Synthesis and Properties of Nanosized Stoichiometric Cobalt Ferrite Spinel
Abstract
:1. Introduction
2. Materials and Methods
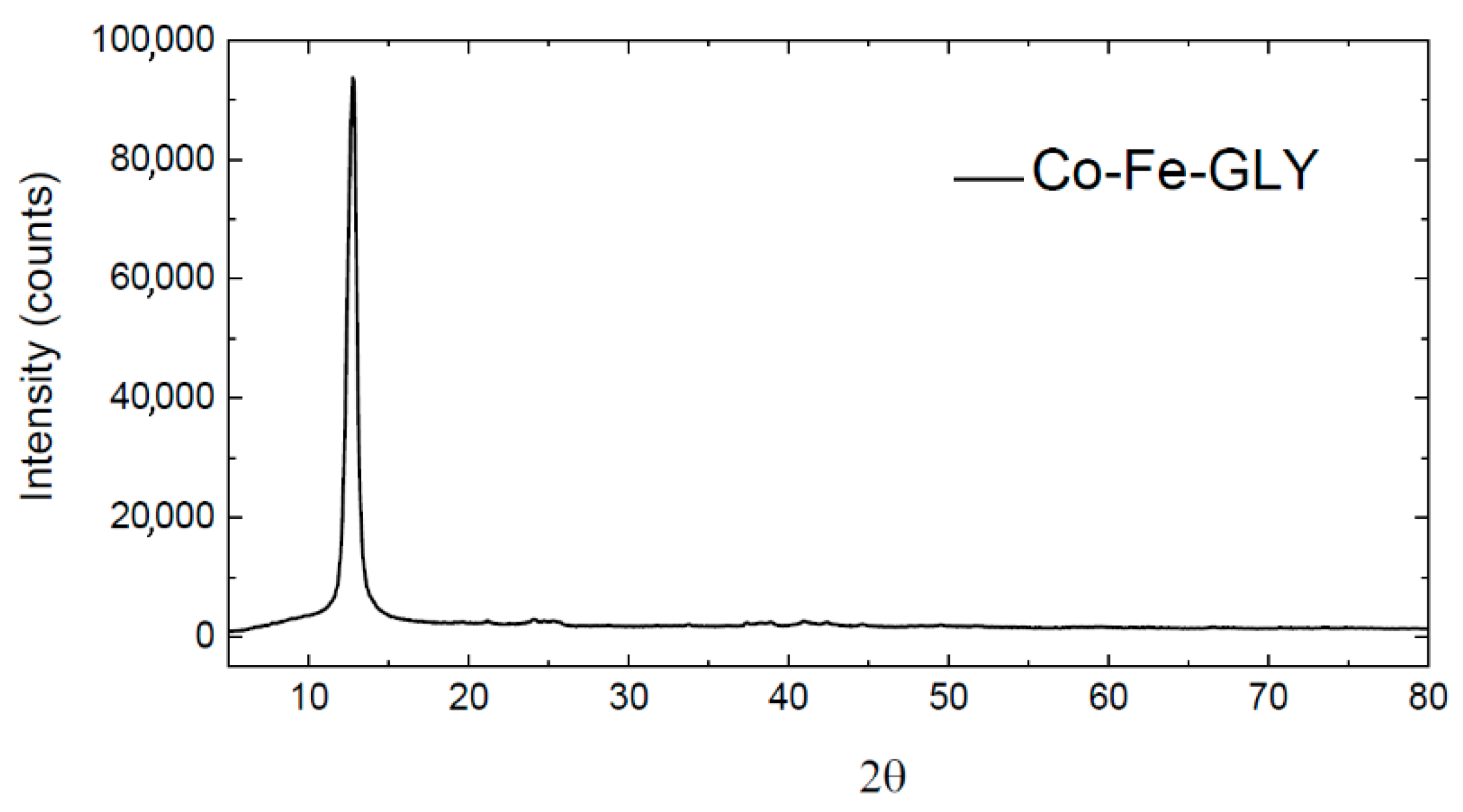
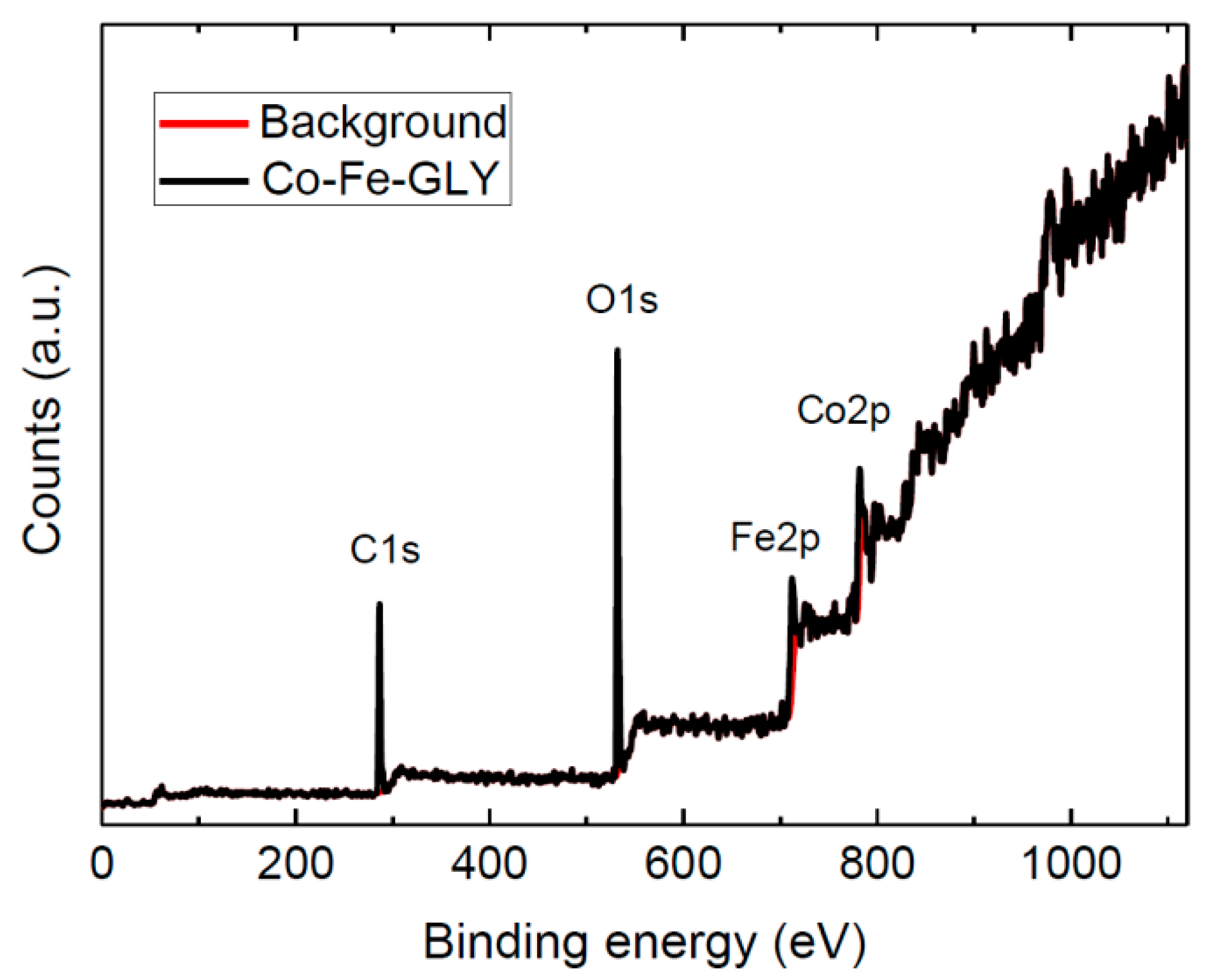
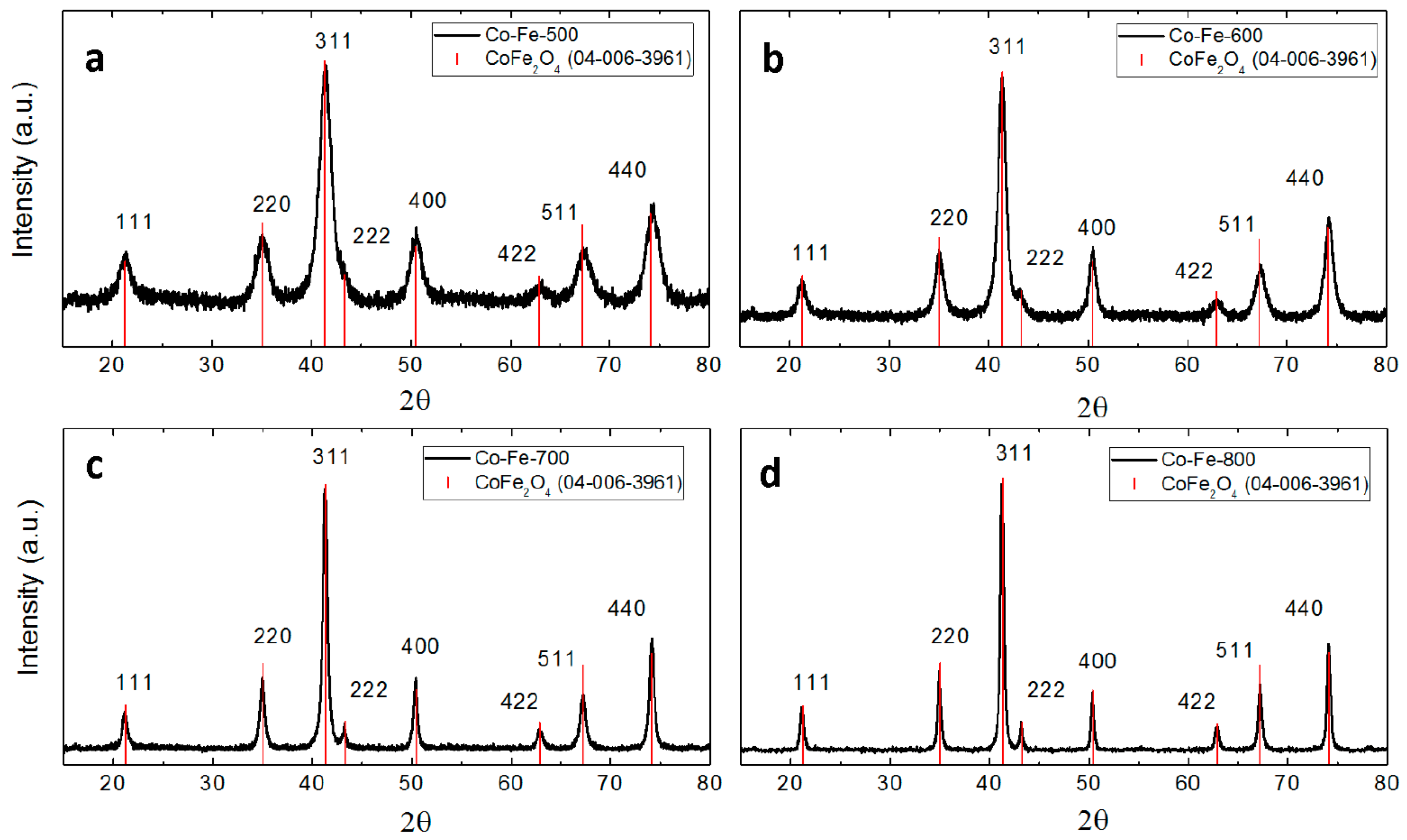
3. Results and Discussion
4. Conclusions
Author Contributions
Conflicts of Interest
References
- He, S.H.; Shi, W.B.; Zhang, X.D.; Li, J.A.; Huang, Y.M. Beta-cyclodextrins-based inclusion complexes of CoFe2O4 magnetic nanoparticles as catalyst for the luminol chemiluminescence system and their applications in hydrogen peroxide detection. Talanta 2010, 82, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Rajput, J.K.; Kaur, G. Synthesis and applications of CoFe2O4 nanoparticles for multicomponent reactions. Catal. Sci. Technol. 2014, 4, 142–151. [Google Scholar] [CrossRef]
- Huang, Q.S.; Zhou, P.J.; Yang, H.; Zhu, L.L.; Wu, H.Y. In situ generation of inverse spinel CoFe2O4 nanoparticles onto nitrogen-doped activated carbon for an effective cathode electrocatalyst of microbial fuel cells. Chem. Eng. J. 2017, 325, 466–473. [Google Scholar] [CrossRef]
- Mordina, B.; Tiwari, R.K.; Setua, D.K.; Sharma, A. Superior elastomeric nanocomposites with electrospun nanofibers and nanoparticles of CoFe2O4 for magnetorheological applications. RSC Adv. 2015, 5, 19091–19105. [Google Scholar] [CrossRef]
- Saffari, J.; Ghanbari, D.; Mir, N.; Khandan-Barani, K. Sonochemical synthesis of CoFe2O4 nanoparticles and their application in magnetic polystyrene nanocomposites. J. Ind. Eng. Chem. 2014, 20, 4119–4123. [Google Scholar] [CrossRef]
- Santhosh, C.; Daneshvar, E.; Kollu, P.; Peraniemi, S.; Grace, A.N.; Bhatnagar, A. Magnetic SiO2-CoFe2O4 nanoparticles decorated on graphene oxide as efficient adsorbents for the removal of anionic pollutants from water. Chem. Eng. J. 2017, 322, 472–487. [Google Scholar] [CrossRef]
- Lima-Tenorio, M.K.; Tenorio-Neto, E.T.; Hechenleitner, A.A.W.; Fessi, H.; Pineda, E.A.G. CoFe2O4 and ZnFe2O4 nanoparticles: An overview about structure, properties, synthesis and biomedical applications. J. Colloid Sci. Biotechnol. 2016, 5, 45–54. [Google Scholar] [CrossRef]
- Momin, N.; Deshmukh, A.; Radha, S. Synthesis and characterization of CoFe2O4-NiFe2O4 magnetic nanoparticles for various biomedical applications: cell viability and cell death evaluations. J. Nano Res. 2015, 34, 1–8. [Google Scholar] [CrossRef]
- Munjal, S.; Khare, N.; Nehate, C.; Koul, V. Water dispersible CoFe2O4 nanoparticles with improved colloidal stability for biomedical applications. J. Magn. Magn. Mater. 2016, 404, 166–169. [Google Scholar] [CrossRef]
- Prabhakaran, T.; Mangalaraja, R.V.; Denardin, J.C.; Jimenez, J.A. The effect of calcination temperature on the structural and magnetic properties of co-precipitated CoFe2O4 nanoparticles. J. Alloys Compd. 2017, 716, 171–183. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, N.; Rana, D.S.; Kumar, P.; Arora, M.; Pant, R.P. Structural and magnetic studies of the nickel doped CoFe2O4 ferrite nanoparticles synthesized by the chemical co-precipitation method. J. Magn. Magn. Mater. 2015, 394, 379–384. [Google Scholar] [CrossRef]
- Safi, R.; Ghasemi, A.; Shoja-Razavi, R. Factors controlling magnetic properties of CoFe2O4 nanoparticles synthesized by chemical co-precipitation: Modeling and optimization using response surface methodology. Ceram. Int. 2016, 42, 15818–15825. [Google Scholar] [CrossRef]
- Safi, R.; Ghasemi, A.; Shoja-Razavi, R.; Ghasemi, E.; Sodaee, T. Rietveld structure refinement, cations distribution and magnetic features of CoFe2O4 nanoparticles synthesized by co-precipitation, hydrothermal, and combustion methods. Ceram. Int. 2016, 42, 6375–6382. [Google Scholar] [CrossRef]
- Jalalian, M.; Mirkazemi, S.M.; Alamolhoda, S. Phase constituents and magnetic properties of the CoFe2O4 nanoparticles prepared by polyvinylpyrrolidone (PVP)-assisted hydrothermal route. Appl. Phys. A 2016, 122, 835. [Google Scholar] [CrossRef]
- Liu, M.; Lu, M.; Wang, L.; Xu, S.C.; Zhao, J.L.; Li, H.B. Mossbauer study on the magnetic properties and cation distribution of CoFe2O4 nanoparticles synthesized by hydrothermal method. J. Mater. Sci. 2016, 51, 5487–5492. [Google Scholar] [CrossRef]
- Rafique, M.Y.; Pan, L.Q.; Javed, Q.U.A.; Iqbal, M.Z.; Yang, L.H. Influence of NaBH4 on the size, composition, and magnetic properties of CoFe2O4 nanoparticles synthesized by hydrothermal method. J. Nanopart. Res. 2012, 14, 1189. [Google Scholar] [CrossRef]
- Suwanchawalit, C.; Somjit, V. A facile hydrothermal synthesis of magnetic CoFe2O4 nanoparticles and photocatalytic performance. Dig. J. Nanomater. Biostruct. 2015, 10, 705–713. [Google Scholar]
- Yadav, R.S.; Havlica, J.; Masilko, J.; Kalina, L.; Hajduchova, M.; Enev, V.; Wasserbauer, J.; Kuritka, I.; Kozakova, Z. Structural, cation distribution, and magnetic properties of CoFe2O4 spinel ferrite nanoparticles synthesized using a starch-assisted sol-gel auto-combustion method. J. Supercond. Nov. Magn. 2015, 28, 1851–1861. [Google Scholar] [CrossRef]
- Yadav, R.S.; Havlica, J.; Ptacek, P.; Kuritka, I.; Kozakova, Z.; Palou, M.; Bartonickova, E.; Bohac, M.; Frajkorova, F.; Masilko, J.; et al. Structural and magnetic properties of CoFe2O4 nanoparticles synthesized by starch-assisted sol-gel auto-combustion method in air, argon, nitrogen and vacuum atmospheres. J. Supercond. Nov. Magn. 2015, 28, 249–258. [Google Scholar] [CrossRef]
- Amiri, G.R.; Yousefi, M.H.; Fatahian, S. Magnetic properties of CoFe2O4 and Co0.5 Zn0.5Fe2O4 ferrite nanoparticles synthesized by microwave method. Optoelectron. Adv. Mater.-Rapid Commun. 2012, 6, 158–161. [Google Scholar]
- Bartolome, E.; Cayado, P.; Solana, E.; Ricart, S.; Gazquez, J.; Mundet, B.; Coll, M.; Puig, T.; Obradors, X.; Valvidares, M.; et al. Magnetic stability against calcining of microwave-synthesized CoFe2O4 nanoparticles. New J. Chem. 2016, 40, 6890–6898. [Google Scholar] [CrossRef]
- Bartunek, V.; Huber, S.; Sedmidubsky, D.; Sofer, Z.; Simek, P.; Jankovsky, O. CoO and Co3O4 nanoparticles with a tunable particle size. Ceram. Int. 2014, 40, 12591–12595. [Google Scholar] [CrossRef]
- Bartunek, V.; Prucha, D.; Svecova, M.; Ulbrich, P.; Huber, S.; Sedmidubsky, D.; Jankovsky, O. Ultrafine ferromagnetic iron oxide nanoparticles: Facile synthesis by low temperature decomposition of iron glycerolate. Mater. Chem. Phys. 2016, 180, 272–278. [Google Scholar] [CrossRef]
- Jankovsky, O.; Sedmidubsky, D.; Simek, P.; Sofer, Z.; Ulbrich, P.; Bartunek, V. Synthesis of MnO, Mn2O3 and Mn3O4 nanocrystal clusters by thermal decomposition of manganese glycerolate. Ceram. Int. 2015, 41, 595–601. [Google Scholar] [CrossRef]
- Pinc, J.; Jankovský, O.; Bartůněk, V. Preparation of manganese oxide nanoparticles by thermal decomposition of nanostructured manganese carbonate. Chem. Pap. 2017, 71, 1031–1035. [Google Scholar] [CrossRef]
- Jankovsky, O.; Sedmidubsky, D.; Sofer, Z.; Luxa, J.; Bartunek, V. Simple synthesis of Cr2O3 nanoparticles with a tunable particle size. Ceram. Int. 2015, 41, 4644–4650. [Google Scholar] [CrossRef]
- Jankovský, O.; Rach, V.; Sedmidubský, D.; Huber, Š.; Ulbrich, P.; Švecová, M.; Bartůněk, V. Simple synthesis of free surface nanostructured spinel NiFe2O4 with a tunable particle size. J. Alloys Compd. 2017, 723, 58–63. [Google Scholar] [CrossRef]
- Bartůněk, V.; Huber, S.; Luxa, J.; Sofer, Z.; Kuchař, M.; Dobrovolny, K.; Jankovský, O. Facile synthesis of magnetic Co nanofoam by low-temperature thermal decomposition of Co glycerolate. IET Micro Nano Lett. 2017, 12, 278–280. [Google Scholar] [CrossRef]
- Tudorache, F.; Popa, P.D.; Dobromir, M.; Iacomi, F. Studies on the structure and gas sensing properties of nickel–cobalt ferrite thin films prepared by spin coating. Mater. Sci. Eng. B 2013, 178, 1334–1338. [Google Scholar] [CrossRef]
- Hill, A.H.; Harrison, A.; Dickinson, C.; Zhou, W.; Kockelmann, W. Crystallographic and magnetic studies of mesoporous eskolaite, Cr2O3. Microporous Mesoporous Mater. 2010, 130, 280–286. [Google Scholar] [CrossRef]
- Nica, V.; Daniel, G.; Ursu, C.; Tudorache, F.; Brinza, F.; Pui, A. Synthesis and characterization of Co-substituted ferrite nanocomposites. IEEE Trans. Magn. 2013, 49, 26–29. [Google Scholar] [CrossRef]
- Wang, Z.W.; Downs, R.T.; Pischedda, V.; Shetty, R.; Saxena, S.K.; Zha, C.S.; Zhao, Y.S.; Schiferl, D.; Waskowska, A. High-pressure X-ray diffraction and Raman spectroscopic studies of the tetragonal spinel CoFe2O4. Phys. Rev. B 2003, 68, 094101. [Google Scholar] [CrossRef]
- Chandramohan, P.; Srinivasan, M.; Velmurugan, S.; Narasimhan, S. Cation distribution and particle size effect on Raman spectrum of CoFe2O4. J. Solid State Chem. 2011, 184, 89–96. [Google Scholar] [CrossRef]
- Lazzeri, M.; Thibaudeau, P. Ab initio Raman spectrum of the normal and disordered MgAl2O4 spinel. Phys. Rev. B 2006, 74, 140301. [Google Scholar] [CrossRef]
- Repko, A.; Nižňanský, D.; Poltierová-Vejpravová, J. A study of oleic acid-based hydrothermal preparation of CoFe2O4 nanoparticles. J. Nanopart. Res. 2011, 13, 5021. [Google Scholar] [CrossRef]
- Bozorth, R.M.; Tilden, E.F.; Williams, A.J. Anisotropy and Magnetostriction of Some Ferrites. Phys. Rev. 1955, 99, 1788–1798. [Google Scholar] [CrossRef]








| Raman Shift (cm−1) | Vibrational Mode | Assignment | ||
|---|---|---|---|---|
| Experimental | Wang et al. [32] | Chandramohan et al. [33] | ||
| 1325 | - | - | A1g | 2nd order bands |
| 1135 | - | - | T2g(2) | |
| 692 | 683 | 695 | A1g (1) | symmetric stretching Fe-O |
| 615 | 617 | 624 | A1g (2) | symmetric stretching Fe(Co)-O |
| 553 | 563 | 575 | T2g(2) | asymmetric stretching Fe-O |
| 473 | 471 | 470 | T2g(3) | asymmetric bending Fe(Co)-O |
| 297 | 300 | 312 | Eg | symmetric bending Fe(Co)-O |
| 183 | 188 | 210 | T2g(1) | translation motion of the whole tetrahedron |
| Co-Fe | μ°Hc (300 K) mT | Mr (300 K) μB/f.u. | M7T (300 K) μB/f.u. | μ°Hc (4.5 K) T | Mr (4.5 K) μB/f.u. | M7T (4.5 K) μB/f.u. | TB K |
|---|---|---|---|---|---|---|---|
| 500 | 45 | 0.20 | 2.00 | 1.95 | 1.36 | 2.23 | 340 |
| 600 | 90 | 0.51 | 2.41 | 1.58 | 1.72 | 2.65 | 328 |
| 700 | 138 | 0.81 | 2.89 | 1.28 | 2.13 | 3.15 | 300 |
| 800 | 130 | 1.20 | 3.20 | 0.99 | 2.47 | 3.46 | 300 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartůněk, V.; Sedmidubský, D.; Huber, Š.; Švecová, M.; Ulbrich, P.; Jankovský, O. Synthesis and Properties of Nanosized Stoichiometric Cobalt Ferrite Spinel. Materials 2018, 11, 1241. https://doi.org/10.3390/ma11071241
Bartůněk V, Sedmidubský D, Huber Š, Švecová M, Ulbrich P, Jankovský O. Synthesis and Properties of Nanosized Stoichiometric Cobalt Ferrite Spinel. Materials. 2018; 11(7):1241. https://doi.org/10.3390/ma11071241
Chicago/Turabian StyleBartůněk, Vilém, David Sedmidubský, Štěpán Huber, Marie Švecová, Pavel Ulbrich, and Ondřej Jankovský. 2018. "Synthesis and Properties of Nanosized Stoichiometric Cobalt Ferrite Spinel" Materials 11, no. 7: 1241. https://doi.org/10.3390/ma11071241






