Corrosion Behaviors of Q345R Steel at the Initial Stage in an Oxygen-Containing Aqueous Environment: Experiment and Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimens and Solution
2.2. Electrochemical Measurements
3. Modeling Description
3.1. Cathodic Reactions
3.1.1. Reduction of Oxygen
3.1.2. Reduction of H+
3.1.3. Reduction of Water
3.2. Anodic Reaction
3.3. The Mixed Potential Theory
4. Results and Discussion
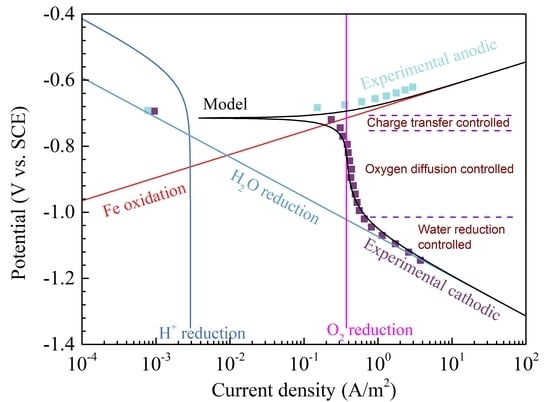
4.1. The Characteristics of the Polarization Curves and Modeling Curves
4.2. Effect of Oxygen Concentration on the Corrosion of Q345R Steel
4.3. Effect of Temperature on the Corrosion of Q345R Steel
4.4. Effect of pH on the Corrosion of Q345R Steel
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Visser, E.; Hendriks, C.; Barrio, M.; Mølnvik, M.J.; Koeijer, G.; Ligemark, S.; Gallo, Y.L. Dynamis CO2 quality recommendations. Int. J. Greenh. Gas Control 2008, 2, 478–484. [Google Scholar] [CrossRef]
- Yan, L.; Peng, Z.; Qiang, L.; Hou, B. Berberine as a natural source inhibitor for mild steel in 1 M H2SO4. Appl. Surf. Sci. 2005, 252, 1245–1253. [Google Scholar] [CrossRef]
- Baylis, J.R. Factors other than dissolved oxygen influencing the corrosion of iron pipes. Ind. Eng. Chem. 1926, 18, 370–380. [Google Scholar] [CrossRef]
- Liao, G.; Yang, H.; Jiang, Y.; Ren, S.; Li, D.; Wang, L.; Wang, Z.; Wang, B.; Liu, W. Applicable scope of oxygen-reduced air flooding and the limit of oxygen content. Pet. Explor. Dev. 2018, 45, 111–117. [Google Scholar] [CrossRef]
- Li, W.; Pots, B.F.M.; Zhong, X.; Nesic, S. Inhibition of CO2 Corrosion of Mild Steel—Study of Mechanical Effects of Highly Turbulent Disturbed Flow. Corros. Sci. 2017, 126, 208–226. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, C.; Long, Z.; Guan, C.; Wang, W.; Hesitao, W. Electrochemical behavior of valve steel in a CO2 /sulfurous acid solution. Electrochim. Acta 2017, 258, 909–918. [Google Scholar] [CrossRef]
- Sun, J.; Sun, C.; Lin, X.; Cheng, X.; Liu, H. Effect of Chromium on Corrosion Behavior of P110 Steels in CO2-H2S Environment with High Pressure and High Temperature. Materials 2016, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Brown, B.; Young, D.; Nesic, S.; Singer, M. Formation Mechanisms of Iron Oxide and Iron Sulfide at High Temperature in Aqueous H2S Corrosion Environment. J. Electrochem. Soc. 2018, 165, C171–C179. [Google Scholar] [CrossRef]
- Liu, H.; Zhong, X.; Liu, H.; Cheng, Y.F. Microbiologically-enhanced galvanic corrosion of the steel beneath a deposit in simulated oilfield-produced water containing Desulfotomaculum nigrificans. Electrochem. Commun. 2018, 90, 1–5. [Google Scholar] [CrossRef]
- Oguzie, E.E.; Ogbulie, J.N.; Nwanebu, F.C.; Adindu, C.B.; Udeze, I.O.; Oguzie, K.L.; Eze, F.C. Broad spectrum corrosion inhibition: corrosion and microbial (SRB) growth inhibiting effects of Piper guineense extract. J. Mater. Sci. 2012, 47, 3592–3601. [Google Scholar] [CrossRef]
- Kahyarian, A.; Schumaker, A.; Brown, B.; Nesic, S. Acidic corrosion of mild steel in the presence of acetic acid: Mechanism and prediction. Electrochim. Acta 2017, 258, 639–652. [Google Scholar] [CrossRef]
- Peng, J.; Robbins, W.; Bota, G. Effect of sulfur compounds on formation of protective scales in naphthenic acid corrosion in non-turbulent flow. Corros. Sci. 2018, 131, 223–234. [Google Scholar] [CrossRef]
- Rosli, N.R. The Effect of Oxygen in Sweet Corrosion of Carbon Steel for Enhanced Oil Recovery Applications. Ph.D. Thesis, Ohio University, Athens, OH, USA, 2015. [Google Scholar]
- Cáceres, L.; Vargas, T.; Herrera, L. Determination of electrochemical parameters and corrosion rate for carbon steel in un-buffered sodium chloride solutions using a superposition model. Corros. Sci. 2007, 49, 3168–3184. [Google Scholar] [CrossRef]
- Baek, W.C.; Kang, T.; Sohn, H.J.; Kho, Y.T. In situ surface enhanced Raman spectroscopic study on the effect of dissolved oxygen on the corrosion film on low carbon steel in 0.01 M NaCl solution. Electrochim. Acta 2001, 46, 2321–2325. [Google Scholar] [CrossRef]
- He, J.; Li, B.; Mao, J.; Liang, Y.; Yang, X.; Cui, Z.; Zhu, S.; Li, Z. Four-electron oxygen reduction from mesoporous carbon modified with Fe2O3 nanocrystals. J. Mater. Sci. 2017, 52, 10938–10947. [Google Scholar] [CrossRef]
- Lou, X.; Singh, P.M. Cathodic activities of oxygen and hydrogen on carbon steel in simulated fuel-grade ethanol. Electrochim. Acta 2001, 56, 2312–2320. [Google Scholar] [CrossRef]
- Wiberg, G.K.H.; Arenz, M. On the influence of hydronium and hydroxide ion diffusion on the hydrogen and oxygen evolution reactions in aqueous media. Electrochim. Acta 2015, 158, 13–17. [Google Scholar] [CrossRef]
- Choi, Y.S.; Nesic, S.; Young, D. Effect of impurities on the corrosion behavior of CO2 transmission pipeline steel in supercritical CO2-water environments. Environ. Sci. Technol. 2010, 44, 9233–9238. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, L.; Vargas, T.; Herrera, L. Influence of pitting and iron oxide formation during corrosion of carbon steel in unbuffered NaCl solutions. Corros. Sci. 2009, 51, 971–978. [Google Scholar] [CrossRef]
- Krawiec, H.; Vignal, V.; Akid, R. Numerical modelling of the electrochemical behaviour of 316 stainless steel based upon static and dynamic experimental microcapillary based techniques: effect of electrolyte flow and capillary size. Surf. Interface Anal. 2008, 40, 315–319. [Google Scholar] [CrossRef]
- Krawiec, H.; Vignal, V.; Akid, R. Numerical modelling of the electrochemical behaviour of 316L stainless steel based upon static and dynamic experimental microcapillary-based techniques. Electrochim. Acta 2008, 53, 5252–5259. [Google Scholar] [CrossRef]
- Briega-Martos, V.; Herrero, E.; Feliu, J.M. Effect of pH and water structure on the oxygen reduction reaction on platinum electrodes. Electrochim. Acta 2017, 241, 497–509. [Google Scholar] [CrossRef]
- Zheng, Y.; Ning, J.; Brown, B.; Nesic, S. Electrochemical Model of Mild Steel Corrosion in a Mixed H2S/CO2 Aqueous Environment in the Absence of Protective Corrosion Product Layers. Corrosion 2015, 71, 316–325. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, Y.F. Modeling of corrosion of steel tubing in CO2 storage. Greenh. Gases 2016, 6, 797–811. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 5-1–5-811. ISBN 978-1-4987-5429-3. [Google Scholar]
- Song, C.; Tang, Y.; Zhang, J.L.; Zhang, J.; Wang, H.; Shen, J.; McDermid, S.; Li, J.; Kozak, P. PEM fuel cell reaction kinetics in the temperature range of 23–120 °C. Electrochim. Acta 2007, 52, 2552–2561. [Google Scholar] [CrossRef]
- Zheng, Y.; Brown, B.; Nesic, S. Electrochemical Study and Modeling of H2S Corrosion of Mild Steel. Corrosion 2014, 70, 351–365. [Google Scholar] [CrossRef]
- Jovancicevic, V.; Bockris, J.O. The Mechanism of Oxygen Reduction on Iron in Neutral Solutions. J. Electrochem. Soc. 1986, 133, 1797–1807. [Google Scholar] [CrossRef]
- Kahyarian, A.; Singer, M.; Nesic, S. Modeling of uniform CO2 corrosion of mild steel in gas transportation systems: A review. J. Nat. Gas Sci. Eng. 2016, 29, 530–549. [Google Scholar] [CrossRef]
- Vilekar, S.A.; Fishtik, I.; Datta, R. Kinetics of the Hydrogen Electrode Reaction. J. Electrochem. Soc. 2010, 157, B1040–B1050. [Google Scholar] [CrossRef]
- Kharaka, Y.K.; Gunter, W.D.; Aggarwal, P.K.; Perkins, E.H.; Dedraal, J.D. Solmineq 88: A Computer Program for Geochemical Modelling of Water-Rock Interaction; U.S. Water-Resources Investigations Report; United States Geological Survey: Reston, VA, USA, 1998; pp. 88–4227.
- Steen, N.V.D.; Simillion, H.; Dolgikh, O.; Terryn, H.; Deconinck, J. An integrated modeling approach for atmospheric corrosion in presence of a varying electrolyte film. Electrochim. Acta 2016, 187, 714–723. [Google Scholar] [CrossRef]
- Auinger, M.; Katsounaros, I.; Meier, J.C.; Klemm, S.O.; Biedermann, P.U.; Topalov, A.A.; Rohwerder, M.; Mayrhofer, K.J.J. Near-surface ion distribution and buffer effects during electrochemical reactions. Phys. Chem. Chem. Phys. 2001, 13, 16384–16394. [Google Scholar] [CrossRef] [PubMed]
- Abodi, L.C.; Dolgikh, O.; Terryn, H.; Deconinck, J. The influence of the capillary size and shape on the readings of the electrochemical microcapillary technique: A parametric study by means of the multi-ion modeling. Electrochim. Acta 2016, 189, 128–136. [Google Scholar] [CrossRef]
- Skaperdas, G.T.; Uhlig, H.H. Corrosion of Steel by Dissolved Carbon Dioxide and Oxygen. Ind. Eng. Chem. 1942, 34, 748–754. [Google Scholar] [CrossRef]
- Bockris, J.O.; Drazic, D.; Despic, A.R. The electrode kinetics of the deposition and dissolution of iron. Electrochim. Acta 1961, 4, 325–361. [Google Scholar] [CrossRef]
- Nesic, S.; Postlethwaite, J.; Olsen, S. An Electrochemical Model for Prediction of Corrosion of Mild Steel in Aqueous Carbon Dioxide Solutions. Corrosion 1996, 52, 280–294. [Google Scholar] [CrossRef] [Green Version]
- Felloni, L. The effect of pH on the electrochemical behaviour of iron in hydrochloric acid. Corros. Sci. 1968, 8, 133–148. [Google Scholar] [CrossRef]
- Mandel, M.; Kietov, V.; Dubberstein, T.; Krüger, L. The Potentiodynamic Polarisation of a High-Alloy Steel—An Analysis by Acoustic Emission Testing and Long-Distance Microscopy. Electrochim. Acta 2017, 240, 350–360. [Google Scholar] [CrossRef]
- Vogt, H.; Balzerm, R.J. The bubble coverage of gas-evolving electrodes in stagnant electrolytes. Electrochim. Acta 2005, 50, 2073–2079. [Google Scholar] [CrossRef]








| Symbol | Value | Unit | Definition | Reference |
|---|---|---|---|---|
| Standard potential (vs. SHE) | ||||
| −0.447 | V | [26] | ||
| 0.401 | V | [26] | ||
| 0 | V | [26] | ||
| −0.828 | V | [26] | ||
| Reference exchange current density | ||||
| 1 | A/m2 | [24] | ||
| 2.8 × 10−3 | A/m2 | [27] | ||
| 0.03 | A/m2 | [24] | ||
| 1.4 × 10−5 | A/m2 | [24] | ||
| Active enthalpy for exchange current density | ||||
| 37,500 | J/mol | Fe oxidation | [24] | |
| 23,200 | J/mol | O2 reduction | [27] | |
| 30,000 | J/mol | H+ reduction | [24] | |
| 30,000 | J/mol | H2O reduction | [24] | |
| Reference temperature | ||||
| 298.15 | K | Exchange current density of | [24] | |
| 303.15 | K | Exchange current density of | [27] | |
| 298.15 | K | Exchange current density of | [24] | |
| 293.15 | K | Exchange current density of | [24] | |
| 293.15 | K | Water dynamic viscosity | [28] | |
| Reference physical parameters | ||||
| 0.1 | mol/m3 | H+ concentration for exchange current density | [24] | |
| 1.002 | kg/m·s | Water dynamic viscosity at 293.15K | [26] | |
| Reference diffusion coefficient at 298.15K | ||||
| 2.3 × 10−9 | m2/s | O2 | [26] | |
| 9.31 × 10−9 | m2/s | H+ | [26] | |
| Constants | ||||
| F | 96,485 | C/mol | Faraday’s constant | [26] |
| R | 8.314 | J/mol·K | Universal gas constant | [26] |
| M | 56 | g/mol | Molecular mass of Fe | [26] |
| ρ | 7.8 | g/cm3 | Density of Fe | [26] |
| Net current density | ||||
| A/m2 | Corrosion | |||
| A/m2 | Fe oxidation | |||
| A/m2 | O2 reduction | |||
| A/m2 | H+ reduction | |||
| A/m2 | H2O reduction | |||
| Exchange current density | ||||
| A/m2 | ||||
| A/m2 | ||||
| A/m2 | ||||
| A/m2 | ||||
| Charge transfer current density | ||||
| A/m2 | O2 reduction | |||
| A/m2 | H+ reduction | |||
| Limited current density | ||||
| A/m2 | O2 reduction | |||
| A/m2 | H+ reduction | |||
| Potential (vs. SHE) | ||||
| V | Steel potential | |||
| V | Corrosion | |||
| V | Reversible potential for | |||
| V | Reversible potential for | |||
| V | Reversible potential for | |||
| V | Reversible potential for | |||
| Absolute value for Tafel slope | ||||
| 70 | mV/decade | Fe oxidation | ||
| 120 | mV/decade | O2 reduction | [29] | |
| mV/decade | H+ reduction | |||
| mV/decade | H2O reduction | |||
| Diffusion coefficient | ||||
| m2/s | O2 | |||
| m2/s | H+ | |||
| Concentration in bulk solution | ||||
| mol/m3 | O2 | |||
| mol/m3 | H+ | |||
| Physical parameters | ||||
| μ | kg/m·s | Water dynamic viscosity | ||
| T | K | Experiment temperature | ||
| δ | mm | Diffusion boundary layer thickness | ||
| Kw | mol2/L2 | Ionic product of water | ||
| CR | mm/y | Corrosion rate |
(mg/L) | Experimental Data | Modeling Result | ||||
|---|---|---|---|---|---|---|
(V vs. SCE) | (A/m2) | (mm/y) | (V vs. SCE) | (A/m2) | (mm/y) | |
| 0.08 | −0.807 ± 0.004 | 0.017 ± 0.002 | 0.019 ± 0.002 | −0.810 | 0.017 | 0.019 |
| 0.34 | −0.787 ± 0.002 | 0.038 ± 0.002 | 0.046 ± 0.002 | −0.785 | 0.037 | 0.044 |
| 1.16 | −0.752 ± 0.004 | 0.097 ± 0.004 | 0.113 ± 0.005 | −0.752 | 0.109 | 0.128 |
| 4.16 | −0.694 ± 0.002 | 0.377 ± 0.005 | 0.443 ± 0.006 | −0.715 | 0.377 | 0.443 |
(°C) | Experimental Data | Modeling Result | ||||
|---|---|---|---|---|---|---|
(V vs. SCE) | (A/m2) | (mm/y) | (V vs. SCE) | (A/m2) | (mm/y) | |
| 30 | −0.807 ± 0.004 | 0.017 ± 0.002 | 0.019 ± 0.002 | −0.810 | 0.017 | 0.019 |
| 40 | −0.802 ± 0.006 | 0.022 ± 0.002 | 0.026 ± 0.002 | −0.808 | 0.023 | 0.027 |
| 50 | −0.793 ± 0.006 | 0.030 ± 0.002 | 0.035 ± 0.002 | −0.806 | 0.031 | 0.036 |
| 80 | −0.801 ± 0.008 | 0.075 ± 0.004 | 0.088 ± 0.005 | −0.796 | 0.073 | 0.086 |
| pH | Experimental Data | Modeling Result | ||||
|---|---|---|---|---|---|---|
(V vs. SCE) | (A/m2) | (mm/y) | (V vs. SCE) | (A/m2) | (mm/y) | |
| 9.0 | −0.815 ± 0.003 | 0.010 ± 0.001 | 0.012 ± 0.001 | −0.814 | 0.014 | 0.017 |
| 8.0 | −0.812 ± 0.002 | 0.012 ± 0.001 | 0.014 ± 0.001 | −0.814 | 0.014 | 0.017 |
| 7.0 | −0.805 ± 0.006 | 0.015 ± 0.001 | 0.018 ± 0.001 | −0.814 | 0.014 | 0.017 |
| 6.0 | −0.807 ± 0.004 | 0.017 ± 0.002 | 0.019 ± 0.002 | −0.810 | 0.017 | 0.019 |
| 5.0 | −0.787 ± 0.004 | 0.040 ± 0.002 | 0.047 ± 0.002 | −0.783 | 0.040 | 0.047 |
| Condition | Experimental Data | Modeling Result | ||||
|---|---|---|---|---|---|---|
(V vs. SCE) | (A/m2) | (mm/y) | (V vs. SCE) | (A/m2) | (mm/y) | |
| pH 9.0, 4.10 mg/L oxygen | −0.718 ± 0.004 | 0.353 ± 0.008 | 0.414 ± 0.010 | −0.715 | 0.369 | 0.433 |
| pH 8.0, 4.22 mg/L oxygen | −0.707 ± 0.008 | 0.376 ± 0.005 | 0.441 ± 0.006 | −0.715 | 0.380 | 0.446 |
| pH 7.0, 4.07 mg/L oxygen | −0.717 ± 0.004 | 0.323 ± 0.009 | 0.379 ± 0.010 | −0.715 | 0.367 | 0.430 |
| pH 6.0, 4.16 mg/L oxygen | −0.694 ± 0.008 | 0.377 ± 0.004 | 0.443 ± 0.005 | −0.715 | 0.377 | 0.443 |
| pH 5.0, 4.11 mg/L oxygen | −0.729 ± 0.005 | 0.337 ± 0.012 | 0.396 ± 0.014 | −0.713 | 0.397 | 0.466 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Hu, J.; Zhong, X.; Zhang, Q.; Zheng, Y.; Zhang, Z.; Zeng, D. Corrosion Behaviors of Q345R Steel at the Initial Stage in an Oxygen-Containing Aqueous Environment: Experiment and Modeling. Materials 2018, 11, 1462. https://doi.org/10.3390/ma11081462
Chen L, Hu J, Zhong X, Zhang Q, Zheng Y, Zhang Z, Zeng D. Corrosion Behaviors of Q345R Steel at the Initial Stage in an Oxygen-Containing Aqueous Environment: Experiment and Modeling. Materials. 2018; 11(8):1462. https://doi.org/10.3390/ma11081462
Chicago/Turabian StyleChen, Longjun, Junying Hu, Xiankang Zhong, Qiang Zhang, Yan Zheng, Zhi Zhang, and Dezhi Zeng. 2018. "Corrosion Behaviors of Q345R Steel at the Initial Stage in an Oxygen-Containing Aqueous Environment: Experiment and Modeling" Materials 11, no. 8: 1462. https://doi.org/10.3390/ma11081462