Microstructures, Mechanical and Corrosion Properties of the Extruded AZ31-xCaO Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Effect of CaO on Microstructure of AZ31 Alloy
3.2. Effect of Extrusion on Microstructure and Properties of AZ31 Alloy
3.2.1. Effect of Extrusion Temperature on Microstructure of AZ31-xCaO Alloy
3.2.2. Effect of Extrusion Ratio on Microstructure of AZ31-xCaO Alloy
3.2.3. Orientations of Extruded AZ31-xCaO Alloy
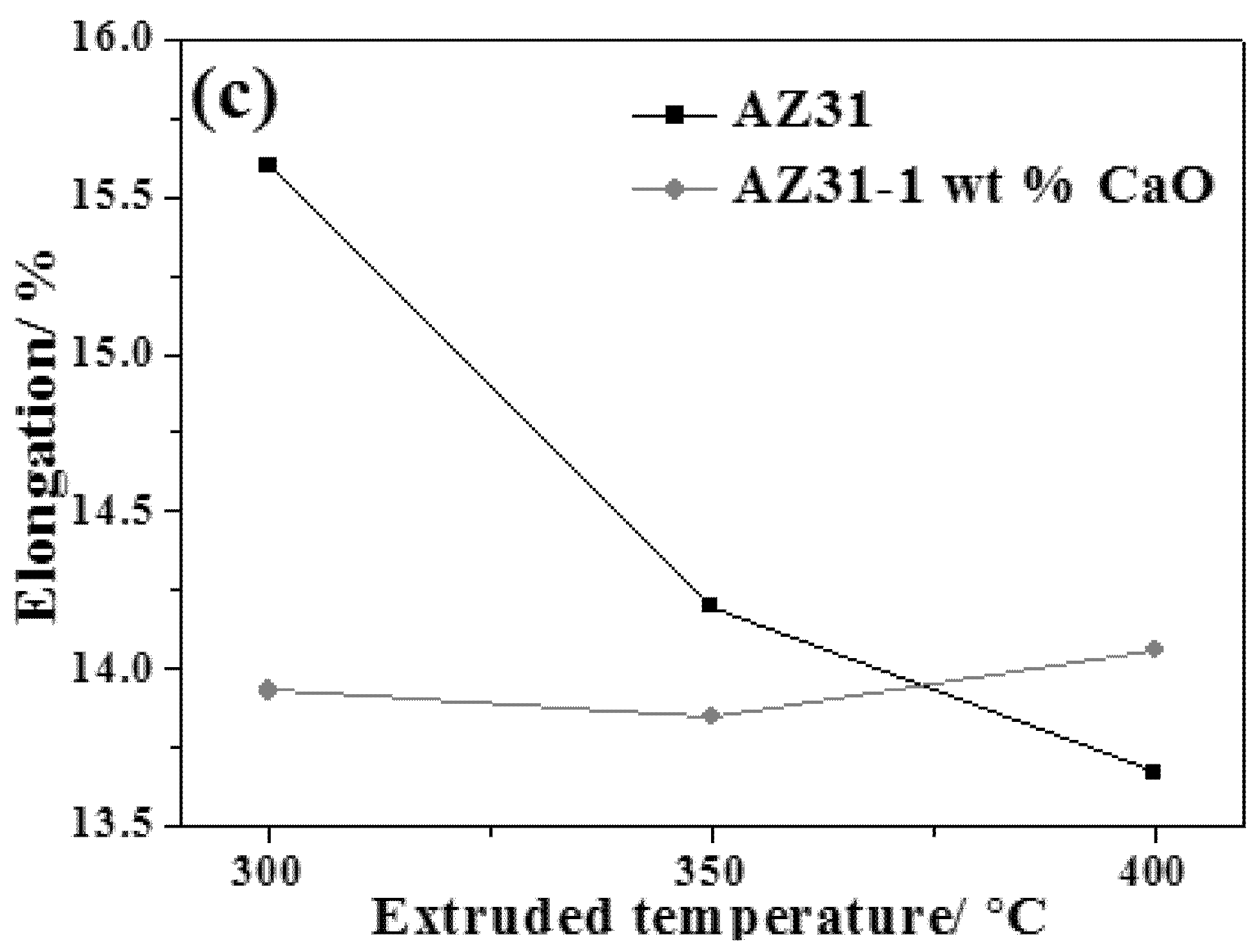
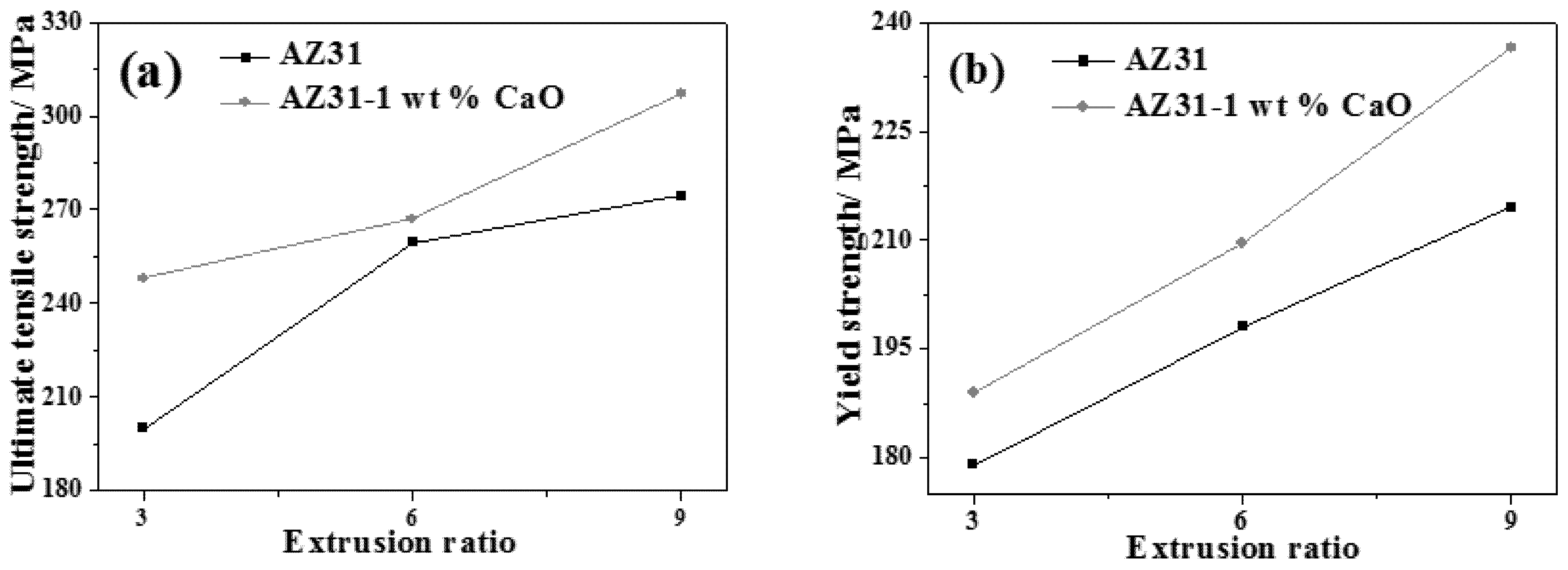
3.2.4. Effect of Extrusion on Mechanical Properties of AZ31-xCaO Alloy
3.2.5. Corrosion Properties of AZ31-xCaO Alloys
4. Conclusions
- (1)
- The grain size of AZ31 or AZ31-1%CaO alloy becomes larger with increasing extrusion temperature. The grain size of AZ31-1%CaO alloy exhibits much smaller than that of AZ31 alloy at the same extrusion temperature. The recrystallization grains distribute more uniformly as the increase of extrusion ratio, and the completely recrystallized grains distribute uniformly in the form of equiaxed crystals as an extrusion ratio of 9.
- (2)
- The yield strength and elongation of extruded AZ31 alloy at room temperature decrease with increasing of extrusion temperature, while ultimate tensile strength decreases firstly and then increases. The ultimate tensile strength, yield strength, and elongation of AZ31-xCaO alloys all increase with the increase of extrusion ratio. The extruded AZ31-1%CaO alloy at the extrusion temperature of 300 °C and an extrusion ratio of 9 exhibits the best mechanical properties.
- (3)
- Corrosion potential of extended alloys becomes smaller with increasing CaO addition, and shifts to negative direction. Corrosion degree of extruded alloys increase over time, while their corrosion rates decrease gradually. The weight loss rate of extruded alloy with CaO is significantly higher than that of AZ31 alloy. Corrosion resistance of extruded alloys decreases with increasing CaO addition, which can be attributed to the addition of CaO.
Author Contributions
Funding
Conflicts of Interest
References
- Liu, X.; Osawa, Y.; Takamori, S.; Mukai, T. Microstructure and mechanical properties of AZ91 alloy produced with ultrasonic vibration. Mater. Sci. Eng. A 2008, 487, 120–123. [Google Scholar] [CrossRef]
- Nie, J.F. Precipitation and hardening in magnesium alloys. Metall. Mater. Trans. A 2012, 43, 3891–3939. [Google Scholar] [CrossRef]
- Mordike, B.L. Properties-Applications-Potential. Mater. Sci. Eng. Ser. A 2001, 37, 302. [Google Scholar]
- Lee, J.K.; Kim, S.K. Effect of CaO addition on the ignition resistance of Mg-Al alloys. Mater. Trans. 2011, 52, 1483–1488. [Google Scholar] [CrossRef]
- Prasad, A.; Shi, Z.; Atrens, A. Influence of Al and Y on the ignition and flammability of Mg alloys. Corros. Sci. 2012, 55, 153–163. [Google Scholar] [CrossRef]
- Zeng, X.Q.; Wang, Q.D.; Lü, Y.Z.; Ding, W.J.; Zhu, Y.P.; Zhai, C.Q.; Xu, X.P. Study on ignition proof magnesium alloy with beryllium and rare earth additions. Scr. Mater. 2000, 43, 403–409. [Google Scholar] [CrossRef]
- Rao, J.S.; Li, H.J.; Xue, H.S. Ignition-proof mechanism of ZM5 magnesium alloy added with rare earth. J. Cent. South Univ. 2010, 17, 28–33. [Google Scholar] [CrossRef]
- Fan, J.F.; Yang, C.L.; Han, G.; Fang, S.; Yang, W.D.; Xu, B.S. Oxidation behavior of ignition-proof magnesium alloys with rare earth addition. J. Alloys Compd. 2011, 509, 2137–2142. [Google Scholar] [CrossRef]
- Sakamoto, M.; Akiyama, S.; Ogi, K. Suppression of ignition and burning of molten Mg alloys by Ca bearing stable oxide film. J. Mater. Sci. Lett. 1997, 16, 1048–1050. [Google Scholar] [CrossRef]
- Jung, Y.G.; Lim, H.K.; Yoon, Y.O.; Kim, S.K.; Kim, D.H. Effects of alloying elements and cooling rate on morphology of phases in CaO added Mg-Al-Si alloys. Magnes. Technol. 2013, 333–339. [Google Scholar] [CrossRef]
- Lee, J.K.; Hyung-Ho, J.O.; Shae, K.K. Effect of CaO addition on ignition behavior in molten AZ31 and AZ91D Magnesium alloys. Rare Metals 2006, 25, 155–159. [Google Scholar] [CrossRef]
- Lee, T.W.; Kim, H.G.; So, M.G.; Lee, J.K.; Shae, K.K.; Park, W.J.; Kim, W.Y.; Kim, S.; Lim, S.H. Microstructural evaluation of oxide layers in CaO-added Mg alloys. J. Alloys Compd. 2015, 635, 5–10. [Google Scholar] [CrossRef]
- Kwak, T.Y.; Lim, H.K.; Kim, W.J. Effect of Ca and CaO on the microstructure and hot compressive deformation behavior of Mg-9.5Zn-2.0Y alloy. Mater. Sci. Eng. A 2015, 648, 146–156. [Google Scholar] [CrossRef]
- Jang, D.I.; Lee, J.K.; Kim, D.U.; Kim, S.K. Influence of CaO on damping capacity and mechanical properties of Mg alloy. Trans. Nonferrous Met. Soc. China 2009, 19, s76–s79. [Google Scholar] [CrossRef]
- Choi, D.H.; Kim, S.K.; Jung, S.B. The microstructures and mechanical properties of friction stir welded AZ31 with CaO Mg alloys. J. Alloys Compd. 2013, 554, 162–168. [Google Scholar] [CrossRef]
- Choi, D.H.; Ahn, B.W.; Kim, S.K.; Yeon, Y.M.; Kim, Y.J.; Park, S.K.; Jung, S.B. Microstructure evaluation of friction stir welded AZ91 with CaO Mg alloy. Mater. Trans. 2011, 52, 802–805. [Google Scholar] [CrossRef]
- Joong-Hwan, J. Effect of CaO addition on microstructure and damping capacity of AM50 magnesium alloy. Mater. Trans. 2013, 54, 409–411. [Google Scholar]
- Bae, S.H.; Jung, K.H.; Shin, Y.C.; Yoon, D.J.; Kawasaki, M. Development of mechanical properties in a CaO added AZ31 magnesium alloy processed by equal-channel angular pressing. Mater. Charact. 2016, 112, 105–112. [Google Scholar] [CrossRef]
- Apps, P.J.; Bowen, J.R.; Prangnell, P.B. The effect of coarse second-phase particles on the rate of grain refinement during severe deformation processing. Acta Mater. 2003, 51, 2811–2822. [Google Scholar] [CrossRef]
- Wang, H.L.; Wang, G.J.; Lian-Xi, H.U.; Wang, Q.; Wang, E.D. Effect of hot rolling on grain refining and mechanical properties of AZ40 magnesium alloy. Trans. Nonferrous Met. Soc. China 2011, 21, s229–s234. [Google Scholar] [CrossRef]
- Chino, Y.; Kimura, K.; Mabuchi, M. Twinning behavior and deformation mechanisms of extruded AZ31 Mg alloy. Mater. Sci. Eng. A 2008, 486, 481–488. [Google Scholar] [CrossRef]
- Bohlen, J.; Yi, S.B.; Swiostek, J.; Letzig, D.; Brokmeier, H.G.; Kainer, K.U. Microstructure and texture development during hydrostatic extrusion of magnesium alloy AZ31. Scr. Mater. 2005, 53, 259–264. [Google Scholar] [CrossRef]
- Azeem, M.A.; Tewari, A.; Ramamurty, U. Effect of recrystallization and grain growth on the mechanical properties of an extruded AZ21 Mg alloy. Mater. Sci. Eng. A 2010, 527, 898–903. [Google Scholar] [CrossRef]
- Chen, Y.J.; Wang, Q.D.; Lin, J.B.; Zhang, L.J.; Zhai, C.Q. Microstructure and mechanical properties ofAZ31 Mg alloy processed by high ratio extrusion. Trans. Nonferrous Met. Soc. China 2006, 16, 1875–1878. [Google Scholar]
- Chen, X.; Li, Q.A.; Chen, J.; Jiang, H.; Zhou, Y. Influence of trace CaO on microstructure and corrosion resistance of AZ81 magnesium alloy. Corros. Sci. Prot. Technol. 2016, 28, 68–72. [Google Scholar]













| Al | Zn | Mn | Fe | Cu | Ni | Si | Mg |
|---|---|---|---|---|---|---|---|
| 2.5-3.5 | 0.6-1.4 | 0.2-1.0 | 0.003 | 0.01 | 0.001 | 0.08 | Bal. |
| Extruded Alloys | Self-Corrosion Potential Ecorr/V |
|---|---|
| AZ31 | −1.406 |
| AZ31-0.5%CaO | −1.409 |
| AZ31-1%CaO | −1.441 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Zhang, Y.; Cong, M.; Li, X.; Xu, W.; Song, L. Microstructures, Mechanical and Corrosion Properties of the Extruded AZ31-xCaO Alloys. Materials 2018, 11, 1467. https://doi.org/10.3390/ma11081467
Lu Y, Zhang Y, Cong M, Li X, Xu W, Song L. Microstructures, Mechanical and Corrosion Properties of the Extruded AZ31-xCaO Alloys. Materials. 2018; 11(8):1467. https://doi.org/10.3390/ma11081467
Chicago/Turabian StyleLu, Yalin, Yang Zhang, Mengqi Cong, Xingcheng Li, Wenting Xu, and Leipeng Song. 2018. "Microstructures, Mechanical and Corrosion Properties of the Extruded AZ31-xCaO Alloys" Materials 11, no. 8: 1467. https://doi.org/10.3390/ma11081467





