Synthesis and Physical Property Characterisation of Spheroidal and Cuboidal Nuclear Waste Simulant Dispersions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Formation Tracking
2.2. Chemical Composition
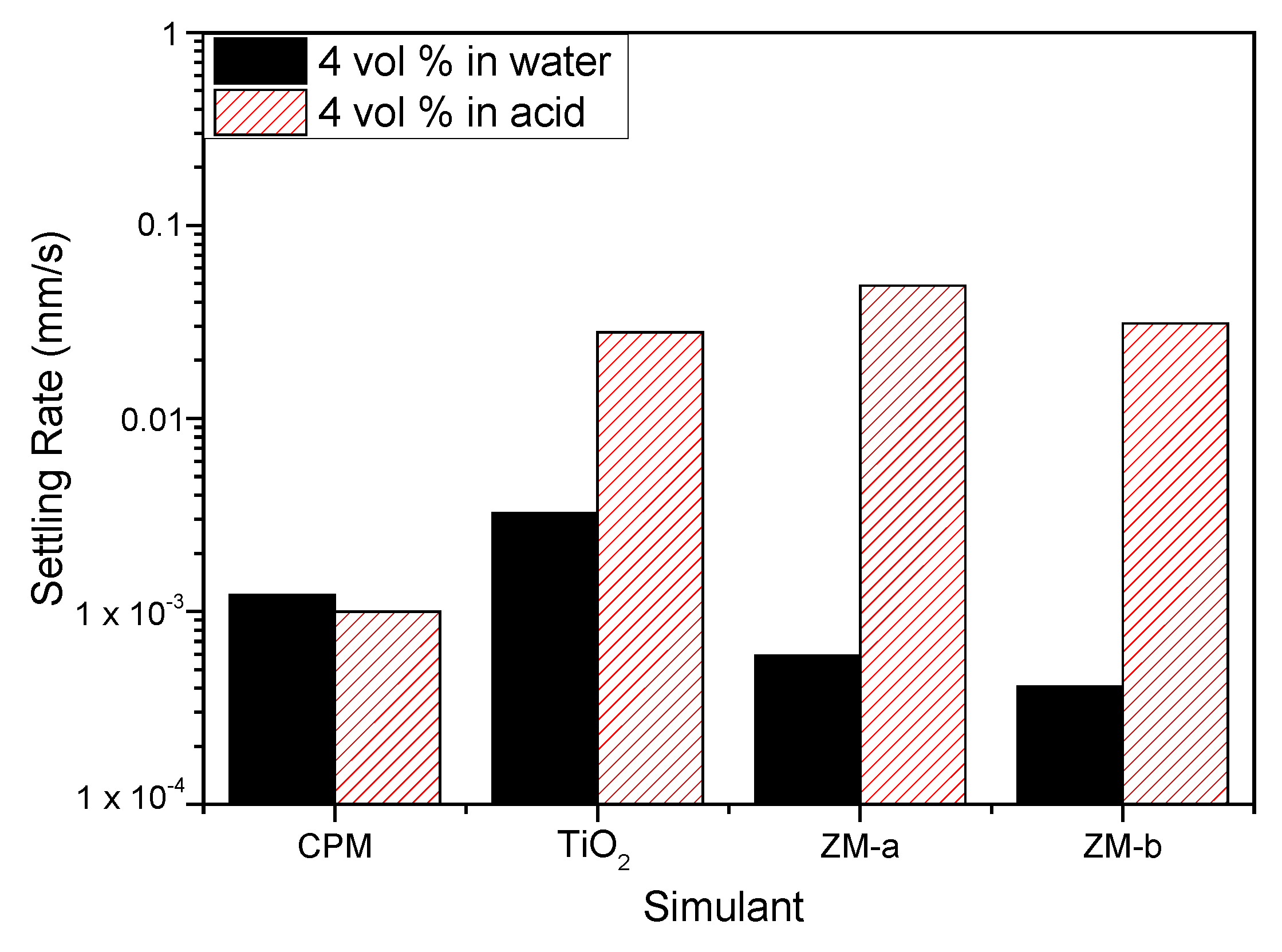
2.3. Size, Stability and Settling Behaviour
3. Materials and Methods
3.1. Synthesis and Materials
3.2. Ultraviolet-Visible Spectroscopy
3.3. Particle Shape, Density and Size Characterisation
3.4. Power X-ray Diffraction, Infrared Spectroscopy and Thermogravimetric Analysis
3.5. Zeta Potential Measurements and Sedimentation Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Edmondson, M.; Maxwell, L.; Ward, T.R. A methodology for POCO of a highly active facility including solids behaviour. In Waste Management; Waste Management Symposium: Phonenix, AZ, USA, 2012. [Google Scholar]
- Harrison, M.T.; Brown, G.C. Chemical durability of UK vitrified high level waste in Si-saturated solutions. Mater. Lett. 2018, 221, 154–156. [Google Scholar] [CrossRef]
- Dobson, A.J.; Phillips, C. High level waste processing in the U.K.—Hard won experience that can benefit U.S. Nuclear cleanup work. In Waste Management; Waste Management Symposium: Tucson, AZ, USA, 2006. [Google Scholar]
- Evans, J.V.; Moore, W.; Shying, M.E.; Sodeau, J.M. Zirconium molybdate gel as a generator for technetium-99m. I. The concept and its evaluation. Appl. Radiat. Isot. 1987, 38, 19–23. [Google Scholar] [CrossRef]
- Monroy-Guzmán, F.; Dı́az-Archundia, L.V.; Contreras Ramı́rez, A. Effect of Zr:Mo ratio on 99mTc generator performance based on zirconium molybdate gels. Appl. Radiat. Isot. 2003, 59, 27–34. [Google Scholar] [CrossRef]
- Monroy-Guzman, F.; Díaz-Archundia, L.V.; Hernández-Cortés, S. 99Mo/99mTc generators performances prepared from zirconium molybate gels. J. Braz. Chem. Soc. 2008, 19, 380–388. [Google Scholar] [CrossRef] [Green Version]
- Monroy-Guzman, F.; Rivero Gutierrez, T.; Lopez Malpica, I.Z.; Hernandez Cortes, S.; Rojas Nava, P.; Vazquez Maldonado, J.C.; Vazquez, A. Production optimization of 99Mo/99mTc zirconium molybate gel generators at semi-automatic device: Disigeg. Appl. Radiat. Isot. 2011, 70, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Lind, C.; Wilkinson, A.P.; Rawn, C.J.; Payzant, E.A. Preparation of the negative thermal expansion material cubic ZrMo2O8. J. Mater. Chem. 2001, 11, 3354–3359. [Google Scholar] [CrossRef]
- Varga, T.; Wilkinson, A.P.; Lind, C.; Bassett, W.A.; Zha, C.-S. Pressure-induced amorphization of cubic ZrMo2O8 studied in situ by x-ray absorption spectroscopy and diffraction. Solid State Commun. 2005, 135, 739–744. [Google Scholar] [CrossRef]
- Rao, B.S.M.; Gantner, E.; Muller, H.G.; Reinhardt, J.; Steinert, D.; Ache, H.J. Solids formation from synthetic fuel reprocessing solutions charaterisation of zirconium molybdate. Appl. Spectrosc. 1986, 40, 330–336. [Google Scholar] [CrossRef]
- Rao, B.S.M.; Gantner, E.; Reinhardt, J.; Steinert, D.; Ache, H.J. Characterization of the solids formed from simulated nuclear fuel reprocessing solutions. J. Nucl. Mater. 1990, 170, 39–49. [Google Scholar] [CrossRef]
- Doucet, F.J.; Goddard, D.T.; Taylor, C.M.; Denniss, I.S.; Hutchison, S.M.; Bryan, N.D. The formation of hydrated zirconium molybdate in simulated spent nuclear fuel reprocessing solutions. Phys. Chem. Chem. Phys. 2002, 4, 3491–3499. [Google Scholar] [CrossRef]
- Magnaldo, A.; Noire, M.H.; Esbelin, E.; Dancausse, J.P.; Picart, S. Zirconium molybdate hydrate precipitates in spent nuclear fuel reprocessing. In Proceedings of the ATALANTE Conference on Nuclear Chemistry for Sustainable Fuel Cycles, Nimes, France, 5–10 June 2004; pp. 1–4. [Google Scholar]
- Magnaldo, A.; Masson, M.; Champion, R. Nucleation and crystal growth of zirconium molybdate hydrate in nitric acid. Chem. Eng. Sci. 2007, 62, 766–774. [Google Scholar] [CrossRef]
- Usami, T.; Tsukada, T.; Inoue, T.; Moriya, N.; Hamada, T.; Serrano Purroy, D.; Malmbeck, R.; Glatz, J.P. Formation of zirconium molybdate sludge from an irradiated fuel and its dissolution into mixture of nitric acid and hydrogen peroxide. J. Nucl. Mater. 2010, 402, 130–135. [Google Scholar] [CrossRef]
- Vereshchagina, T.A.; Fomenko, E.V.; Vasilieva, N.G.; Solovyov, L.A.; Vereshchagin, S.N.; Bazarova, Z.G.; Anshits, A.G. A novel layered zirconium molybdate as a precursor to a ceramic zirconomolybdate host for lanthanide bearing radioactive waste. J. Mater. Chem. 2011, 21, 12001–12007. [Google Scholar] [CrossRef]
- Zhang, L.; Takeuchi, M.; Koizumi, T.; Hirasawa, I. Evaluation of precipitation behavior of zirconium molybdate hydrate. Front. Chem. Sci. Eng. 2013, 7, 65–71. [Google Scholar] [CrossRef]
- Liu, X.; Chen, J.; Zhang, Y.; Wang, J. Precipitation of zirconium and molybdenum in simulated high-level liquid waste concentration and denitration process. Procedia Chem. 2012, 7, 575–580. [Google Scholar]
- Arai, T.; Ito, D.; Hirasawa, I.; Miyazaki, Y.; Takeuchi, M. Encrustation prevention of zirconium molybdate hydrate by changing temperature, nitric acid, or solution concentration. Chem. Eng. Technol. 2018, 41, 1199–1204. [Google Scholar] [CrossRef]
- Izumida, T.; Kawamura, F. Precipitates formation behavior in simulated high level liquid waste of fuel reprocessing. J. Nucl. Sci. Technol. 1990, 27, 267–274. [Google Scholar] [CrossRef]
- Paul, N.; Hammond, R.B.; Hunter, T.N.; Edmondson, M.; Maxwell, L.; Biggs, S. Synthesis of nuclear waste simulants by reaction precipitation: Formation of caesium phosphomolybdate, zirconium molybdate and morphology modification with citratomolybdate complex. Polyhedron 2015, 89, 129–141. [Google Scholar] [CrossRef]
- Paul, N.; Biggs, S.; Shiels, J.; Hammond, R.B.; Edmondson, M.; Maxwell, L.; Harbottle, D.; Hunter, T.N. Influence of shape and surface charge on the sedimentation of spheroidal, cubic and rectangular cuboid particles. Powder Technol. 2017, 322, 75–83. [Google Scholar] [CrossRef]
- Paul, N.; Biggs, S.; Edmondson, M.; Hunter, T.N.; Hammond, R.B. Characterising highly active nuclear waste simulants. Chem. Eng. Res. Des. 2013, 91, 742–751. [Google Scholar] [CrossRef]
- Clearfield, A.; Blessing, R.H. The preparation and crystal structure of a basic zirconium molybdate and its relationship to ion exchange gels. J. Inorg. Nucl. Chem. 1972, 34, 2643–2663. [Google Scholar] [CrossRef]
- Krtil, J.; Kouřím, V. Exchange properties of ammonium salts of 12-heteropolyacids. Sorption of caesium on ammonium phosphotungstate and phosphomolybdate. J. Inorg. Nucl. Chem. 1960, 12, 367–369. [Google Scholar] [CrossRef]
- Lento, J.; Harjula, R. Separation of cesium from nuclear waste solutions with hexacyanoferrate(ii)s and ammonium phosphomolybdate. Solvent Extr. Ion Exch. 1987, 5, 343–352. [Google Scholar] [CrossRef]
- Bykhovskii, D.N.; Kol’tsova, T.I.; Kuz’mina, M.A. Phases of variable composition in crystallization of cesium phosphomolybdate. Radiochemistry 2006, 48, 429–433. [Google Scholar] [CrossRef]
- Bykhovskii, D.N.; Kol’tsova, T.I.; Roshchinskaya, E.M. Cesium preconcentration by recovery from solutions in the form of phosphomolybdate. Radiochemistry 2009, 51, 159–164. [Google Scholar] [CrossRef]
- Bykhovskii, D.N.; Kol’tsova, T.I.; Roshchinskaya, E.M. Reduction of radioactive waste volume using selective crystallization processes. Radiochemistry 2010, 52, 530–536. [Google Scholar] [CrossRef]
- Rezaei Ghalebi, H.; Aber, S.; Karimi, A. Keggin type of cesium phosphomolybdate synthesized via solid-state reaction as an efficient catalyst for the photodegradation of a dye pollutant in aqueous phase. J. Mol. Catal. A Chem. 2016, 415, 96–103. [Google Scholar] [CrossRef]
- Bradley, D.F.; Quayle, M.J.; Ross, E.; Ward, T.R.; Watson, N. Promoting the conversion of caesium phosphomolybdate to zirconium molybdate. In Proceedings of the ATALANTE Conference on Nuclear Chemistry for Sustainable Fuel Cycles, Nimes, France, 5–10 June 2004. [Google Scholar]
- Jiang, J.; May, I.; Sarsfield, M.J.; Ogden, M.; Fox, D.O.; Jones, C.J.; Mayhew, P. A spectroscopic study of the dissolution of cesium phosphomolybdate and zirconium molybdate by ammonium carbamate. J. Solut. Chem. 2005, 34, 443–468. [Google Scholar] [CrossRef]
- Dunnett, B.; Ward, T.; Roberts, R.; Cheesewright, J. Physical properties of highly active liquor containing molybdate solids. In Proceedings of the ATALANTE Conference on Nuclear Chemistry for Sustainable Fuel Cycles, Montpellier, France, 5–10 June 2016. [Google Scholar]
- Biggs, S.; Fairweather, M.; Hunter, T.; Peakall, J.; Omokanye, Q. Engineering properties of nuclear waste slurries. In Proceedings of the ASME 2009 12th International Conference on Environmental Remediation and Radioactive Waste Management, Liverpool, UK, 11–15 October 2009. [Google Scholar]
- Mandzy, N.; Grulke, E.; Druffel, T. Breakage of TiO2 agglomerates in electrostatically stabilized aqueous dispersions. Powder Technol. 2005, 160, 121–126. [Google Scholar] [CrossRef]
- Liao, D.L.; Wu, G.S.; Liao, B.Q. Zeta potential of shape-controlled TiO2 nanoparticles with surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2009, 348, 270–275. [Google Scholar] [CrossRef]
- Richardson, J.F.; Zaki, W.N. The sedimentation of a suspension of uniform spheres under conditions of viscous flow. Chem. Eng. Sci. 1954, 3, 65–73. [Google Scholar] [CrossRef]
- Bargieł, M.; Tory, E.M. Extension of the richardson–zaki equation to suspensions of multisized irregular particles. Int. J. Miner. Process. 2013, 120, 22–25. [Google Scholar] [CrossRef]
- Turney, M.A.; Cheung, M.K.; Powell, R.L.; McCarthy, M.J. Hindered settling of rod-like particles measured with magnetic resonance imaging. AIChE J. 1995, 41, 251–257. [Google Scholar] [CrossRef]
- Chong, Y.S.; Ratkowsky, D.A.; Epstein, N. Effect of particle shape on hindered settling in creeping flow. Powder Technol. 1979, 55–66. [Google Scholar] [CrossRef]
- Lau, R.; Chuah, H.K.L. Dynamic shape factor for particles of various shapes in the intermediate settling regime. Adv. Powder Technol. 2013, 24, 306–310. [Google Scholar] [CrossRef]
- Tomkins, M.R.; Baldock, T.E.; Nielsen, P. Hindered settling of sand grains. Sedimentology 2005, 52, 1425–1432. [Google Scholar] [CrossRef]
- Loth, E. Drag of non-spherical solid particles of regular and irregular shape. Powder Technol. 2008, 182, 342–353. [Google Scholar] [CrossRef]
- Dogonchi, A.S.; Hatami, M.; Hosseinzadeh, K.; Domairry, G. Non-spherical particles sedimentation in an incompressible Newtonian medium by Padé approximation. Powder Technol. 2015, 278, 248–256. [Google Scholar] [CrossRef]
- Lerche, D.; Sobisch, T. Direct and accelerated characterization of formulation stability. J. Dispers. Sci. Technol. 2011, 32, 1799–1811. [Google Scholar] [CrossRef]









| Material | Formula | Purity | Supplier |
|---|---|---|---|
| Phosphomolybdic acid hydrate Caesium nitrate Nitric acid Zirconyl nitrate | H3PMo12O40 CsNO3 HNO3 ZrO(NO3)2 | Solid—80% Solid—99.9% Solution—70% Solution—35 wt. % in dilute HNO3 | Acros Organics Aldrich Fisher Scientific Sigma-Aldrich |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiels, J.; Harbottle, D.; Hunter, T.N. Synthesis and Physical Property Characterisation of Spheroidal and Cuboidal Nuclear Waste Simulant Dispersions. Materials 2018, 11, 1235. https://doi.org/10.3390/ma11071235
Shiels J, Harbottle D, Hunter TN. Synthesis and Physical Property Characterisation of Spheroidal and Cuboidal Nuclear Waste Simulant Dispersions. Materials. 2018; 11(7):1235. https://doi.org/10.3390/ma11071235
Chicago/Turabian StyleShiels, Jessica, David Harbottle, and Timothy N. Hunter. 2018. "Synthesis and Physical Property Characterisation of Spheroidal and Cuboidal Nuclear Waste Simulant Dispersions" Materials 11, no. 7: 1235. https://doi.org/10.3390/ma11071235





