Copolymerization of UF Resins with Dimethylurea for Improving Storage Stability without Impairing Adhesive Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of UF Resins
2.3. Resin Characterization
2.4. 13C NMR Spectroscopy
2.5. Formaldehyde Content of the Resins
2.6. Automated Bonding Evaluation System (ABES)
2.7. Particleboard Production
3. Results
3.1. Incorporation of DMeU at Different Stages
3.2. Effect of DMeU Concentration
3.3. Physico-Mechanical Tests
4. Discussion and Conclusions
- DMeU did not react with the polymer when it was added only at the end of the condensation step.
- About 75% of DMeU added to the resin during condensation reacted with the polymer.
- Incorporation of DMeU in the polymer resulted in a higher percentage of methylene and methylene-ether branched bridges.
- Virtually all DMeU reacted when it was added during condensation.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dunky, M. Urea-Formaldehyde (UF) adhesive resins for wood. Int. J. Adhes. Adhes. 1998, 18, 95–107. [Google Scholar] [CrossRef]
- Christjanson, P.; Siimer, K.; Pehk, T.; Lasn, I. Structural changes in urea-formaldehyde resins during storage. Holz als Roh- und Werkst. 2002, 60, 379–384. [Google Scholar] [CrossRef]
- Kim, M.G.; Wan, H.; No, B.Y.; Nieh, W.L. Examination of selected synthesis and room-temperature storage parameters for wood adhesive-type urea-formaldehyde resins by 13C-NMR spectroscopy. IV. J. Appl. Polym. Sci. 2001, 82, 155–1169. [Google Scholar] [CrossRef]
- Siimer, K.; Kaljuvee, T.; Christjanson, P.; Pehk, T. Changes in curing behaviour of aminoresins during storage. J. Therm. Anal. Calorim. 2005, 80, 123–130. [Google Scholar] [CrossRef]
- Christjanson, P.; Pehk, T.; Siimer, K. Structure formation in urea-formaldehyde resin synthesis. Proc. Estonian Acad. Sci. Chem. 2006, 55, 212–225. [Google Scholar]
- Kim, M.G.; No, B.Y.; Lee, S.M.; Nieh, W.L. Examination of Selected Synthesis and Room-Temperature Storage Parameters for Wood Adhesive-Type Urea-Formaldehyde Resins by 13C-NMR Spectroscopy. V. J. Appl. Polym. Sci. 2003, 89, 1896–1917. [Google Scholar] [CrossRef]
- Li, T.; Guo, X.; Liang, J.; Wang, H.; Xie, X.; Du, G. Competitive formation of the methylene and methylene ether bridges in the urea–formaldehyde reaction in alkaline solution: A combined experimental and theoretical study. Wood Sci. Technol. 2015, 49, 475–493. [Google Scholar] [CrossRef]
- Cao, M.; Li, T.; Liang, J.; Du, G. The Influence of pH on the Melamine-Dimethylurea-Formaldehyde Co-Condensations: A Quantitative 13C-NMR Study. Poymers 2017, 9, 109. [Google Scholar] [CrossRef]
- Ferra, J.M.; Mena, P.C.; Martins, J.; Mendes, A.M.; Costa, M.R.C.; Magalhaes, F.D.; Carvalho, L.H. Optimization of the synthesis of urea-formaldehyde resins using response surface methodology. J. Adhes. Sci. Technol. 2010, 24, 1455–1472. [Google Scholar] [CrossRef]
- CEN-European Committee for Standardization. Adhesives-Determination of Free Formaldehyde in Amino and Amidoformaldehyde Condensates; EN 1243; CEN-CENELEC: Brussels, Switzerland, 2011. [Google Scholar]
- Ferra, J.M.; Ohlmeyer, M.; Mendes, A.M.; Costa, M.R.N.; Carvalho, L.H.; Magalhães, F.D. Evaluation of urea-formaldehyde adhesives performance by recently developed mechanical tests. Int. J. Adhes. Adhes. 2011, 31, 127–134. [Google Scholar] [CrossRef]
- Costa, N.A.; Pereira, J.; Ferra, J.; Cruiz, J.; Martins, J.; Magalhaes, F.D.; Mendes, A.; Carvalho, L.H. Evaluation of Bonding Performance of Amino Polymers Using ABES. J. Adhes. 2014, 90, 80–88. [Google Scholar] [CrossRef]
- CEN-European Committee for Standardization. Wood-Based Panels—Determination of Density; EN 323; CEN-CENELEC: Brussels, Switzerland, 1993. [Google Scholar]
- CEN-European Committee for Standardization. Wood-Based Panels—Determination of Moisture Content; EN 322; CEN-CENELEC: Brussels, Switzerland, 1993. [Google Scholar]
- CEN-European Committee for Standardization. Particleboards and Fibreboards—Determination of Tensile Strength Perpendicular to the Plane of the Board; EN 319; CEN-CENELEC: Brussels, Switzerland, 1993. [Google Scholar]
- CEN-European Committee for Standardization. Particleboards and Fibreboards—Determination of Swelling in Thickness after Immersion in Water; EN 317; CEN-CENELEC: Brussels, Switzerland, 1993. [Google Scholar]
- CEN-European Committee for Standardization. Wood-Based Panels-Determination of Formaldehyde Release—Part 5: Extraction Method (Called the Perforator Method) (ISO 12460-5:2015); EN ISO 12460-5; CEN-CENELEC: Brussels, Switzerland, 2015. [Google Scholar]
- Li, T.; Liang, J.; Cao, M.; Guo, X.; Xie, X.; Du, G. Re-elucidation of the acid-catalyzed urea-formaldehyde reactions: A theoretical and 13C-NMR study. J. Appl. Polym. Sci. 2016, 133, 1097–4628. [Google Scholar] [CrossRef]
- Carvalho, L.M.H.; Costa, R.P.F.N.; Costa, C.A.V. A Very Simple Empirical Kinetic Model of the Acid-Catalyzed Cure of Urea-Formaldehyde Resins. J. Appl. Polym. Sci. 2006, 102, 5977–5987. [Google Scholar] [CrossRef]
- Steinhof, O.; Scherr, G.; Hasse, H. Investigation of the reaction of 1, 3-dimethylurea with formaldehyde by quantitative on-line NMR spectroscopy: A model for the urea-ormaldehyde system. MagnResonChem 2016, 54, 457–476. [Google Scholar] [CrossRef] [PubMed]
- CEN-European Committee for Standardization. Wood-Based Panels for Use in Construction-Characteristics, Evaluation of Conformity and Marking; EN 13986+A1; CEN-CENELEC: Brussels, Switzerland, 2004. [Google Scholar]





| Structure | Chemical Shift (ppm) | Relative Peak Area for Resin IC (%) | Relative Peak Area for Resin AC (%) |
|---|---|---|---|
| Methyl groups | |||
| NH(CH3)–CO–NH(CH3) (1) | 26.38 | - | 2.5 |
| NH(CH3)–CO–N(CH3)– (2) | 26.98 | 3.9 | 3.1 |
| HO–N(CH3)–CO–NH(CH3) (3) | 32.82 | 1.1 | 2.7 |
| –N(CH3)–CO–NH(CH3) (4) | 33.41 | 3.0 | - |
| Methylene groups | |||
| –NH–CH2–NH– (5) | 45–46 | 6.4 | 7.0 |
| –NH–CH2–N= (6) | 51–53 | 10.5 | 9.0 |
| –NH–CH2–N(CH3)– (7) | 53–54 | 4.1 | - |
| Hydroxymethyl groups | |||
| –NH–CH2–OH (8) | 63–64 | 11.9 | 14.3 |
| =N–CH2–OH (9) | 68–70 | 8.4 | 7.4 |
| Methylene-ether groups | |||
| –NH–CH2–O–CH2–NH– (10) | 67–68 | 7.7 | 9.7 |
| =N–CH2–O–CH2–NH– (11) | 72 | 3.4 | 0.8 |
| Formaldehyde | |||
| HO–CH2–OH | 82 | 1.6 | 1.1 |
| Carbonyl groups | |||
| H2N–CO–NH2 | - | - | - |
| H2N–CO–NH– | 158–159 | 21.7 | 21.3 |
| =N–CO–NH–; –HN–CO–NH– | 157–158 | 19.2 | 17.3 |
| NH(CH3)–CO–NH(CH3) | 159.5 | - | 1.4 |
| Resins | Reference | 1.25% | 2.5% | 5% |
|---|---|---|---|---|
| Gel time (s) | 54 | 59 | 59 | 67 |
| Storage Time/Properties | Fresh | 1 Month | 2 Months | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DMeU % | REF | 1.25% | 2.5% | 5% | 1.25% | 2.5% | 5% | 1.25% | 2.5% | 5% |
| Density (kg/m3) | 667 ± 6 | 661 ± 8 | 660 ± 9 | 679 ± 7 | 656 ± 7 | 650 ± 8 | 645 ± 6 | 650 ± 7 | 670 ± 4 | 651 ± 8 |
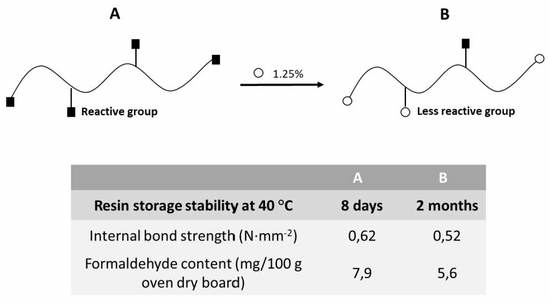
| Internal bond strength (N∙mm−2) | 0.62 ± 0.01 | 0.54 ± 0.02 | 0.50 ± 0.02 | 0.51 ± 0.06 | 0.53 ± 0.05 | 0.52 ± 0.02 | 0.47 ± 0.04 | 0.52 ± 0.04 | 0.52 ± 0.06 | 0.43 ± 0.05 |
| Thickness swelling (%) | 43.7 ± 0.9 | 37.1 ± 1.1 | 37.1 ± 1.1 | 42.7 ± 3.3 | 35.4 ± 2.1 | 32.8 ± 0.8 | 39.8 ± 1.1 | 36.7 ± 1.3 | 36.7 ± 1.8 | 37.6 ± 3.1 |
| Moisture content (%) | 6.5 ± 0.5 | 7.1 ±.5 | 6.8 ± 0.2 | 6.5 ± 0.5 | 5.5 ± 0.1 | 5.4 ± 0.1 | 5.6 ± 0.1 | 6.4 ± 0.1 | 7.5 ± 0.2 | 6.4 ± 0.4 |
| Formaldehyde content (mg/100 g oven dry board) | 7.9 | 6.9 | 6.5 | 5.9 | - | - | - | 5.6 | 5.5 | 5.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, P.; Pereira, J.; Paiva, N.T.; Ferra, J.M.; Martins, J.M.; Carvalho, L.H.; Magalhães, F.D. Copolymerization of UF Resins with Dimethylurea for Improving Storage Stability without Impairing Adhesive Performance. Materials 2018, 11, 1032. https://doi.org/10.3390/ma11061032
Pereira P, Pereira J, Paiva NT, Ferra JM, Martins JM, Carvalho LH, Magalhães FD. Copolymerization of UF Resins with Dimethylurea for Improving Storage Stability without Impairing Adhesive Performance. Materials. 2018; 11(6):1032. https://doi.org/10.3390/ma11061032
Chicago/Turabian StylePereira, Pedro, João Pereira, Nádia. T. Paiva, João. M. Ferra, Jorge M. Martins, Luísa. H. Carvalho, and Fernão. D. Magalhães. 2018. "Copolymerization of UF Resins with Dimethylurea for Improving Storage Stability without Impairing Adhesive Performance" Materials 11, no. 6: 1032. https://doi.org/10.3390/ma11061032