piperazine-based simple structure for selective sensing of Hg2+ and glutathione and construction of a logic circuit mimicking an INHIBIT gate. New J. Chem. 2013, 37, 4206–4213. [Google Scholar] [CrossRef]
- Sun, Z.; Li, H.; Guo, D.; Liu, Y.; Tian, Z.; Yan, S. A novel piperazine-bis(rhodamine-B)-based chemosensor for highly sensitive and selective naked-eye detection of Cu2+ and its application as an INHIBIT logic device. J. Lumin. 2015, 167, 156–162. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, X.; Jiang, L.; Liang, C.; Zhu, R.; Li, H. Piperazine-functionalized ordered mesoporous polymer as highly active and reusable organocatalyst for water-medium organic synthesis. Green Chem. 2013, 15, 1665–1672. [Google Scholar] [CrossRef]
- Sachdev, D.; Maheshwari, P.H.; Dubey, A. Piperazine functionalized mesoporous silica for selective and sensitive detection of ascorbic acid. J. Porous Mater. 2016, 23, 123–129. [Google Scholar] [CrossRef]
- Ramachandran, R.; Balasubramanian, S.; Aridoss, G.; Parthiban, P.; Yamuna, G.; Kabilan, S. Synthesis and studies of semiconducting piperazine–aniline copolymer. Eur. Polym. J. 2006, 42, 1885–1892. [Google Scholar] [CrossRef]
- Horányi, G.; Bakos, I. Experimental evidence demonstrating the occurrence of reduction processes of ClO4− ions in an acid medium at platinized platinum electrodes. J. Electroanal. Chem. 1992, 331, 727–737. [Google Scholar] [CrossRef]
- Heacock, R.A.; Marion, L. The infrared spectra of secondary amines and their salts. Can. J. Chem. 1956, 34, 1782–1795. [Google Scholar] [CrossRef]
- Wang, S.L.; Lin, S.Y.; Chen, T.F. Thermal-Dependent dehydration process and intramolecular cyclization of lisinopril dihydrate in the solid state. Chem. Pharm. Bull. 2000, 48, 1890–1893. [Google Scholar] [CrossRef] [PubMed]
- Cheam, T.C.; Krimm, S. Vibrational analysis of crystalline diketopiperazine—I. Raman and i.r. spectra, Spectrochim. Acta Part A Mol. Spectrosc. 1984, 40, 481–501. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons: Chichester, UK, 2004. [Google Scholar]
- Pattar, V.P.; Magdum, P.A.; Patill, D.G.; Nandibewoor, S.T. Thermodynamic, kinetic and mechanistic investigations of Piperazine oxidation by Diperiodatocuprate(III) complex in aqueous alkaline médium. J. Chem. Sci. 2016, 128, 477–485. [Google Scholar] [CrossRef]
- Shim, Y.-B.; Won, M.; Park, S. Electrochemistry of Conductive Polymers VIII. J. Electrochem. Soc. 1990, 137, 538. [Google Scholar] [CrossRef]
- Cotarelo, M.; Huerta, F.; Quijada, C.; Cases, F.; Vázquez, J. The electrocatalytic behaviour of poly(aniline-co-4adpa) thin films in weakly acidic médium. Synth. Met. 2004, 144, 207–211. [Google Scholar] [CrossRef]
- Owens, J.L.; Dryhurst, G. Electrochemical reduction of tetraketopiperazine. Anal. Chim. Acta 1976, 87, 37–50. [Google Scholar] [CrossRef]
- Ping, Z.; Nauer, G.E.; Neugebauer, H.; Theiner, J.; Neckel, A. In situ Fourier transform infrared attenuated total reflection (FTIR-ATR) spectroscopic investigations on the base-acid transitions of leucoemeraldine. Electrochim. Acta 1997, 42, 1693–1700. [Google Scholar] [CrossRef]
- Louarn, G.; Lapkowski, M.; Quillard, S.; Pron, A.; Buisson, J.P.; Lefrant, S. Vibrational properties of polyaniline—Isotope effects. J. Phys. Chem. 1996, 100, 6998–7006. [Google Scholar] [CrossRef]
- Abidi, M.; López-Bernabeu, S.; Huerta, F.; Montilla, F.; Besbes-Hentati, S.; Morallón, E. The chemical and electrochemical oxidative polymerization of 2-amino-4-tert-butylphenol. Electrochim. Acta 2016, 212, 958–965. [Google Scholar] [CrossRef]
- Quillard, S.; Berrada, K.; Louarn, G.; Lefrant, S.; Lapkowski, M.; Pron, A. In situ Raman spectroscopic studies of the electrochemical behavior of polyaniline. New J. Chem. 1995, 19, 365–374. [Google Scholar]
- Hendra, P.J.; Powell, D.B. The infra-red and Raman spectra of piperazine. Spectrochim. Acta 1962, 18, 299–306. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database, Version 4.1 (Web Version), 2012. Available online: http://Srdata.Nist.Gov/Xps/ (accessed on 15 January 2018).
- Langer, J.J. Polyaniline micro- and nanostructure. Adv. Mater. Opt. Electron. 1999, 9, 1–7. [Google Scholar] [CrossRef]
- Yonezawa, S. Effects of the Solvent for the Electropolymerization of Aniline on Discharge and Charge Characteristics of Polyaniline. J. Electrochem. Soc. 1995, 142, 3309. [Google Scholar] [CrossRef]
- Stern, D.A.; Salaita, G.N.; Lu, F.; McCargar, J.W.; Batina, N.; Frank, D.G.; Laguren-Davidson, L.; Lin, C.H.; Walton, N. Studies of L-DOPA and related compounds adsorbed from aqueous solutions at platinum(100) and platinum(111): Electron energy-loss spectroscopy, Auger spectroscopy, and electrochemistry. Langmuir 1988, 4, 711–722. [Google Scholar] [CrossRef]
- Kavanoz, M.; Ülker, E.; Bük, U. A Novel Polyaniline–Poly(3-Methylthiophene)–Poly(3,3′-Diaminobenzidine) Electrode for the Determination of Dopamine in Human Serum. Anal. Lett. 2015, 48, 75–88. [Google Scholar] [CrossRef]
- Březina, M.; Koryta, J.; Loučka, T.; Maršíková, D.; Pradáč, J. Adsorption and kinetics of oxidation of ascorbic acid at platinum electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1972, 40, 13–17. [Google Scholar] [CrossRef]
- Xing, X.; Bae, I.T.; Shao, M.; Liu, C.-C. Electro-oxidation of l-ascorbic acid on platinum in acid solutions: An in-situ FTIRRAS study. J. Electroanal. Chem. 1993, 346, 309–321. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dkhili, S.; López-Bernabeu, S.; Kedir, C.N.; Huerta, F.; Montilla, F.; Besbes-Hentati, S.; Morallon, E. An Electrochemical Study on the Copolymer Formed from Piperazine and Aniline Monomers. Materials 2018, 11, 1012. https://doi.org/10.3390/ma11061012
Dkhili S, López-Bernabeu S, Kedir CN, Huerta F, Montilla F, Besbes-Hentati S, Morallon E. An Electrochemical Study on the Copolymer Formed from Piperazine and Aniline Monomers. Materials. 2018; 11(6):1012. https://doi.org/10.3390/ma11061012
Chicago/Turabian StyleDkhili, Samiha, Sara López-Bernabeu, Chahineze Nawel Kedir, Francisco Huerta, Francisco Montilla, Salma Besbes-Hentati, and Emilia Morallon. 2018. "An Electrochemical Study on the Copolymer Formed from Piperazine and Aniline Monomers" Materials 11, no. 6: 1012. https://doi.org/10.3390/ma11061012