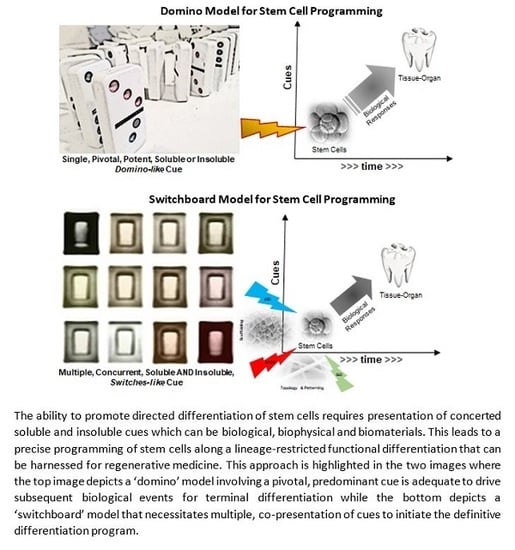
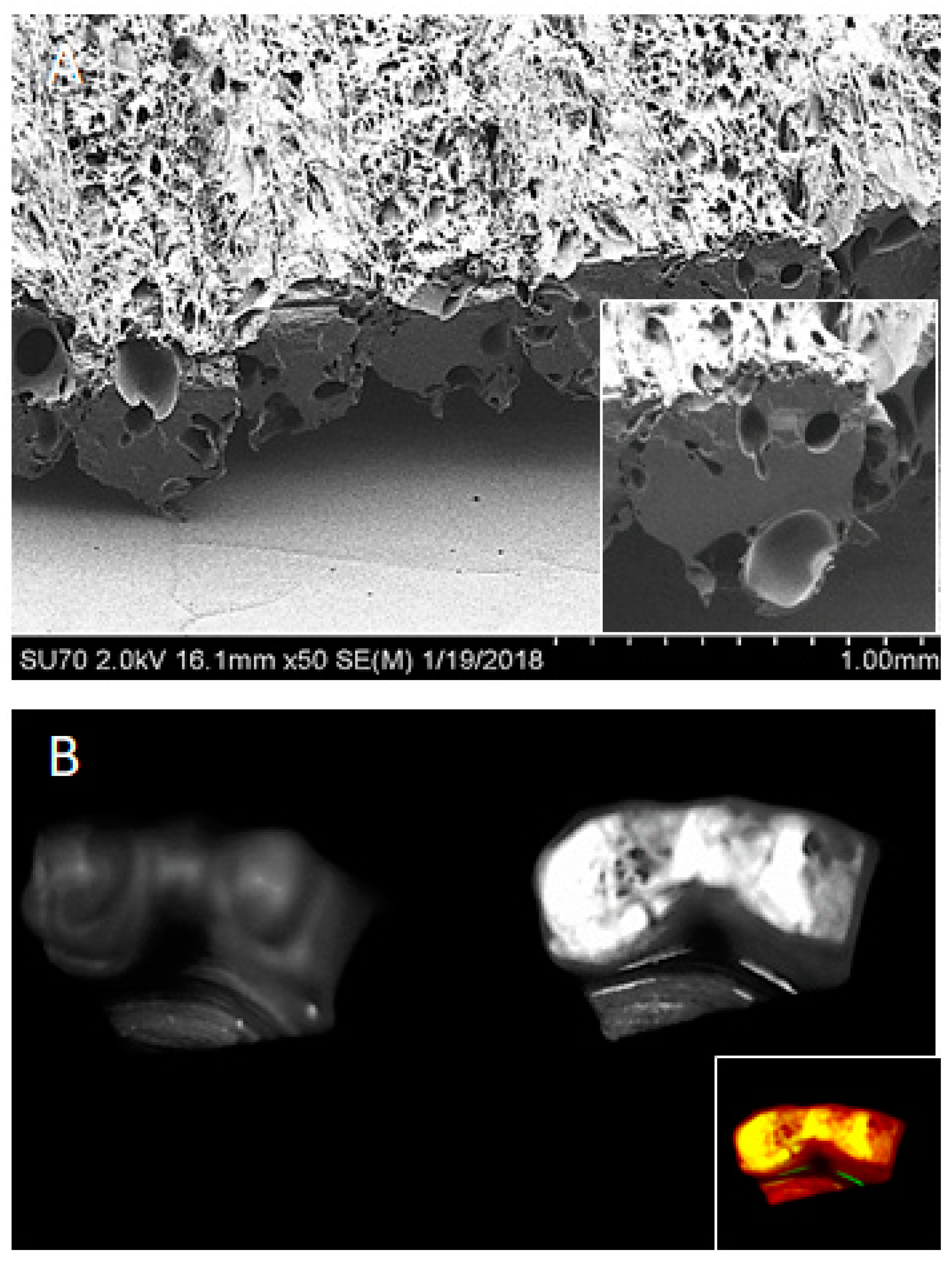
Nanoscale and Macroscale Scaffolds with Controlled-Release Polymeric Systems for Dental Craniomaxillofacial Tissue Engineering
Abstract
:1. Introduction
2. Fundamental Principles of Engineering Tissues and Organs
2.1. A Simplistic View of the Time–Space Paradigm in Tissue Engineering
2.2. Biomaterial Fabrication Approaches
3. Material Selection for Biomaterial Systems
4. Polymeric Microencapsulation for Nano- and Microspheres
5. Electrospinning to Generate Nanofiber Scaffolds
5.1. Nanofiber Scaffold
5.2. Roles of Extracellular Nanostructures in Cell Differentiation
5.3. Microsphere- or Nanosphere-Incorporated Nanofibrous Scaffolds
6. Gas Foaming and Water Leaching
7. 3D Printing
7.1. Designing Bioactive Systems with 3D Printing
7.2. Sense-and-Respond “Smart” Biomaterials for Theranostics
8. Applications for Dental and Craniofacial Tissue Engineering
8.1. Pulp–Dentin Tissue Engineering
8.2. Temporomandibular Joint
8.3. Periodontium Bioengineering
8.4. Salivary Glands and Taste Bud Engineering
9. Applications of Materials for Osteoblast Differentiation and Bone Regeneration
10. Future Perspectives
11. Conclusions
Funding
Conflicts of Interest
References
- Goonoo, N.; Bhaw-Luximon, A.; Jhurry, D. In vitro and in vivo cytocompatibility of electrospun nanofiber scaffolds for tissue engineering applications. RSC Adv. 2014, 4, 31618–31642. [Google Scholar] [CrossRef]
- Pelipenko, J.; Kocbek, P.; Kristl, J. Critical attributes of nanofibers: Preparation, drug loading, and tissue regeneration. Int. J. Pharm. 2015, 484, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.J.; Giannobile, W.V.; Helms, J.A.; Hollister, S.J.; Krebsbach, P.H.; Longaker, M.T.; Shi, S. Craniofacial tissue engineering by stem cells. J. Dent. Res. 2006, 85, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth factors, matrices, and forces combine and control stem cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef] [PubMed]
- Arany, P.R.; Mooney, D.J. At the edge of translation—Materials to program cells for directed differentiation. Oral Dis. 2011, 17, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Fiorellini, J.P.; Howell, T.H.; Cochran, D.; Malmquist, J.; Lilly, L.C.; Spagnoli, D.; Toljanic, J.; Jones, A.; Nevins, M. Randomized study evaluating recombinant human bone morphogenetic protein-2 for extraction socket augmentation. J. Periodontol. 2005, 76, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Boyne, P.J.; Marx, R.E.; Nevins, M.; Triplett, G.; Lazaro, E.; Lilly, L.C.; Alder, M.; Nummikoski, P. A feasibility study evaluating rhBMP-2/absorbable collagen sponge for maxillary sinus floor augmentation. Int. J. Periodontics Restor. Dent. 1997, 17, 11–25. [Google Scholar]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, P.; Schweller, R.M.; Khademhosseini, A.; West, J.L.; Bashir, R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu. Rev. Biomed. Eng. 2014, 16, 247–276. [Google Scholar] [CrossRef] [PubMed]
- Van de Witte, P.; Dijkstra, P.J.; Van den Berg, J.; Feijen, J. Phase separation processes in polymer solutions in relation to membrane formation. J. Membr. Sci. 1996, 117, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Chen, P. Self-assembly of ionic-complementary peptides: A physicochemical viewpoint. Colloids Surf. A Physicochem. Eng. Asp. 2005, 261, 3–24. [Google Scholar] [CrossRef]
- Donnet, J.-B.; Park, S.-J. Surface characteristics of pitch-based carbon fibers by inverse gas chromatography method. Carbon 1991, 29, 955–961. [Google Scholar] [CrossRef]
- Jung, M.-J.; Jeong, E.; Kim, Y.; Lee, Y.-S. Influence of the textual properties of activated carbon nanofibers on the performance of electric double-layer capacitors. J. Ind. Eng. Chem. 2013, 19, 1315–1319. [Google Scholar] [CrossRef]
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3D printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Jang, T.-S.; Jung, H.-D.; Pan, H.M.; Han, W.T.; Chen, S.; Song, J. 3D printing of hydrogel composite systems: Recent advances in technology for tissue engineering. Int. J. Bioprint. 2018, 4. [Google Scholar] [CrossRef] [Green Version]
- Jordan, A.M.; Viswanath, V.; Kim, S.-E.; Pokorski, J.K.; Korley, L.T. Processing and surface modification of polymer nanofibers for biological scaffolds: A review. J. Mater. Chem. B 2016, 4, 5958–5974. [Google Scholar] [CrossRef]
- Quirós, J.; Boltes, K.; Rosal, R. Bioactive applications for electrospun fibers. Polym. Rev. 2016, 56, 631–667. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, H.W.; Kang, I.-K.; Lee, H.B. Cell behaviour on polymer surfaces with different functional groups. Biomaterials 1994, 15, 705–711. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Tan, A.; Pastorin, G.; Ho, H.K. Nanomaterial scaffolds for stem cell proliferation and differentiation in tissue engineering. Biotechnol. Adv. 2013, 31, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Massia, S.P.; Stark, J. Immobilized RGD peptides on surface-grafted dextran promote biospecific cell attachment. J. Biomed. Mater. Res. Part A 2001, 56, 390–399. [Google Scholar] [CrossRef]
- VandeVondele, S.; Vörös, J.; Hubbell, J.A. RGD-grafted poly-l-lysine-graft-(polyethylene glycol) copolymers block non-specific protein adsorption while promoting cell adhesion. Biotechnol. Bioeng. 2003, 82, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Gombotz, W.R.; Pettit, D.K. Biodegradable polymers for protein and peptide drug delivery. Bioconj. Chem. 1995, 6, 332–351. [Google Scholar] [CrossRef]
- Wang, H.; Leeuwenburgh, S.C.; Li, Y.; Jansen, J.A. The use of micro-and nanospheres as functional components for bone tissue regeneration. Tissue Eng. Part B Rev. 2011, 18, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Uludağ, H. Nanoparticulate systems for growth factor delivery. Pharm. Res. 2009, 26, 1561. [Google Scholar] [CrossRef] [PubMed]
- Richardson, T.P.; Peters, M.C.; Ennett, A.B.; Mooney, D.J. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 2001, 19, 1029. [Google Scholar] [CrossRef] [PubMed]
- Obregon, F.; Vaquette, C.; Ivanovski, S.; Hutmacher, D.; Bertassoni, L. Three-dimensional bioprinting for regenerative dentistry and craniofacial tissue engineering. J. Dent. Res. 2015, 94 (Suppl. 9), 143S–152S. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yao, R.; Ouyang, L.; Ding, H.; Zhang, T.; Zhang, K.; Cheng, S.; Sun, W. Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication 2014, 6, 035001. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.; Visconti, R.P.; Kasyanov, V.; Forgacs, G.; Drake, C.J.; Markwald, R.R. Organ printing: Tissue spheroids as building blocks. Biomaterials 2009, 30, 2164–2174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, S.; Grunert, R.; Mohr, F.W.; Falk, V. 3D-Imaging of cardiac structures using 3D heart models for planning in heart surgery: A preliminary study. Interact. Cardiovasc. Thorac. Surg. 2008, 7, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.R.; Mooney, D.J. Polymeric growth factor delivery strategies for tissue engineering. Pharm. Res. 2003, 20, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.S.; Park, T.G. Biodegradable polymeric microcellular foams by modified thermally induced phase separation method. Biomaterials 1999, 20, 1783–1790. [Google Scholar] [CrossRef]
- Ma, P.X. Biomimetic materials for tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; John, B. Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog. Polym. Sci. 2013, 38, 1487–1503. [Google Scholar] [CrossRef]
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Gerhardt, L.-C.; Boccaccini, A.R. Bioactive glass and glass-ceramic scaffolds for bone tissue engineering. Materials 2010, 3, 3867–3910. [Google Scholar] [CrossRef] [PubMed]
- Kumada, Y.; Zhang, S. Significant type I and type III collagen production from human periodontal ligament fibroblasts in 3D peptide scaffolds without extra growth factors. PLoS ONE 2010, 5, e10305. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Yamada, Y.; Shimojima, K.; Chino, Y.; Hosokawa, H.; Mabuchi, M. Novel titanium foam for bone tissue engineering. J. Mater. Res. 2002, 17, 2633–2639. [Google Scholar] [CrossRef]
- Rajzer, I.; Menaszek, E.; Castano, O. Electrospun polymer scaffolds modified with drugs for tissue engineering. Mater. Sci. Eng. C 2017, 77, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.M.; Jun, J.-H.; Chen, V.J.; Seo, J.; Baek, J.-H.; Ryoo, H.-M.; Kim, G.-S.; Somerman, M.J.; Ma, P.X. Nano-fibrous scaffolding promotes osteoblast differentiation and biomineralization. Biomaterials 2007, 28, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Krishna, B.V.; Bandyopadhyay, A.; Bose, S. Processing and biocompatibility evaluation of laser processed porous titanium. Acta Biomater. 2007, 3, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Sagomonyants, K.B.; Hakim-Zargar, M.; Jhaveri, A.; Aronow, M.S.; Gronowicz, G. Porous tantalum stimulates the proliferation and osteogenesis of osteoblasts from elderly female patients. J. Orthop. Res. 2011, 29, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Chawla, J.S.; Amiji, M.M. Biodegradable poly (ε-caprolactone) nanoparticles for tumor-targeted delivery of tamoxifen. Int. J. Pharm. 2002, 249, 127–138. [Google Scholar] [CrossRef]
- Shuai, X.; Ai, H.; Nasongkla, N.; Kim, S.; Gao, J. Micellar carriers based on block copolymers of poly (ε-caprolactone) and poly (ethylene glycol) for doxorubicin delivery. J. Control. Release 2004, 98, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Shive, M.S. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 1997, 28, 5–24. [Google Scholar] [CrossRef]
- Jain, R.A. The manufacturing techniques of various drug loaded biodegradable poly (lactide-co-glycolide)(PLGA) devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef]
- Wischke, C.; Schwendeman, S.P. Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles. Int. J. Pharm. 2008, 364, 298–327. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.-S.; Huang, G. Effects of emulsifiers on the controlled release of paclitaxel (Taxol®) from nanospheres of biodegradable polymers. J. Control. Release 2001, 71, 53–69. [Google Scholar] [CrossRef]
- Rosca, I.D.; Watari, F.; Uo, M. Microparticle formation and its mechanism in single and double emulsion solvent evaporation. J. Control. Release 2004, 99, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Grijpma, D.W.; Feijen, J. Porous polymeric structures for tissue engineering prepared by a coagulation, compression moulding and salt leaching technique. Biomaterials 2003, 24, 1937–1947. [Google Scholar] [CrossRef]
- Coombes, A.; Rizzi, S.; Williamson, M.; Barralet, J.; Downes, S.; Wallace, W. Precipitation casting of polycaprolactone for applications in tissue engineering and drug delivery. Biomaterials 2004, 25, 315–325. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. In The Biomaterials: Silver Jubilee Compendium; Elsevier: New York, NY, USA, 2006. [Google Scholar]
- Arany, P.; Huang, G.; Gadish, O.; Feliz, J.; Weaver, J.; Kim, J.; Yuen, W.; Mooney, D. Multi-lineage MSC differentiation via engineered morphogen fields. J. Dent. Res. 2014, 93, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Kang, S.G.; Kim, E.S.; Cho, S.H.; Lee, J.H. Fabrication and characterization of hydrophilic poly (lactic-co-glycolic acid)/poly (vinyl alcohol) blend cell scaffolds by melt-molding particulate-leaching method. Biomaterials 2003, 24, 4011–4021. [Google Scholar] [CrossRef]
- Nayani, K.; Katepalli, H.; Sharma, C.S.; Sharma, A.; Patil, S.; Venkataraghavan, R. Electrospinning combined with nonsolvent-induced phase separation to fabricate highly porous and hollow submicrometer polymer fibers. Ind. Eng. Chem. Res. 2011, 51, 1761–1766. [Google Scholar] [CrossRef]
- Wu, J.; Meredith, J.C. Assembly of chitin nanofibers into porous biomimetic structures via freeze drying. ACS Macro Lett. 2014, 3, 185–190. [Google Scholar] [CrossRef]
- Rahman, S.U.; Oh, J.-H.; Cho, Y.-D.; Chung, S.H.; Lee, G.; Baek, J.-H.; Ryoo, H.-M.; Woo, K.M. Fibrous topography-potentiated canonical Wnt signaling directs the odontoblastic differentiation of dental pulp-derived stem cells. ACS Appl. Mater. Interfaces 2018, 10, 17526–17541. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Kim, S.E.; Wei, J.; Hyun, Y.T.; Yun, H.S.; Kim, D.H.; Shin, J.W.; Shin, J.W. Fabrication and characterization of novel nano-and micro-HA/PCL composite scaffolds using a modified rapid prototyping process. J. Biomed. Mater. Res. Part A 2009, 89, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Ong, Y.X.J.; Lee, L.Y.; Davoodi, P.; Wang, C.-H. Production of drug-releasing biodegradable microporous scaffold using a two-step micro-encapsulation/supercritical foaming process. J. Supercrit. Fluids 2018, 133, 263–269. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216. [Google Scholar] [CrossRef]
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. Part A 2002, 60, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Webster, T.J. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Taipale, J.; Keski-Oja, J. Growth factors in the extracellular matrix. FASEB J. 1997, 11, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials 2000, 21, 1803–1810. [Google Scholar] [CrossRef]
- Buttiglieri, S.; Pasqui, D.; Migliori, M.; Johnstone, H.; Affrossman, S.; Sereni, L.; Wratten, M.; Barbucci, R.; Tetta, C.; Camussi, G. Endothelization and adherence of leucocytes to nanostructured surfaces. Biomaterials 2003, 24, 2731–2738. [Google Scholar] [CrossRef]
- Fan, Y.; Cui, F.; Hou, S.; Xu, Q.; Chen, L.; Lee, I.-S. Culture of neural cells on silicon wafers with nano-scale surface topograph. J. Neurosci. Methods 2002, 120, 17–23. [Google Scholar] [CrossRef]
- Lord, M.; Cousins, B.; Doherty, P.; Whitelock, J.; Simmons, A.; Williams, R.; Milthorpe, B. The effect of silica nanoparticulate coatings on serum protein adsorption and cellular response. Biomaterials 2006, 27, 4856–4862. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.; Hunt, J.; Gallagher, J.; Hanarp, P.; Sutherland, D.; Gold, J. Quantitative assessment of the response of primary derived human osteoblasts and macrophages to a range of nanotopography surfaces in a single culture model in vitro. Biomaterials 2003, 24, 4799–4818. [Google Scholar] [CrossRef]
- El-Ghannam, A.; Ducheyne, P.; Shapiro, I. Formation of surface reaction products on bioactive glass and their effects on the expression of the osteoblastic phenotype and the deposition of mineralized extracellular matrix. Biomaterials 1997, 18, 295–303. [Google Scholar] [CrossRef]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.O.; Herzyk, P.; Wilkinson, C.D.; Oreffo, R.O. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007, 6, 997. [Google Scholar] [CrossRef] [PubMed]
- You, M.-H.; Kwak, M.K.; Kim, D.-H.; Kim, K.; Levchenko, A.; Kim, D.-Y.; Suh, K.-Y. Synergistically enhanced osteogenic differentiation of human mesenchymal stem cells by culture on nanostructured surfaces with induction media. Biomacromolecules 2010, 11, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.Y.; Wen, J.; Yim, E.K.; Leong, K.W. Sustained release of proteins from electrospun biodegradable fibers. Biomacromolecules 2005, 6, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Wen, Y.; Zong, M.H.; Linhardt, R.J.; Wu, H. Encapsulation of Bioactive Compound in Electrospun Fibers and Its Potential Application. J. Agric. Food Chem. 2017, 65, 9161–9179. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, Y.; Liu, S.; Tan, L.; Mo, X.; Ramakrishna, S. Encapsulation of proteins in poly(l-lactide-co-caprolactone) fibers by emulsion electrospinning. Colloids Surf. B Biointerfaces 2010, 75, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, T.; Li, M.; Fu, N.; Fu, Y.; Ba, K.; Deng, S.; Jiang, Y.; Hu, J.; Peng, Q.; et al. Electrospun fibers for dental and craniofacial applications. Curr. Stem Cell Res. Ther. 2014, 9, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.X.; Arany, P.R.; Mooney, D.J. Modeling and Validation of Multilayer Poly(Lactide-Co-Glycolide) Scaffolds for In Vitro Directed Differentiation of Juxtaposed Cartilage and Bone. Tissue Eng. Part A 2015, 21, 2228–2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farahani, R.D.; Dubé, M.; Therriault, D. Three-dimensional printing of multifunctional nanocomposites: Manufacturing techniques and applications. Adv. Mater. 2016, 28, 5794–5821. [Google Scholar] [CrossRef] [PubMed]
- Utela, B.; Storti, D.; Anderson, R.; Ganter, M. A review of process development steps for new material systems in three dimensional printing (3DP). J. Manuf. Process. 2008, 10, 96–104. [Google Scholar] [CrossRef]
- Guillemot, F.; Souquet, A.; Catros, S.; Guillotin, B.; Lopez, J.; Faucon, M.; Pippenger, B.; Bareille, R.; Rémy, M.; Bellance, S. High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater. 2010, 6, 2494–2500. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Patanwala, H.S.; Bognet, B.; Ma, A.W. Inkjet and inkjet-based 3D printing: Connecting fluid properties and printing performance. Rapid Prototyp. J. 2017, 23, 562–576. [Google Scholar] [CrossRef]
- Rahman, S.U.; Arany, P.R. 3D bioprinting: Prostheses-restorations … now, tissues and organ systems! Oral Dis. 2017, 23, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Seol, Y.-J.; Kang, H.-W.; Lee, S.J.; Atala, A.; Yoo, J.J. Bioprinting technology and its applications. Eur. J. Cardio-Thorac. Surg. 2014, 46, 342–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, A.V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D printing of scaffolds for tissue regeneration applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312. [Google Scholar] [CrossRef] [PubMed]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Yoon, M.C.; Jeong, C.M.; Jang, J.; Jeong, S.I.; Cho, D.W.; Huh, J.B. Efficacy of rhBMP-2 loaded PCL/PLGA/beta-TCP guided bone regeneration membrane fabricated by 3D printing technology for reconstruction of calvaria defects in rabbit. Biomed. Mater. 2014, 9, 065006. [Google Scholar] [CrossRef] [PubMed]
- Fahimipour, F.; Rasoulianboroujeni, M.; Dashtimoghadam, E.; Khoshroo, K.; Tahriri, M.; Bastami, F.; Lobner, D.; Tayebi, L. 3D printed TCP-based scaffold incorporating VEGF-loaded PLGA microspheres for craniofacial tissue engineering. Dent. Mater. 2017, 33, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Nagrath, M.; Sikora, A.; Graca, J.; Chinnici, J.; Rahman, S.U.; Reddy, S.G.; Ponnusamy, S.; Maddi, A.; Arany, P.R. Functionalized Prosthetic Interfaces using 3D Printing: Generating Infection-Neutralizing Prosthesis for Dentistry. Mater. Commun. 2018, 15, 114–119. [Google Scholar] [CrossRef]
- Hsu, C.C.; Wobus, C.E.; Steffen, E.K.; Riley, L.K.; Livingston, R.S. Development of a microsphere-based serologic multiplexed fluorescent immunoassay and a reverse transcriptase PCR assay to detect murine norovirus 1 infection in mice. Clin. Diagn. Lab. Immunol. 2005, 12, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.M.-C.; Cuda, G.; Bunimovich, Y.L.; Gaspari, M.; Heath, J.R.; Hill, H.D.; Mirkin, C.A.; Nijdam, A.J.; Terracciano, R.; Thundat, T. Nanotechnologies for biomolecular detection and medical diagnostics. Curr. Opin. Chem. Biol. 2006, 10, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2012, 64, 24–36. [Google Scholar] [CrossRef]
- Chen, J.; Sheu, A.Y.; Li, W.; Zhang, Z.; Kim, D.H.; Lewandowski, R.J.; Omary, R.A.; Shea, L.D.; Larson, A.C. Poly(lactide-co-glycolide) microspheres for MRI-monitored transcatheter delivery of sorafenib to liver tumors. J. Control. Release 2014, 184, 10–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, J.; Shao, R.; Wei, X.; Gupta, S.; Li, C. Near-infrared light triggers release of Paclitaxel from biodegradable microspheres: Photothermal effect and enhanced antitumor activity. Small 2010, 6, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- McCabe, J.F.; Yan, Z.; Al Naimi, O.T.; Mahmoud, G.; Rolland, S.L. Smart materials in dentistry. Aust. Dent. J. 2011, 56 (Suppl. 1), 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 21, 745–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moraes, C.; Mehta, G.; Lesher-Perez, S.C.; Takayama, S. Organs-on-a-chip: A focus on compartmentalized microdevices. Ann. Biomed. Eng. 2012, 40, 1211–1227. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, A.D.; van den Berg, A. Organs-on-chips: Breaking the in vitro impasse. Integr. Biol. 2012, 4, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, P.; Zhou, L.; Qin, Z.; Gao, K.; Yao, J.; Li, C.; Wang, P. A biomimetic bioelectronic tongue: A switch for On- and Off-response of acid sensations. Biosens. Bioelectron. 2017, 92, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Imura, Y.; Yoshimura, E.; Sato, K. Micro total bioassay system for oral drugs: Evaluation of gastrointestinal degradation, intestinal absorption, hepatic metabolism, and bioactivity. Anal. Sci. 2012, 28, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Mauk, M.G.; Ziober, B.L.; Chen, Z.; Thompson, J.A.; Bau, H.H. Lab-on-a-chip technologies for oral-based cancer screening and diagnostics: Capabilities, issues, and prospects. Ann. N. Y. Acad. Sci. 2007, 1098, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson-Bouchard, K.; Vunjak-Novakovic, G. Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development. Cell Stem Cell 2018, 22, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Lind, J.U.; Busbee, T.A.; Valentine, A.D.; Pasqualini, F.S.; Yuan, H.; Yadid, M.; Park, S.-J.; Kotikian, A.; Nesmith, A.P.; Campbell, P.H. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat. Mater. 2017, 16, 303. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, E.; Morita, R.; Nakao, K.; Ishida, K.; Nakamura, T.; Takano-Yamamoto, T.; Ogawa, M.; Mizuno, M.; Kasugai, S.; Tsuji, T. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 13475–13480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, M.; Oshima, M.; Ogawa, M.; Sonoyama, W.; Hara, E.S.; Oida, Y.; Shinkawa, S.; Nakajima, R.; Mine, A.; Hayano, S.; et al. Practical whole-tooth restoration utilizing autologous bioengineered tooth germ transplantation in a postnatal canine model. Sci. Rep. 2017, 7, 44522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bursac, N.; Juhas, M.; Rando, T.A. Synergizing Engineering and Biology to Treat and Model Skeletal Muscle Injury and Disease. Annu. Rev. Biomed. Eng. 2015, 17, 217–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, W.M.; Jang, Y.C.; Garcia, A.J. Engineered matrices for skeletal muscle satellite cell engraftment and function. Matrix Biol. 2017, 60–61, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Handschin, C.; Mortezavi, A.; Plock, J.; Eberli, D. External physical and biochemical stimulation to enhance skeletal muscle bioengineering. Adv. Drug Deliv. Rev. 2015, 82–83, 168–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, X.; Ding, F.; Williams, D.F. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials 2014, 35, 6143–6156. [Google Scholar] [CrossRef] [PubMed]
- Spivey, E.C.; Khaing, Z.Z.; Shear, J.B.; Schmidt, C.E. The fundamental role of subcellular topography in peripheral nerve repair therapies. Biomaterials 2012, 33, 4264–4276. [Google Scholar] [CrossRef] [PubMed]
- Pfister, B.J.; Gordon, T.; Loverde, J.R.; Kochar, A.S.; Mackinnon, S.E.; Cullen, D.K. Biomedical engineering strategies for peripheral nerve repair: Surgical applications, state of the art, and future challenges. Crit. Rev. Biomed. Eng. 2011, 39, 81–124. [Google Scholar] [CrossRef] [PubMed]
- Carriel, V.; Alaminos, M.; Garzon, I.; Campos, A.; Cornelissen, M. Tissue engineering of the peripheral nervous system. Expert Rev. Neurother. 2014, 14, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Saracino, G.A.; Cigognini, D.; Silva, D.; Caprini, A.; Gelain, F. Nanomaterials design and tests for neural tissue engineering. Chem. Soc. Rev. 2013, 42, 225–262. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.A.; Liu, X.; Hu, J.; Ma, P.X. The enhancement of human embryonic stem cell osteogenic differentiation with nano-fibrous scaffolding. Biomaterials 2010, 31, 5526–5535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Prabhakaran, M.P.; Qin, X.; Ramakrishna, S. Biocomposite scaffolds for bone regeneration: Role of chitosan and hydroxyapatite within poly-3-hydroxybutyrate-co-3-hydroxyvalerate on mechanical properties and in vitro evaluation. J. Mech. Behav. Biomed. Mater. 2015, 51, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Reddy, V.J.; Wong, S.Y.; Li, X.; Su, B.; Ramakrishna, S.; Lim, C.T. Enhanced biomineralization in osteoblasts on a novel electrospun biocomposite nanofibrous substrate of hydroxyapatite/collagen/chitosan. Tissue Eng. Part A 2010, 16, 1949–1960. [Google Scholar] [CrossRef] [PubMed]
- Pangon, A.; Saesoo, S.; Saengkrit, N.; Ruktanonchai, U.; Intasanta, V. Hydroxyapatite-hybridized chitosan/chitin whisker bionanocomposite fibers for bone tissue engineering applications. Carbohydr. Polym. 2016, 144, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Frohbergh, M.E.; Katsman, A.; Botta, G.P.; Lazarovici, P.; Schauer, C.L.; Wegst, U.G.; Lelkes, P.I. Electrospun hydroxyapatite-containing chitosan nanofibers crosslinked with genipin for bone tissue engineering. Biomaterials 2012, 33, 9167–9178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, P.N.; Dwivedi, N.; Phillips, L.M.; Yu, X.; Herberg, S.; Bowerman, C.; Solorio, L.D.; Murphy, W.L.; Alsberg, E. Controlled Dual growth factor delivery from microparticles incorporated within human bone marrow-derived mesenchymal stem cell aggregates for enhanced bone tissue engineering via endochondral ossification. Stem Cells Transl. Med. 2016, 5, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Zhao, F.; Xie, W.; Zhang, W.; Fu, X.; Gao, W.; Lei, B.; Chen, X. 3D Printing Nanoscale Bioactive Glass Scaffolds Enhance Osteoblast Migration and Extramembranous Osteogenesis through Stimulating Immunomodulation. Adv. Healthc. Mater. 2018, e1800361. [Google Scholar] [CrossRef] [PubMed]
- Morales-Gomez, J.A.; Garcia-Estrada, E.; Leos-Bortoni, J.E.; Delgado-Brito, M.; Flores-Huerta, L.E.; De La Cruz-Arriaga, A.A.; Torres-Diaz, L.J.; de Leon, A.R.M. Cranioplasty with a low-cost customized polymethylmethacrylate implant using a desktop 3D printer. J. Neurosurg. 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Sun, M.; Zhang, F.; Liu, A.; He, Y.; Fu, J.; Yang, X.; Wang, H.; Gou, Z. Custom Repair of Mandibular Bone Defects with 3D Printed Bioceramic Scaffolds. J. Dent. Res. 2018, 97, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Mowry, S.E.; Jammal, H.; Myer, C., 4th; Solares, C.A.; Weinberger, P. A Novel Temporal Bone Simulation Model Using 3D Printing Techniques. Otol. Neurotol. 2015, 36, 1562–1565. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hill, R.G.; Mozafari, M. Bioactive Glasses: Sprouting Angiogenesis in Tissue Engineering. Trends Biotechnol. 2018, 36, 430–444. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Sun, Y.; Maciejewska, I.; Qin, D.; Peng, T.; McIntyre, B.; Wygant, J.; Butler, W.T.; Qin, C. Distribution of SIBLING proteins in the organic and inorganic phases of rat dentin and bone. Eur. J. Oral Sci. 2008, 116, 104–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, O.; Smith, A.; Cooper, P.; Shelton, R.; Scheven, B. The effects of cryopreservation on cells isolated from adipose, bone marrow and dental pulp tissues. Cryobiology 2014, 69, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Lan, Y.; Baek, J.-A.; Gao, Y.; Jiang, R. Wnt/beta-catenin signaling plays an essential role in activation of odontogenic mesenchyme during early tooth development. Dev. Biol. 2009, 334, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Bae, C.; Lee, J.; Ko, S.; Yang, X.; Jiang, R.; Cho, E. β-catenin is required in odontoblasts for tooth root formation. J. Dent. Res. 2013, 92, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Haruyama, N.; Hall, B.; Danton, M.J.; Zhang, L.; Arany, P.; Mooney, D.J.; Harichane, Y.; Goldberg, M.; Gibson, C.W.; et al. TGF-ss regulates enamel mineralization and maturation through KLK4 expression. PLoS ONE 2013, 8, e82267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galler, K.M.; D’Souza, R.N.; Hartgerink, J.D.; Schmalz, G. Scaffolds for dental pulp tissue engineering. Adv. Dent. Res. 2011, 23, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Mooney, D.J.; Powell, C.; Piana, J.; Rutherford, B. Engineering dental pulp-like tissue in vitro. Biotechnol. Prog. 1996, 12, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Jin, X.; Ma, H.; Hu, J.; Ni, L.; Ma, P.X. The odontogenic differentiation of human dental pulp stem cells on nanofibrous poly(l-lactic acid) scaffolds in vitro and in vivo. Acta Biomater. 2010, 6, 3856–3863. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, H.; Jin, X.; Hu, J.; Liu, X.; Ni, L.; Ma, P.X. The effect of scaffold architecture on odontogenic differentiation of human dental pulp stem cells. Biomaterials 2011, 32, 7822–7830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordeiro, M.M.; Dong, Z.; Kaneko, T.; Zhang, Z.; Miyazawa, M.; Shi, S.; Smith, A.J.; Nor, J.E. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J. Endod. 2008, 34, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Arany, P.R.; Cho, A.; Hunt, T.D.; Sidhu, G.; Shin, K.; Hahm, E.; Huang, G.X.; Weaver, J.; Chen, A.C.; Padwa, B.L.; et al. Photoactivation of endogenous latent transforming growth factor-beta1 directs dental stem cell differentiation for regeneration. Sci. Transl. Med. 2014, 6, 238ra69. [Google Scholar] [CrossRef] [PubMed]
- Vining, K.H.; Scherba, J.C.; Bever, A.M.; Alexander, M.R.; Celiz, A.D.; Mooney, D.J. Synthetic Light-Curable Polymeric Materials Provide a Supportive Niche for Dental Pulp Stem Cells. Adv. Mater. 2018, 30, 1704486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Nor, F.; Oh, M.; Cucco, C.; Shi, S.; Nor, J.E. Wnt/beta-Catenin Signaling Determines the Vasculogenic Fate of Postnatal Mesenchymal Stem Cells. Stem Cells 2016, 34, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhong, J.; Gong, Q.; Cheng, B.; Kim, S.G.; Ling, J.; Mao, J.J. Regenerative Endodontics by Cell Homing. Dent. Clin. N. Am. 2017, 61, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Xin, X.; Moioli, E.K.; Chung, J.; Lee, C.H.; Chen, M.; Fu, S.Y.; Koch, P.D.; Mao, J.J. Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Eng. Part A 2010, 16, 3023–3031. [Google Scholar] [CrossRef] [PubMed]
- Erisken, C.; Kalyon, D.M.; Zhou, J.; Kim, S.G.; Mao, J.J. Viscoelastic Properties of Dental Pulp Tissue and Ramifications on Biomaterial Development for Pulp Regeneration. J. Endod. 2015, 41, 1711–1717. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Liu, X. Formation of Nanofibrous Matrices, Three-Dimensional Scaffolds, and Microspheres: From Theory to Practice. Tissue Eng. Part C Methods 2017, 23, 50–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Ma, C.; Xie, X.; Sun, H.; Liu, X. Pulp regeneration in a full-length human tooth root using a hierarchical nanofibrous microsphere system. Acta Biomater. 2016, 35, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Qu, T.; Chang, B.; Jing, Y.; Feng, J.Q.; Liu, X. 3D Maskless Micropatterning for Regeneration of Highly Organized Tubular Tissues. Adv. Healthc. Mater. 2018, 7, 1700738. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, D. TMJ Bioengineering: A review. J. Oral Biol. Craniofac. Res. 2013, 3, 140–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helgeland, E.; Shanbhag, S.; Pedersen, T.O.; Mustafa, K.; Rosen, A. Scaffold-Based Temporomandibular Joint Tissue Regeneration in Experimental Animal Models: A Systematic Review. Tissue Eng. Part B Rev. 2018. [Google Scholar] [CrossRef]
- Blunk, T.; Sieminski, A.L.; Gooch, K.J.; Courter, D.L.; Hollander, A.P.; Nahir, A.M.; Langer, R.; Vunjak-Novakovic, G.; Freed, L.E. Differential effects of growth factors on tissue-engineered cartilage. Tissue Eng. 2002, 8, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Thorup, A.-S.; Eldridge, S.; Caxaria, S.; Nalesso, G.; Thomas, B.; Pitzalis, C.; Luyten, F.; Dell’Accio, F. A Potency Assay for Assessing the Chondrogenic Efficiency of Bioactive Molecules in Human Cartilage in vivo. Osteoarthr. Cartil. 2017, 25, S273. [Google Scholar] [CrossRef]
- Fortier, L.A.; Barker, J.U.; Strauss, E.J.; McCarrel, T.M.; Cole, B.J. The role of growth factors in cartilage repair. Clin. Orthop. Relat. Res. 2011, 469, 2706–2715. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.M.; Cukierman, E. Modeling tissue morphogenesis and cancer in 3D. Cell 2007, 130, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Itani, Y.; Asamura, S.; Matsui, M.; Tabata, Y.; Isogai, N. Evaluation of nanofiber-based polyglycolic acid scaffolds for improved chondrocyte retention and in vivo bioengineered cartilage regeneration. Plast. Reconstr. Surg. 2014, 133, 805e–813e. [Google Scholar] [CrossRef] [PubMed]
- Wright, L.D.; McKeon-Fischer, K.D.; Cui, Z.; Nair, L.S.; Freeman, J.W. PDLA/PLLA and PDLA/PCL nanofibers with a chitosan-based hydrogel in composite scaffolds for tissue engineered cartilage. J. Tissue Eng. Regen. Med. 2014, 8, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Abedi, G.; Sotoudeh, A.; Soleymani, M.; Shafiee, A.; Mortazavi, P.; Aflatoonian, M.R. A collagen-poly(vinyl alcohol) nanofiber scaffold for cartilage repair. J. Biomater. Sci. Polym. Ed. 2011, 22, 2445–2455. [Google Scholar] [CrossRef] [PubMed]
- Casper, M.E.; Fitzsimmons, J.S.; Stone, J.J.; Meza, A.O.; Huang, Y.; Ruesink, T.J.; O’Driscoll, S.W.; Reinholz, G.G. Tissue engineering of cartilage using poly-epsilon-caprolactone nanofiber scaffolds seeded in vivo with periosteal cells. Osteoarthr. Cartil. 2010, 18, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Castro, N.J.; Cheng, X.; Keidar, M.; Zhang, L.G. Cold Atmospheric Plasma Modified Electrospun Scaffolds with Embedded Microspheres for Improved Cartilage Regeneration. PLoS ONE 2015, 10, e0134729. [Google Scholar] [CrossRef] [PubMed]
- Legemate, K.; Tarafder, S.; Jun, Y.; Lee, C.H. Engineering Human TMJ Discs with Protein-Releasing 3D-Printed Scaffolds. J. Dent. Res. 2016, 95, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; Gronthos, S.; Ivanovski, S.; Fisher, A.; Hutmacher, D.W. Tissue engineered periodontal products. J. Periodontal Res. 2016, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.F.; Vaquette, C.; Zhang, Q.; Reis, R.L.; Ivanovski, S.; Hutmacher, D.W. Advanced tissue engineering scaffold design for regeneration of the complex hierarchical periodontal structure. J. Clin. Periodontol. 2014, 41, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Nadiri, A.; Kuchler-Bopp, S.; Perrin-Schmitt, F.; Peters, H.; Lesot, H. Tissue engineering of tooth crown, root, and periodontium. Tissue Eng. 2006, 12, 2069–2075. [Google Scholar] [CrossRef] [PubMed]
- Cochran, D.L.; Jones, A.; Heijl, L.; Mellonig, J.T.; Schoolfield, J.; King, G.N. Periodontal regeneration with a combination of enamel matrix proteins and autogenous bone grafting. J. Periodontol. 2003, 74, 1269–1281. [Google Scholar] [CrossRef] [PubMed]
- Fong, H.K.; Foster, B.L.; Popowics, T.E.; Somerman, M.J. The crowning achievement: Getting to the root of the problem. J. Dent. Educ. 2005, 69, 555–570. [Google Scholar] [PubMed]
- Ripamonti, U.; Reddi, A.H. Tissue engineering, morphogenesis, and regeneration of the periodontal tissues by bone morphogenetic proteins. Crit. Rev. Oral Biol. Med. 1997, 8, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, L.; Zhang, D.; Huang, S.; Jing, Z.; Wu, Y.; Zhao, Z.; Zhao, L.; Zhou, S. Incorporation of aligned PCL-PEG nanofibers into porous chitosan scaffolds improved the orientation of collagen fibers in regenerated periodontium. Acta Biomater. 2015, 25, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.G.; Yashiro, R.; Washio, K.; Yamato, M.; Okano, T.; Ishikawa, I. Periodontal ligament cell sheet promotes periodontal regeneration in athymic rats. J. Clin. Periodontol. 2008, 35, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Guo, W.; Ding, Y.; Gong, J.; Zou, Q.; Xie, D.; Chen, Y.; Wu, Y.; Tian, W. Comparative study of human dental follicle cell sheets and periodontal ligament cell sheets for periodontal tissue regeneration. Cell Transpl. 2013, 22, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Y.; Mao, L.; Peng, H. Periodontal healing by periodontal ligament cell sheets in a teeth replantation model. Arch. Oral Biol. 2012, 57, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Correa-Duarte, M.A.; Wagner, N.; Rojas-Chapana, J.; Morsczeck, C.; Thie, M.; Giersig, M. Fabrication and biocompatibility of carbon nanotube-based 3D networks as scaffolds for cell seeding and growth. Nano Lett. 2004, 4, 2233–2236. [Google Scholar] [CrossRef]
- Ratcliffe, A.; Butler, D.L.; Dyment, N.A.; Cagle, P.J., Jr.; Proctor, C.S.; Ratcliffe, S.S.; Flatow, E.L. Scaffolds for tendon and ligament repair and regeneration. Ann. Biomed. Eng. 2015, 43, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Lomas, A.J.; Ryan, C.N.; Sorushanova, A.; Shologu, N.; Sideri, A.I.; Tsioli, V.; Fthenakis, G.C.; Tzora, A.; Skoufos, I.; Quinlan, L.R.; et al. The past, present and future in scaffold-based tendon treatments. Adv. Drug Deliv. Rev. 2015, 84, 257–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinese, C.; Gagnieu, C.; Nottelet, B.; Rondot-Couzin, C.; Hunger, S.; Coudane, J.; Garric, X. In vivo evaluation of hybrid patches composed of PLA based copolymers and collagen/chondroitin sulfate for ligament tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1778–1788. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Han, Y.; Wong, Y.S.; Fuh, J.Y.H. Fibre-based scaffolding techniques for tendon tissue engineering. J. Tissue Eng. Regen. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hochleitner, G.; Chen, F.; Blum, C.; Dalton, P.D.; Amsden, B.; Groll, J. Melt electrowriting below the critical translation speed to fabricate crimped elastomer scaffolds with non-linear extension behaviour mimicking that of ligaments and tendons. Acta Biomater. 2018, 72, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Font Tellado, S.; Chiera, S.; Bonani, W.; Poh, P.S.P.; Migliaresi, C.; Motta, A.; Balmayor, E.R.; van Griensven, M. Heparin functionalization increases retention of TGF-beta2 and GDF5 on biphasic silk fibroin scaffolds for tendon/ligament-to-bone tissue engineering. Acta Biomater. 2018, 72, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.T.; Wikesjo, U.M.; Jung, U.W.; Lee, J.S.; Kim, T.G.; Kim, C.K. Comparison between a beta-tricalcium phosphate and an absorbable collagen sponge carrier technology for rhGDF-5-stimulated periodontal wound healing/regeneration. J. Periodontol. 2013, 84, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Oortgiesen, D.A.; Meijer, G.J.; Bronckers, A.L.; Walboomers, X.F.; Jansen, J.A. Regeneration of the periodontium using enamel matrix derivative in combination with an injectable bone cement. Clin. Oral Investig. 2013, 17, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Windisch, P.; Szendroi-Kiss, D.; Horvath, A.; Rosta, P.; Becker, J.; Gera, I.; Schwarz, F. Clinical and histologic evaluation of an enamel matrix derivative combined with a biphasic calcium phosphate for the treatment of human intrabony periodontal defects. J. Periodontol. 2008, 79, 1991–1999. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Fan, W.; Xiao, Y.; Hamlet, S.; Hutmacher, D.W.; Ivanovski, S. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials 2012, 33, 5560–5573. [Google Scholar] [CrossRef] [PubMed]
- Pitaru, S.; Narayanan, S.A.; Olson, S.; Savion, N.; Hekmati, H.; Alt, I.; Metzger, Z. Specific cementum attachment protein enhances selectively the attachment and migration of periodontal cells to root surfaces. J. Periodontal Res. 1995, 30, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Batool, F.; Morand, D.N.; Thomas, L.; Bugueno, I.M.; Aragon, J.; Irusta, S.; Keller, L.; Benkirane-Jessel, N.; Tenenbaum, H.; Huck, O. Synthesis of a Novel Electrospun Polycaprolactone Scaffold Functionalized with Ibuprofen for Periodontal Regeneration: An In Vitro and In Vivo Study. Materials 2018, 11, 580. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Kang, J.; Ji, B.; Guo, W.; Ding, Y.; Wu, Y.; Tian, W. Periodontal-Derived Mesenchymal Cell Sheets Promote Periodontal Regeneration in Inflammatory Microenvironment. Tissue Eng. Part A 2017, 23, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Asa’ad, F.; Pagni, G.; Pilipchuk, S.P.; Gianni, A.B.; Giannobile, W.V.; Rasperini, G. 3D-Printed Scaffolds and Biomaterials: Review of Alveolar Bone Augmentation and Periodontal Regeneration Applications. Int. J. Dent. 2016, 2016, 1239842. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Gallucci, G.O.; Buser, D.; Bosshardt, D.; Belser, U.C.; Yelick, P.C. Bioengineered periodontal tissue formed on titanium dental implants. J. Dent. Res. 2011, 90, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Gault, P.; Black, A.; Romette, J.L.; Fuente, F.; Schroeder, K.; Thillou, F.; Brune, T.; Berdal, A.; Wurtz, T. Tissue-engineered ligament: Implant constructs for tooth replacement. J. Clin. Periodontol. 2010, 37, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Bloomquist, R.F.; Parnell, N.F.; Phillips, K.A.; Fowler, T.E.; Tian, Y.Y.; Sharpe, P.T.; Streelman, J.T. Coevolutionary patterning of teeth and taste buds. Proc. Natl. Acad. Sci. USA 2015, 112, E5954–E5962. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.P.; Patel, V.N.; Liu, S.; Harrington, D.A.; Hoffman, M.P.; Jia, X.; Witt, R.L.; Farach-Carson, M.C.; Pradhan-Bhatt, S. Primary Salivary Human Stem/Progenitor Cells Undergo Microenvironment-Driven Acinar-Like Differentiation in Hyaluronate Hydrogel Culture. Stem Cells Transl. Med. 2017, 6, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Shubin, A.D.; Felong, T.J.; Schutrum, B.E.; Joe, D.S.; Ovitt, C.E.; Benoit, D.S. Encapsulation of primary salivary gland cells in enzymatically degradable poly (ethylene glycol) hydrogels promotes acinar cell characteristics. Acta Biomater. 2017, 50, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, S.J.; Soscia, D.A.; Oztan, B.; Mosier, A.P.; Jean-Gilles, R.; Gadre, A.; Cady, N.C.; Yener, B.; Castracane, J.; Larsen, M. The regulation of focal adhesion complex formation and salivary gland epithelial cell organization by nanofibrous PLGA scaffolds. Biomaterials 2012, 33, 3175–3186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Z.; Wu, T.; Xu, J.; Liu, G.; Xie, Y.; Zhang, C.; Wang, J.; Wang, S. Generation of bioartificial salivary gland using whole-organ decellularized bioscaffold. Cells Tissues Organs 2014, 200, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-W.; Hsiao, Y.-C.; Young, T.-H.; Yang, T.-L. Maintenance of the spheroid organization and properties of glandular progenitor cells by fabricated chitosan based biomaterials. Biomater. Sci. 2018, 6, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Tsuji, T. Functional Salivary Gland Regeneration. In Organ Regeneration; Springer: Berlin, Germany, 2017; pp. 135–151. [Google Scholar]
- Ozdemir, T.; Srinivasan, P.P.; Zakheim, D.R.; Harrington, D.A.; Witt, R.L.; Farach-Carson, M.C.; Jia, X.; Pradhan-Bhatt, S. Bottom-up assembly of salivary gland microtissues for assessing myoepithelial cell function. Biomaterials 2017, 142, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Furuhashi, A.; Zhang, Q.; Jiang, C.; Chang, T.-H.; Le, A. Induction of Salivary Gland–Like Cells from Dental Follicle Epithelial Cells. J. Dent. Res. 2017, 96, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.; Obana, A.; Usami, Y.; Sakai, M.; Nohara, K.; Egusa, H.; Sakai, T. Regenerating salivary glands in the microenvironment of induced pluripotent stem cells. BioMed Res. Int. 2015, 2015, 293570. [Google Scholar] [CrossRef] [PubMed]
- McCall, A.D.; Nelson, J.W.; Leigh, N.J.; Duffey, M.E.; Lei, P.; Andreadis, S.T.; Baker, O.J. Growth factors polymerized within fibrin hydrogel promote amylase production in parotid cells. Tissue Eng. Part A 2013, 19, 2215–2225. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.; Wang, C.-S.; Maruyama, C.; Lei, P.; Andreadis, S.; Baker, O. L1 Peptide–Conjugated Fibrin Hydrogels Promote Salivary Gland Regeneration. J. Dent. Res. 2017, 96, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Dawson, P.; Zhang, Q.; Harris, Z.; Limesand, K.H. Administration of growth factors promotes salisphere formation from irradiated parotid salivary glands. PLoS ONE 2018, 13, e0193942. [Google Scholar] [CrossRef] [PubMed]
- Lilliu, M.A.; Seo, Y.; Isola, M.; Charbonneau, A.; Zeitouni, A.; El-Hakim, M.; Tran, S. Natural extracellular matrix scaffolds recycled from human salivary digests: A morphometric study. Oral Dis. 2016, 22, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Nigam, S.K. Concise review: Can the intrinsic power of branching morphogenesis be used for engineering epithelial tissues and organs? Stem Cells Transl. Med. 2013, 2, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Cantara, S.I.; Soscia, D.A.; Sequeira, S.J.; Jean-Gilles, R.P.; Castracane, J.; Larsen, M. Selective functionalization of nanofiber scaffolds to regulate salivary gland epithelial cell proliferation and polarity. Biomaterials 2012, 33, 8372–8382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joraku, A.; Sullivan, C.A.; Yoo, J.; Atala, A. In-vitro reconstitution of three-dimensional human salivary gland tissue structures. Differentiation 2007, 75, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Ozdener, M.H.; Rawson, N.E. Primary culture of mammalian taste epithelium. In Epithelial Cell Culture Protocols; Springer: Berlin, Germany, 2012; pp. 95–107. [Google Scholar]
- Nishiyama, M.; Yuki, S.; Fukano, C.; Sako, H.; Miyamoto, T.; Tomooka, Y. Attempt to develop taste bud models in three-dimensional culture. Zool. Sci. 2011, 28, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, C.J.; Stone, L.M.; McPheeters, M.; Ogura, T.; Böttger, B.; Lasher, R.S.; Finger, T.E.; Kinnamon, S.C. Maintenance of rat taste buds in primary culture. Chem. Sens. 2001, 26, 861–873. [Google Scholar] [CrossRef]
- Morris-Wiman, J.; Brinkley, L.; Sego, R. An in vitro model for the study of taste papillae morphogenesis using branchial arch explants. Brain Res. Protoc. 2000, 5, 172–181. [Google Scholar] [CrossRef]
- Mbiene, J.P.; Maccallum, D.K.; Mistretta, C.M. Organ cultures of embryonic rat tongue support tongue and gustatory papilla morphogenesis in vitro without intact sensory ganglia. J. Comp. Neurol. 1997, 377, 324–340. [Google Scholar] [CrossRef]
- Farbman, A.I. Taste bud regeneration in organ culture. Ann. N. Y. Acad. Sci. 1974, 228, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Ookura, T.; Kawamoto, K.; Tsuzaki, H.; Mikami, Y.; Ito, Y.; Oh, S.-H.; Hino, A. Fibroblast and epidermal growth factors modulate proliferation and neural cell adhesion molecule expression in epithelial cells derived from the adult mouse tongue. In Vitro Cell. Dev. Biol.-Anim. 2002, 38, 365–372. [Google Scholar] [CrossRef]
- Kishi, M.; Emori, Y.; Tsukamoto, Y.; Abe, K. Changes in cell morphology and cell-to-cell adhesion induced by extracellular Ca2+ in cultured taste bud cells. Biosci. Biotechnol. Biochem. 2002, 66, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.M.; Jung, H.-M.; Oh, J.-H.; ur Rahman, S.; Kim, S.M.; Baek, J.-H.; Ryoo, H.-M. Synergistic effects of dimethyloxalylglycine and butyrate incorporated into α-calcium sulfate on bone regeneration. Biomaterials 2015, 39, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Oh, J.-H.; Jung, H.-M.; Choi, Y.; Rahman, S.U.; Kim, S.; Kim, T.-I.; Shin, H.-I.; Lee, Y.-S.; Frank, H.Y. Effects of the incorporation of ε-aminocaproic acid/chitosan particles to fibrin on cementoblast differentiation and cementum regeneration. Acta Biomater. 2017, 61, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, H.; Niu, X.; Yu, B.; Fan, Y.; Feng, Q.; Cui, F.-Z.; Watari, F. The use of carbon nanotubes to induce osteogenic differentiation of human adipose-derived MSCs in vitro and ectopic bone formation in vivo. Biomaterials 2012, 33, 4818–4827. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, Y.; Zheng, L.; Liu, H.; Niu, X.; Huang, J.; Zhao, F.; Fan, Y. Effect of substrate stiffness on the functions of rat bone marrow and adipose tissue derived mesenchymal stem cells in vitro. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2014, 102, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Kimira, Y.; Odaira, H.; Nomura, K.; Taniuchi, Y.; Inoue, N.; Nakatani, S.; Shimizu, J.; Wada, M.; Mano, H. Collagen-derived dipeptide prolyl-hydroxyproline promotes osteogenic differentiation through Foxg1. Cell. Mol. Biol. Lett. 2017, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Fu, N.; Liao, J.; Lin, S.; Sun, K.; Tian, T.; Zhu, B.; Lin, Y. PCL-PEG-PCL film promotes cartilage regeneration in vivo. Cell Prolif. 2016, 49, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A.; Ginalska, G. Chitosan/β-1, 3-glucan/hydroxyapatite bone scaffold enhances osteogenic differentiation through TNF-α-mediated mechanism. Mater. Sci. Eng. C 2017, 73, 225–233. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, S.U.; Nagrath, M.; Ponnusamy, S.; Arany, P.R. Nanoscale and Macroscale Scaffolds with Controlled-Release Polymeric Systems for Dental Craniomaxillofacial Tissue Engineering. Materials 2018, 11, 1478. https://doi.org/10.3390/ma11081478
Rahman SU, Nagrath M, Ponnusamy S, Arany PR. Nanoscale and Macroscale Scaffolds with Controlled-Release Polymeric Systems for Dental Craniomaxillofacial Tissue Engineering. Materials. 2018; 11(8):1478. https://doi.org/10.3390/ma11081478
Chicago/Turabian StyleRahman, Saeed Ur, Malvika Nagrath, Sasikumar Ponnusamy, and Praveen R. Arany. 2018. "Nanoscale and Macroscale Scaffolds with Controlled-Release Polymeric Systems for Dental Craniomaxillofacial Tissue Engineering" Materials 11, no. 8: 1478. https://doi.org/10.3390/ma11081478